Effect of Vulcanization and CO2 Plasticization on Cell Morphology of Silicone Rubber in Temperature Rise Foaming Process
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
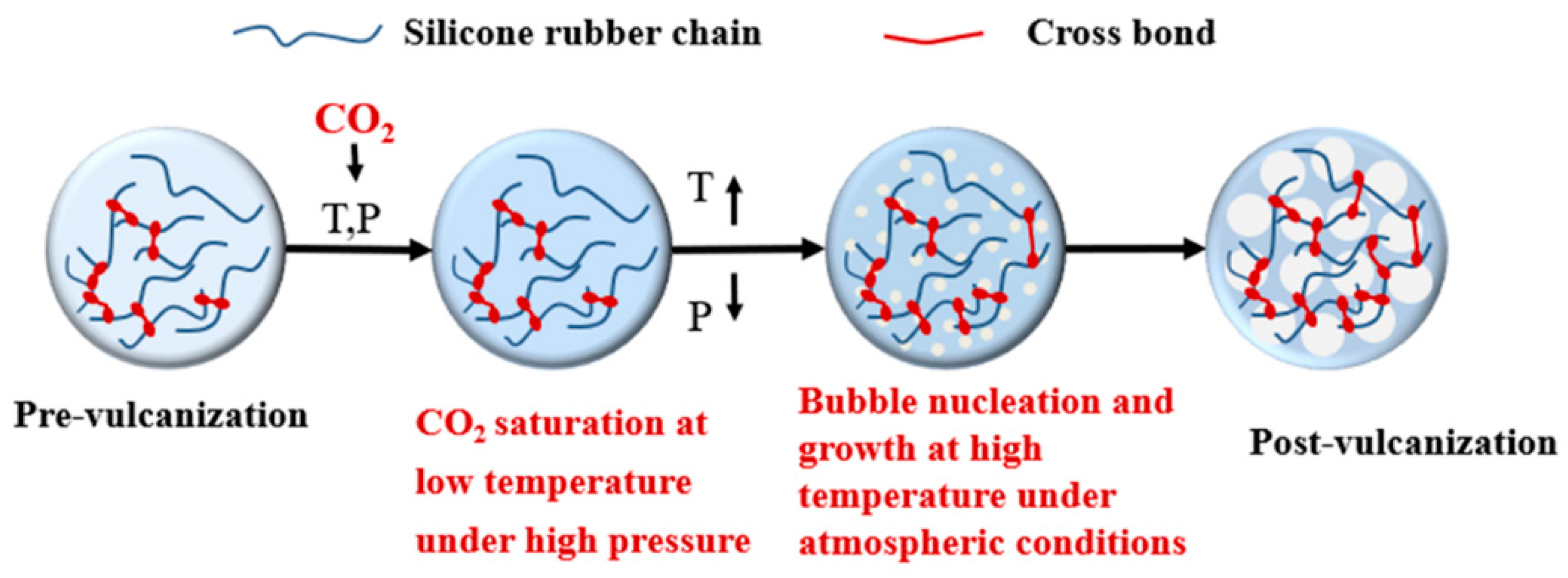
2.2. Pre-Vulcanization Process
2.3. Step Temperature-Rising Foaming Process
2.4. Characterization
