Poloxamer 407 Based Gel Formulations for Transungual Delivery of Hydrophobic Drugs: Selection and Optimization of Potential Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nail Clippings and Ethical Approval
2.3. Screening of Penetration Enhancers
2.4. Topography of Nail
2.5. Preparation of P407 Gel
2.6. Estimation of Drug Loading in Gels
2.7. Experimental Design
2.8. Determination of Gelation Temperature
2.9. Determination of Gel Strength
2.10. Rheology
2.11. Washability/Erosion Profile of P407 Gel
2.12. In Vitro Drug Release
2.13. Kinetic Modelling of Erosion Profile and In Vitro Drug Release
2.14. In Vitro Drug Permeation
3. Results
3.1. Screening of Penetration Enhancers Based on Hydration Enhancement Factor
3.2. Effect of Penetration Enhancer on the Surface Morphology of Human Nail
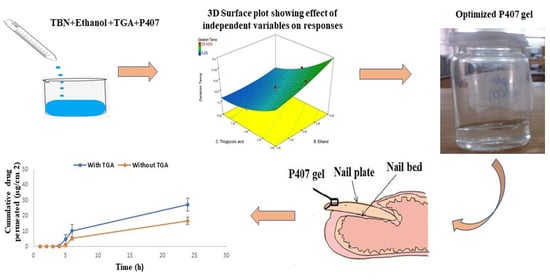
3.3. Effect of Independent Variables on Gelation Temperature of P407 Gel
3.4. Effect of Independent Variables on Gel Strength
3.5. Response Optimization
3.6. Effect of Preparation Method on Loading of Drug
3.7. Rheology
3.8. Washability/Gel Erosion
3.9. In Vitro Release Profile
3.10. Kinetic Modelling of Erosion Profile and Drug Release
3.11. In Vitro Drug Permeation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Mehta, D.M.; Shelat, P.K. Microemulsion-Based Antifungal Gel Delivery to Nail for the Treatment of Onychomycosis: Formulation, Optimization, and Efficacy Studies. Drug Deliv. Transl. Res. 2012, 2, 463–476. [Google Scholar] [CrossRef]
- Bseiso, E.A.; Nasr, M.; Sammour, O.A.; Abd El Gawad, N.A. Novel Nail Penetration Enhancer Containing Vesicles “NPEVs” for Treatment of Onychomycosis. Drug Deliv. 2016, 23, 2813–2819. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Miyamoto, M.; Sugibayashi, K.; Morimoto, Y. Enhancing Effect of N-Acetyl-L-Cysteine or 2-Mercaptoethanol on the in Vitro Permeation of 5-Fluorouracil or Tolnaftate through the Human Nail Plate. Chem. Pharm. Bull. 1998, 46, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Quintanar-Guerrero, D.; Ganem-Quintanar, A.; Tapia-Olguín, P.; Kalia, Y.N.; Buri, P. The Effect of Keratolytic Agents on the Permeability of Three Imidazole Antimycotic Drugs through the Human Nail. Drug Dev. Ind. Pharm. 1998, 24, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Mohorčič, M.; Torkar, A.; Friedrich, J.; Kristl, J.; Murdan, S. An Investigation into Keratinolytic Enzymes to Enhance Ungual Drug Delivery. Int. J. Pharm. 2007, 332, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Vejnovic, I.; Simmler, L.; Betz, G. Investigation of Different Formulations for Drug Delivery through the Nail Plate. Int. J. Pharm. 2010, 386, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.A.; Hao, J.; Li, S.K. Effects of Organic Solvents on the Barrier Properties of Human Nail. J. Pharm. Sci. 2011, 100, 4244–4257. [Google Scholar] [CrossRef]
- Felzenszwalb, I.; da Silva Fernandes, A.; Brito, L.B.; Oliveira, G.A.R.; Silva, P.A.S.; Arcanjo, M.E.; da Costa Marques, M.R.; Vicari, T.; Leme, D.M.; Cestari, M.M. Toxicological Evaluation of Nail Polish Waste Discarded in the Environment. Environ. Sci. Pollut. Res. 2019, 26, 27590–27603. [Google Scholar] [CrossRef]