2.4.1. Differential Scanning Calorimetry (DSC) Analysis
2.4.2. Rheological Properties Measurements
2.4.3. Foam Characterization
3. Results and Discussion
3.1. Foamable Range of Pre-Vulcanization Degree
3.2. Effect of CO2 Plasticization Derived from CO2 Saturation
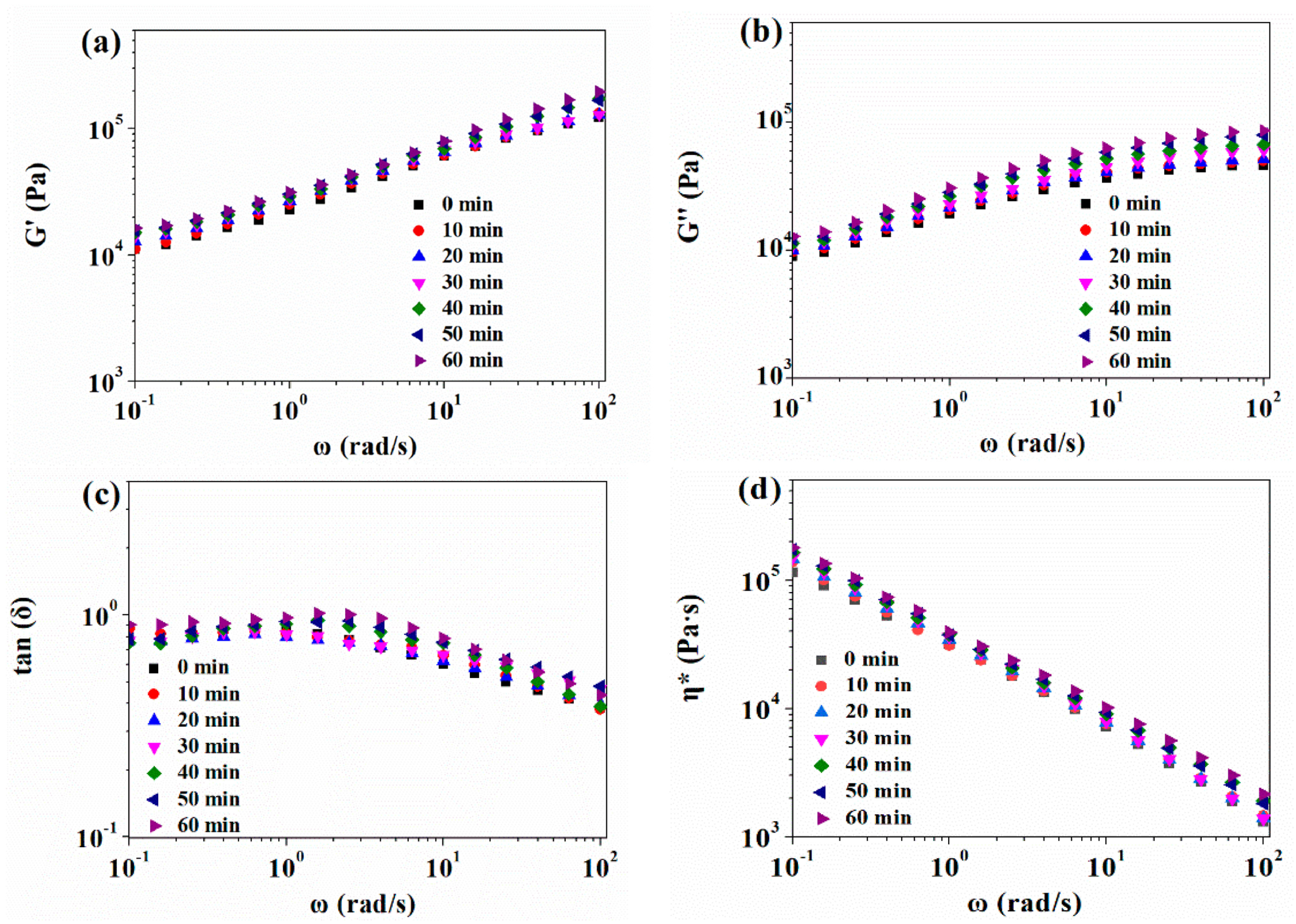
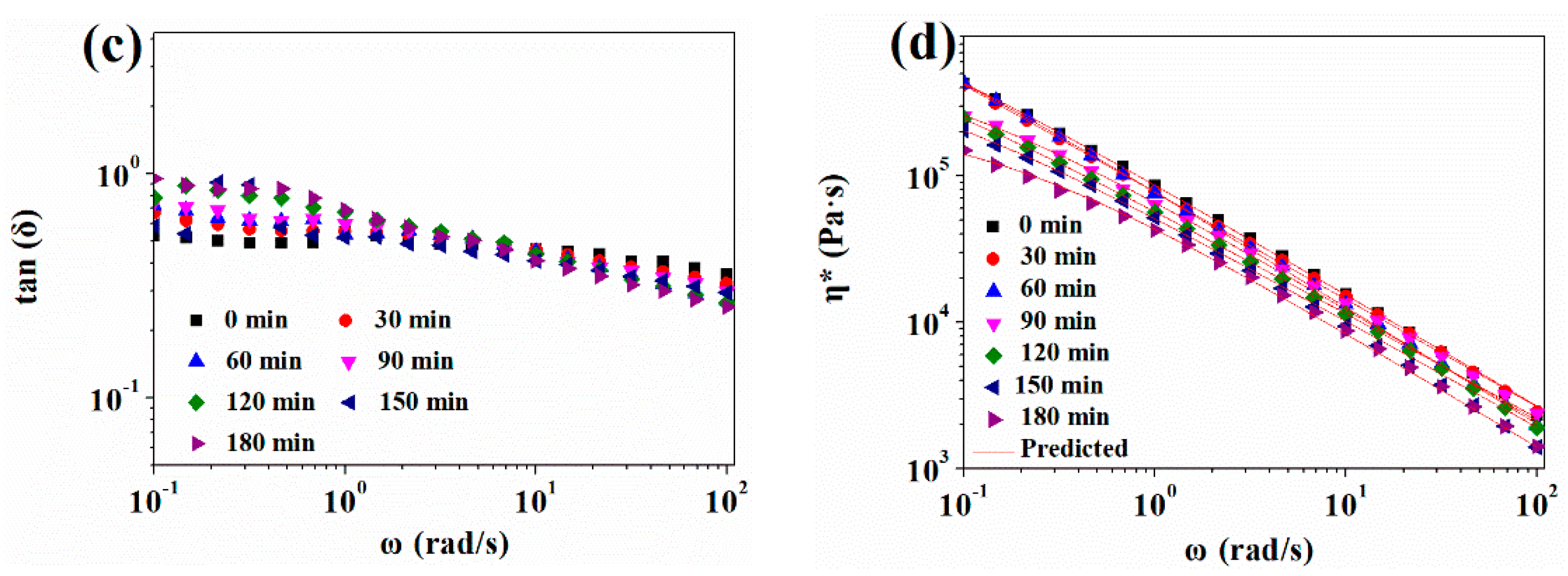