- Elsayed, M.M.A. Development of Topical Therapeutics for Management of Onychomycosis and Other Nail Disorders: A Pharmaceutical Perspective. J. Control. Release 2015, 199, 132–144. [Google Scholar] [CrossRef]
- Ranch, K.M.; Maulvi, F.A.; Naik, M.J.; Koli, A.R.; Parikh, R.K.; Shah, D.O. Optimization of a Novel in Situ Gel for Sustained Ocular Drug Delivery Using Box-Behnken Design: In Vitro, Ex Vivo, in Vivo and Human Studies. Int. J. Pharm. 2019, 554, 264–275. [Google Scholar] [CrossRef]
- Das, P.; Jana, N.R. Biomedical Applications of Functional Polyaspartamide-Based Materials. ACS Appl. Polym. Mater. 2021. [Google Scholar] [CrossRef]
- Hui, X.; Wester, R.C.; Barbadillo, S.; Lee, C.; Patel, B.; Wortzmman, M.; Gans, E.H.; Maibach, H.I. Ciclopirox Delivery into the Human Nail Plate. J. Pharm. Sci. 2004, 93, 2545–2548. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Dubo, J.; Barraza, N.; González, R.; Veloso, E. Hybrid Chitosan-Pluronic F-127 Films with BaTiO3:Co Nanoparticles: Synthesis and Properties. J. Magn. Magn. Mater. 2015, 377, 65–69. [Google Scholar] [CrossRef]
- Jabarian, L.E.; Rouini, M.R.; Atyabi, F.; Foroumadi, A.; Nassiri, S.M.; Dinarvand, R. In Vitro and in Vivo Evaluation of an in Situ Gel Forming System for the Delivery of PEGylated Octreotide. Eur. J. Pharm. Sci. 2013, 48, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, S.T.; Özer, Ö. Novel Topical Formulations of Terbinafine-HCl for Treatment of Onychomycosis. Eur. J. Pharm. Sci. 2013, 48, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Du, F.; Song, L.; Zhang, H.; Yang, J.; Cao, H.; Zheng, Y.; Yang, Z.; Wang, G.; Yang, H.; et al. Synthesis and Characterization of Reactive Poloxamer 407s for Biomedical Applications. J. Control. Release 2009, 138, 49–56. [Google Scholar] [CrossRef]
- Abou-Shamat, M.A.; Calvo-Castro, J.; Stair, J.L.; Cook, M.T. Modifying the Properties of Thermogelling Poloxamer 407 Solutions through Covalent Modification and the Use of Polymer Additives. Macromol. Chem. Phys. 2019, 220, 1900173. [Google Scholar] [CrossRef]
- Ju, C.; Sun, J.; Zi, P.; Jin, X.; Zhang, C. Thermosensitive Micelles–Hydrogel Hybrid System Based on Poloxamer 407 for Localized Delivery of Paclitaxel. J. Pharm. Sci. 2013, 102, 2707–2717. [Google Scholar] [CrossRef]
- Chouhan, P.; Saini, T.R. Hydration of Nail Plate: A Novel Screening Model for Transungual Drug Permeation Enhancers. Int. J. Pharm. 2012, 436, 179–182. [Google Scholar] [CrossRef]
- Jessup, C.J.; Ghannoum, M.A.; Ryder, N.S. An Evaluation of the in Vitro Activity of Terbinafine. Med. Mycol. 2000, 38, 155–159. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J.; Pena-Pereira, F. Environmental Risk-Based Ranking of Solvents Using the Combination of a Multimedia Model and Multi-Criteria Decision Analysis. Green Chem. 2017, 19, 1034–1042. [Google Scholar] [CrossRef]
- Xuan, J.-J.; Balakrishnan, P.; Oh, D.H.; Yeo, W.H.; Park, S.M.; Yong, C.S.; Choi, H.-G. Rheological Characterization and in Vivo Evaluation of Thermosensitive Poloxamer-Based Hydrogel for Intramuscular Injection of Piroxicam. Int. J. Pharm. 2010, 395, 317–323. [Google Scholar] [CrossRef]
- Ur-Rehman, T.; Tavelin, S.; Gröbner, G. Effect of DMSO on Micellization, Gelation and Drug Release Profile of Poloxamer 407. Int. J. Pharm. 2010, 394, 92–98. [Google Scholar] [CrossRef]
- Choi, H.G.; Lee, M.K.; Kim, M.H.; Kim, C.K. Effect of Additives on the Physicochemical Properties of Liquid Suppository Bases. Int. J. Pharm. 1999, 190, 13–19. [Google Scholar] [CrossRef]
- Ur-Rehman, T.; Tavelin, S.; Gröbner, G. Chitosan in Situ Gelation for Improved Drug Loading and Retention in Poloxamer 407 Gels. Int. J. Pharm. 2011, 409, 19–29. [Google Scholar] [CrossRef]
- Vaghasiya, H.; Kumar, A.; Sawant, K. Development of Solid Lipid Nanoparticles Based Controlled Release System for Topical Delivery of Terbinafine Hydrochloride. Eur. J. Pharm. Sci. 2013, 49, 311–322. [Google Scholar] [CrossRef]
- Murdan, S. Enhancing the Nail Permeability of Topically Applied Drugs. Expert Opin. Drug Deliv. 2008, 5, 1267–1282. [Google Scholar] [CrossRef]
- Khengar, R.H.; Jones, S.A.; Turner, R.B.; Forbes, B.; Brown, M.B. Nail Swelling as a Pre-Formulation Screen for the Selection and Optimisation of Ungual Penetration Enhancers. Pharm. Res. 2007, 24, 2207–2212. [Google Scholar] [CrossRef]
- Patel, H.R.; Patel, R.P.; Patel, M.M. Poloxamers: A Pharmaceutical Excipients with Therapeutic Behaviors. Int. J. PharmTech Res. 2009, 1, 299–303. [Google Scholar]
- Meloun, M.; Bordovská, S.; Galla, L. The Thermodynamic Dissociation Constants of Clotrimazole, Terbinafine Hcl, Acetylsalicylic Acid, Salicylic Acid, and Galanthamine by the Nonlinear Regression of Multiwavelength Spectrophotometric Ph-Titration Data. SRX Pharmacol. 2010. [Google Scholar] [CrossRef]
- Kwon, K.-W.; Park, M.J.; Hwang, J.; Char, K. Effects of Alcohol Addition on Gelation in Aqueous Solution of PEO-PPO-PEO Triblock Copolymer. Polym. J. 2001, 33, 404–410. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Cristiano, M.C.; Fresta, M.; Cosco, D. Rutin-Loaded Poloxamer 407-Based Hydrogels for in Situ Administration: Stability Profiles and Rheological Properties. Nanomaterials 2020, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.-J.; Yan, Y.-D.; Oh, D.H.; Choi, Y.K.; Yong, C.S.; Choi, H.-G. Development of Thermo-Sensitive Injectable Hydrogel with Sustained Release of Doxorubicin: Rheological Characterization and in Vivo Evaluation in Rats. Drug Deliv. 2011, 18, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling Properties of Purified Poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final Amended Report on the Safety Assessment of Ammonium Thioglycolate, Butyl Thioglycolate, Calcium Thioglycolate, Ethanolamine Thioglycolate, Ethyl Thioglycolate, Glyceryl Thioglycolate, Isooctyl Thioglycolate, Isopropyl Thioglycolate, Magnesium Thiogl. Int. J. Toxicol. 2009, 28 (Suppl. 4), 68–133. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.W.; El-Ashmawy, A.A.; Mursi, N.M.; Emara, L.H. Optimization of Gliclazide Loaded Alginate-Gelatin Beads Employing Central Composite Design. Drug Dev. Ind. Pharm. 2019, 45, 1959–1972. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines 2019, 6, 7. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of Thermo-Reversible Pluronic F-127 Gels in Pharmaceutical Formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Ranch, K.M.; Maulvi, F.A.; Koli, A.R.; Desai, D.T.; Parikh, R.K.; Shah, D.O. Tailored Doxycycline Hyclate Loaded In Situ Gel for the Treatment of Periodontitis: Optimization, In Vitro Characterization, and Antimicrobial Studies. AAPS PharmSciTech 2021, 22, 1–11. [Google Scholar] [CrossRef]