3.2.1. Effect of CO2 Plasticization on Rheological Behavior
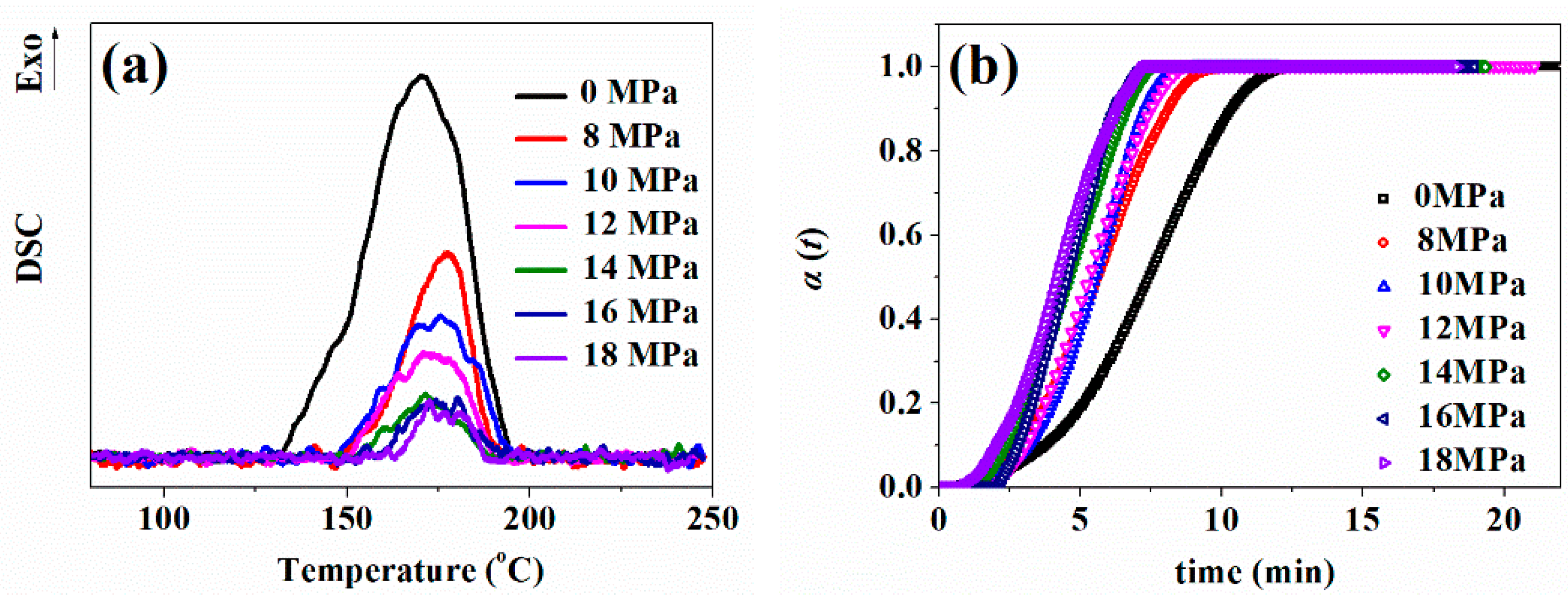
3.2.2. Effect of CO2 Plasticization on Vulcanization Reaction