- Mortensen, K.; Pedersen, J.S. Structural Study on the Micelle Formation of Poly(Ethylene Oxide)–Poly(Propylene Oxide)–Poly(Ethylene Oxide) Triblock Copolymer in Aqueous Solution. Macromolecules 1993, 26, 805–812. [Google Scholar] [CrossRef]
- Li, H.; Yu, G.-E.; Price, C.; Booth, C.; Hecht, E.; Hoffmann, H. Concentrated Aqueous Micellar Solutions of Diblock Copoly(Oxyethylene/Oxybutylene) E 41 B 8: A Study of Phase Behavior. Macromolecules 2002. [Google Scholar] [CrossRef]
- Kjøniksen, A.L.; Calejo, M.T.; Zhu, K.; Nyström, B.; Sande, S.A. Stabilization of Pluronic Gels in the Presence of Different Polysaccharides. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Abdul Rahman, M.N.; Qader, O.A.J.A.; Sukmasari, S.; Ismail, A.F.; Doolaanea, A.A. Rheological Characterization of Different Gelling Polymers for Dental Gel Formulation. J. Pharm. Sci. Res. 2017, 9, 2633–2640. [Google Scholar]
- Goo, Y.T.; Yang, H.M.; Kim, C.H.; Kim, M.S.; Kim, H.K.; Chang, I.H.; Choi, Y.W. Optimization of a Floating Poloxamer 407-Based Hydrogel Using the Box-Behnken Design: In Vitro Characterization and in Vivo Buoyancy Evaluation for Intravesical Instillation. Eur. J. Pharm. Sci. 2021, 163, 105885. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Saccomani, L.; Chetoni, P.; Burgalassi, S.; Senesi, S.; Ghelardi, E.; Mailland, F. Hydrosoluble Medicated Nail Lacquers: In Vitro Drug Permeation and Corresponding Antimycotic Activity. Br. J. Dermatol. 2010, 162, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hui, P.C.L.; Wat, E.; Ng, F.S.F.; Kan, C.W.; Wang, X.; Wong, E.C.W.; Hu, H.; Chan, B.; Lau, C.B.S.; et al. In Vitro Drug Release and Percutaneous Behavior of Poloxamer-Based Hydrogel Formulation Containing Traditional Chinese Medicine. Colloids Surf. B Biointerfaces 2016, 148, 526–532. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Li, N.; Gao, J.Q.; Sun, H.; Chen, S. Sustained Delivery of IL-1Ra from Pluronic F127-Based Thermosensitive Gel Prolongs Its Therapeutic Potentials. Pharm. Res. 2012, 29, 3475–3485. [Google Scholar] [CrossRef]
- Gupta, A.K.; Paquet, M. Improved Efficacy in Onychomycosis Therapy. Clin. Dermatol. 2013, 31, 555–563. [Google Scholar] [CrossRef]
- Darkes, M.J.M.; Scott, L.J.; Goa, K.L. Terbinafine: A Review of Its Use in Onychomycosis in Adults. Am. J. Clin. Dermatol. 2003, 4, 39–65. [Google Scholar] [CrossRef]










| S.No. | Penetration Enhancer | Concentration in the Nail Treatment Solution % w/v | Hydration Enhancement Factor (HEF) Mean ± SD |
|---|---|---|---|
| 1. | Thiourea | 5 | 1.19 ± 0.06 |
| 2. | Sodium lauryl sulphate | 5 | 0.91 ± 0.13 |
| 3. | Tween 20 | 5 | 1.23 ± 0.08 |
| 4. | Tween 80 | 5 | 1.14 ± 0.21 |
| 5. | DMSO | 5 | 1.18 ± 0.46 |
| 6. | Oxalic acid | 5 | 1.42 ± 0.15 |
| 7. | Urea | 5 | 1.04 ± 0.19 |
| 8. | Thioglycolic acid | 5 | 2.73 ± 0.43 |
| 9. | Glycolic acid | 5 | 1.42 ± 0.15 |
| 10. | Mercaptoethanol | 5 | 1.76 ± 0.47 |
| 11. | Resorcinol | 5 | 1.39 ± 0.16 |
| 12. | Alpha cyclodextrin | 5 | 1.22 ± 0.18 |
| 13. | Hydroxy Propyl-β-Cyclodextrin | 5 | 0.87 ± 0.08 |
| 14. | Chitosan | 1 | 1.08 ± 0.28 |
| 15. | Chitosan | 0.5 | 0.86 ± 0.05 |
| 16. | Thiolated chitosan | 0.5 | 1.23 ± 0.17 |
| 17. | β-Cyclodextrin | 5 | 1.09 ± 0.13 |
| 18. | Tartaric acid | 5 | 1.44 ± 0.07 |
| 19. | Methionine | 5 | 0.98 ± 0.12 |
| 20. | Lactic acid | 5 | 1.32 ± 0.10 |
| 21. | Ethanol | 5 | 1.06 ± 0.12 |
| 22. | Ethanol | 20 | 1.24 ± 0.22 |
| 23. | Poloxamer 407 | 5 | 1.20 ± 0.06 |
| Code | P407 (% w/w) | Ethanol (% w/w) | TGA (% w/w) | Gelation Temp (°C) | Gel Strength (s) |
|---|---|---|---|---|---|
| F1 | 26 | 2 | 10 | 13.46 ± 0.28 | 92.66 ± 5.13 |
| F2 | 28 | 6 | 5 | 8.43 ± 0.28 | 122.33 ± 8.50 |
| F3 | 30 | 10 | 0 | 11.85 ± 0.25 | 124.66 ± 9.50 |
| F4 | 26 | 6 | 5 | 15.46 ± 0.18 | 93.66 ± 6.02 |
| F5 | 30 | 2 | 10 | 5.25 ± 0.16 | 156 ± 7.21 |
| F6 | 28 | 6 | 10 | 6.53 ± 0.09 | 119 ± 8.54 |
| F7 | 28 | 10 | 5 | 10.4 ± 0.32 | 111 ± 7.54 |
| F8 | 26 | 10 | 0 | 20.43 ± 0.23 | 69 ± 7.54 |
| F9 | 30 | 10 | 10 | 6.33 ± 0.09 | 149 ± 9.16 |
| F10 | 30 | 6 | 5 | 5.93 ±0.23 | 142.33 ± 6.65 |
| F11 | 30 | 2 | 0 | 7.38± 0.35 | 131 ± 7.54 |
| F12 | 28 | 6 | 5 | 8.53 ± 0.14 | 122 ± 4.35 |
| F13 | 28 | 6 | 5 | 8.66 ± 0.16 | 121.33 ± 9.29 |
| F14 | 26 | 2 | 0 | 17.35 ± 0.25 | 82 ± 5.56 |
| F15 | 28 | 6 | 5 | 8.61 ± 0.32 | 124.3 ± 7.02 |
| F16 | 28 | 6 | 5 | 9.2 ± 0.23 | 126.33 ± 7.57 |
| F17 | 28 | 6 | 0 | 12.7 ± 0.21 | 112.66 ± 5.50 |
| F18 | 28 | 2 | 5 | 7.71 ± 0.21 | 131 ± 8.88 |
| F19 | 28 | 6 | 5 | 8.38 ± 0.22 | 124.33 ± 4.93 |
| F20 | 26 | 10 | 10 | 14.3 ± 0.32 | 84.33 ± 5.50 |
| Model | For Gel Erosion | For Drug Release | |
|---|---|---|---|
| Zero order | K | 3.89 | 6.60 |
| R2 | 0.9989 | 0.8894 | |
| First order | k1 | 0.048 | 0.098 |
| R2 | 0.9814 | 0.9918 | |
| Higuchi | kH | 11.01 | 18.14 |
| R2 | 0.8142 | 0.9475 | |
| Korsmeyer–Peppas | kKP | 3.72 | 12.99 |
| R2 | 0.9990 | 0.9902 | |
| n | 1.02 | 0.67 | |
| Hixson–Crowell | kHC | 0.015 | 0.02 |
| R2 | 0.9898 | 0.9769 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, K.H.; Raza, F.; Munawar, S.M.; Sohail, M.; Zafar, H.; Zafar, M.I.; Ur-Rehman, T. Poloxamer 407 Based Gel Formulations for Transungual Delivery of Hydrophobic Drugs: Selection and Optimization of Potential Additives. Polymers 2021, 13, 3376. https://doi.org/10.3390/polym13193376
Ullah KH, Raza F, Munawar SM, Sohail M, Zafar H, Zafar MI, Ur-Rehman T. Poloxamer 407 Based Gel Formulations for Transungual Delivery of Hydrophobic Drugs: Selection and Optimization of Potential Additives. Polymers. 2021; 13(19):3376. https://doi.org/10.3390/polym13193376
Chicago/Turabian StyleUllah, Kamran Hidayat, Faisal Raza, Syed Mohsin Munawar, Muhammad Sohail, Hajra Zafar, Mazhar Iqbal Zafar, and Tofeeq Ur-Rehman. 2021. "Poloxamer 407 Based Gel Formulations for Transungual Delivery of Hydrophobic Drugs: Selection and Optimization of Potential Additives" Polymers 13, no. 19: 3376. https://doi.org/10.3390/polym13193376
APA StyleUllah, K. H., Raza, F., Munawar, S. M., Sohail, M., Zafar, H., Zafar, M. I., & Ur-Rehman, T. (2021). Poloxamer 407 Based Gel Formulations for Transungual Delivery of Hydrophobic Drugs: Selection and Optimization of Potential Additives. Polymers, 13(19), 3376. https://doi.org/10.3390/polym13193376