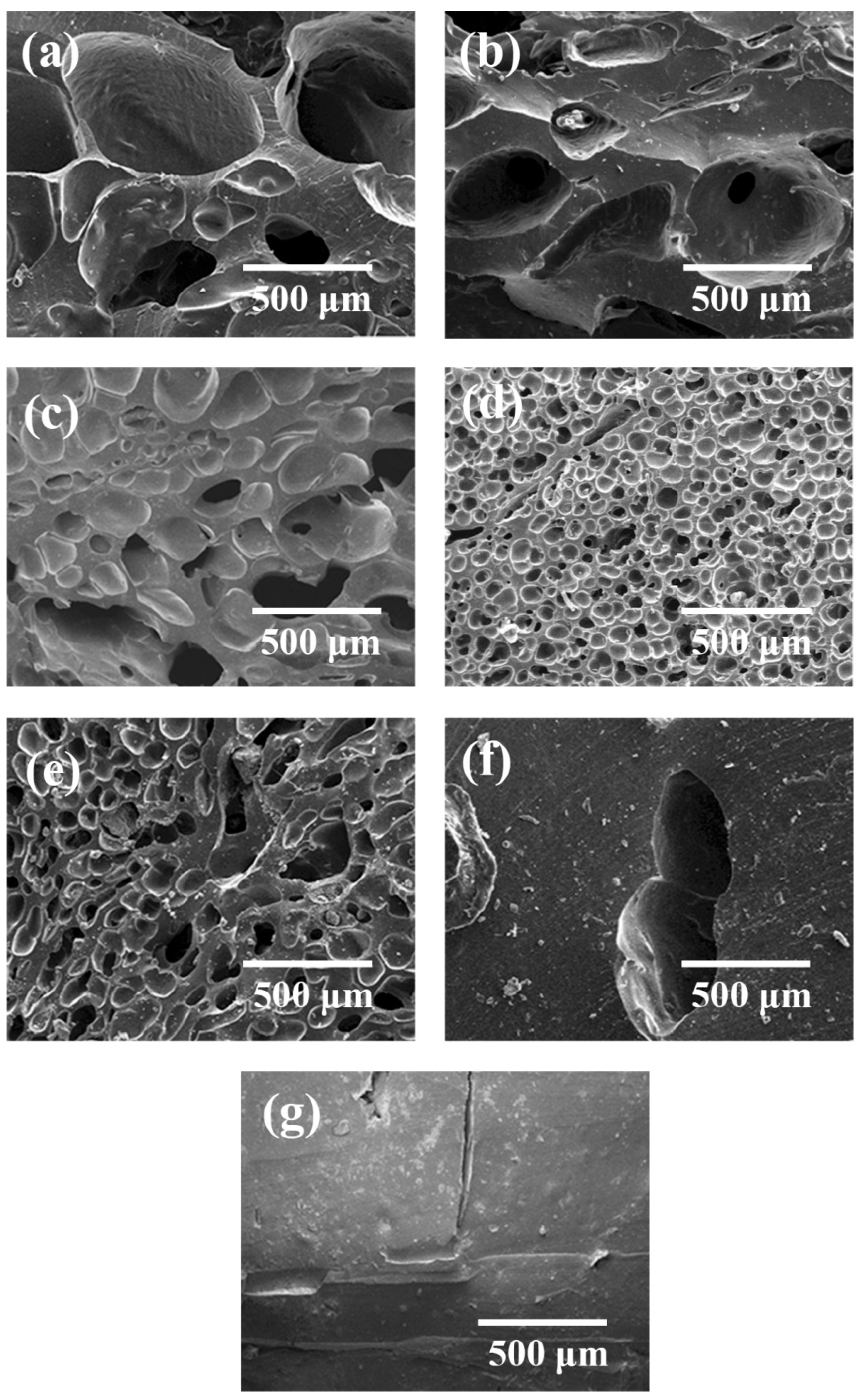
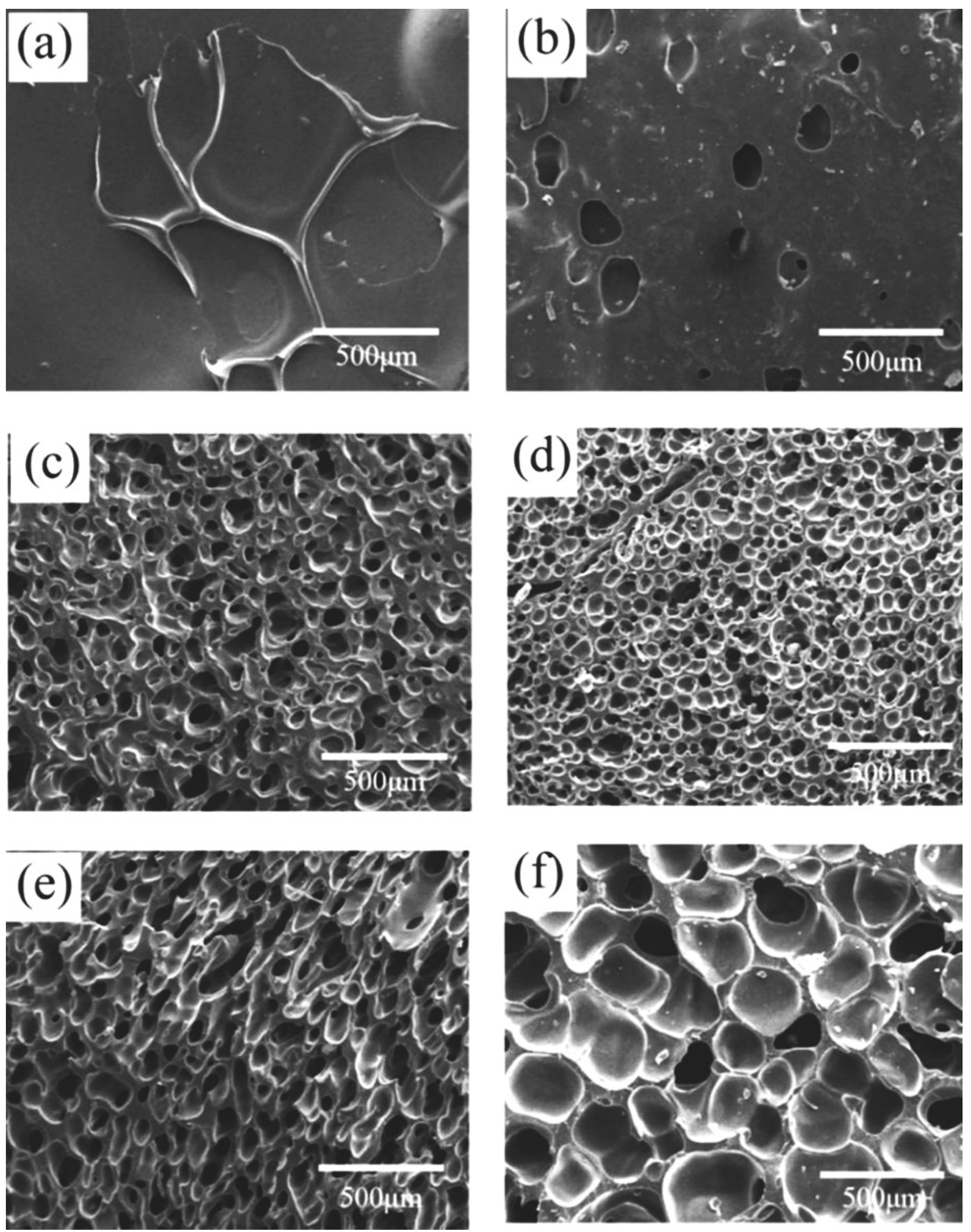
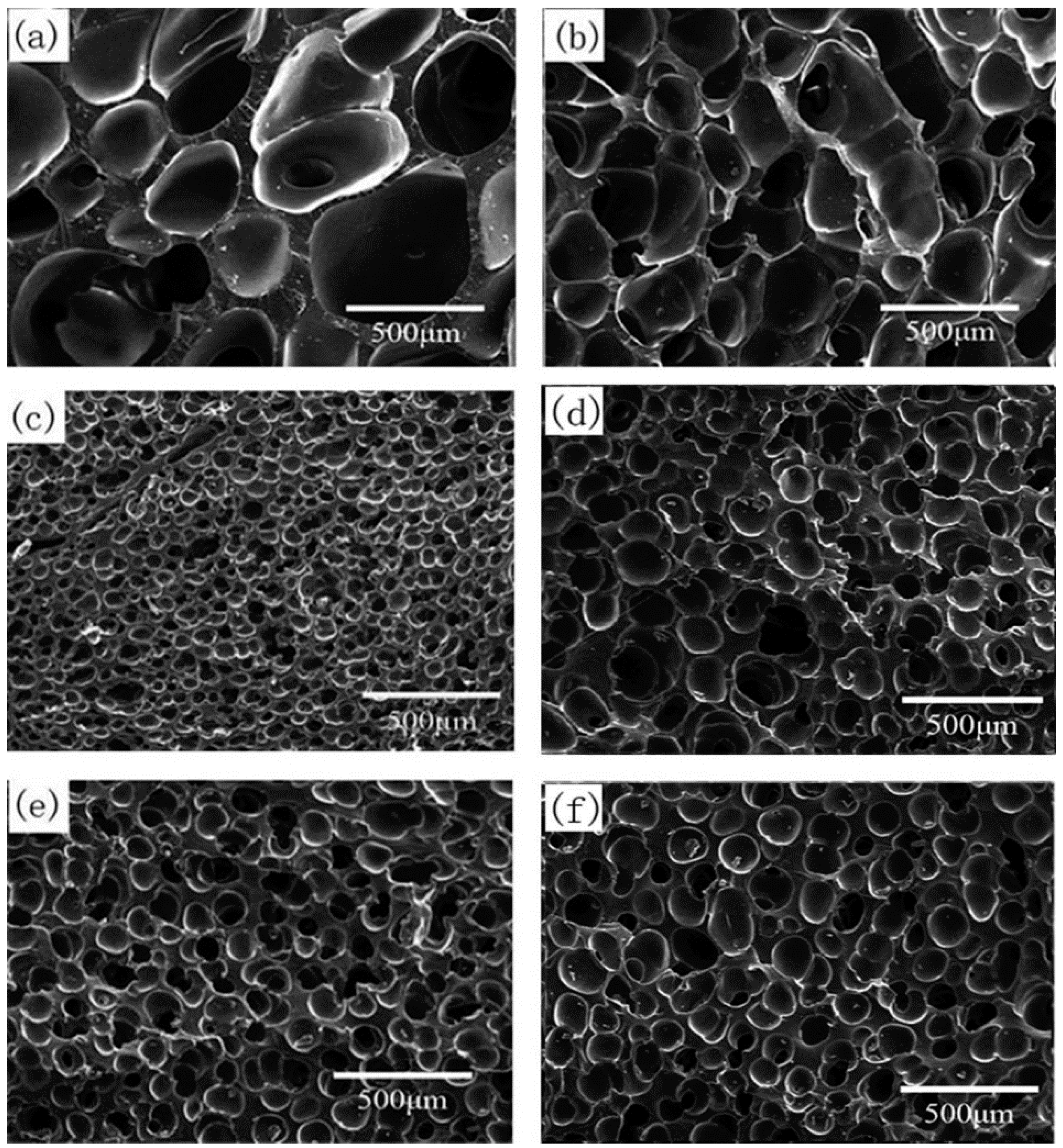
3.2.3. Effect of CO2 Saturation Time on Cell Morphology
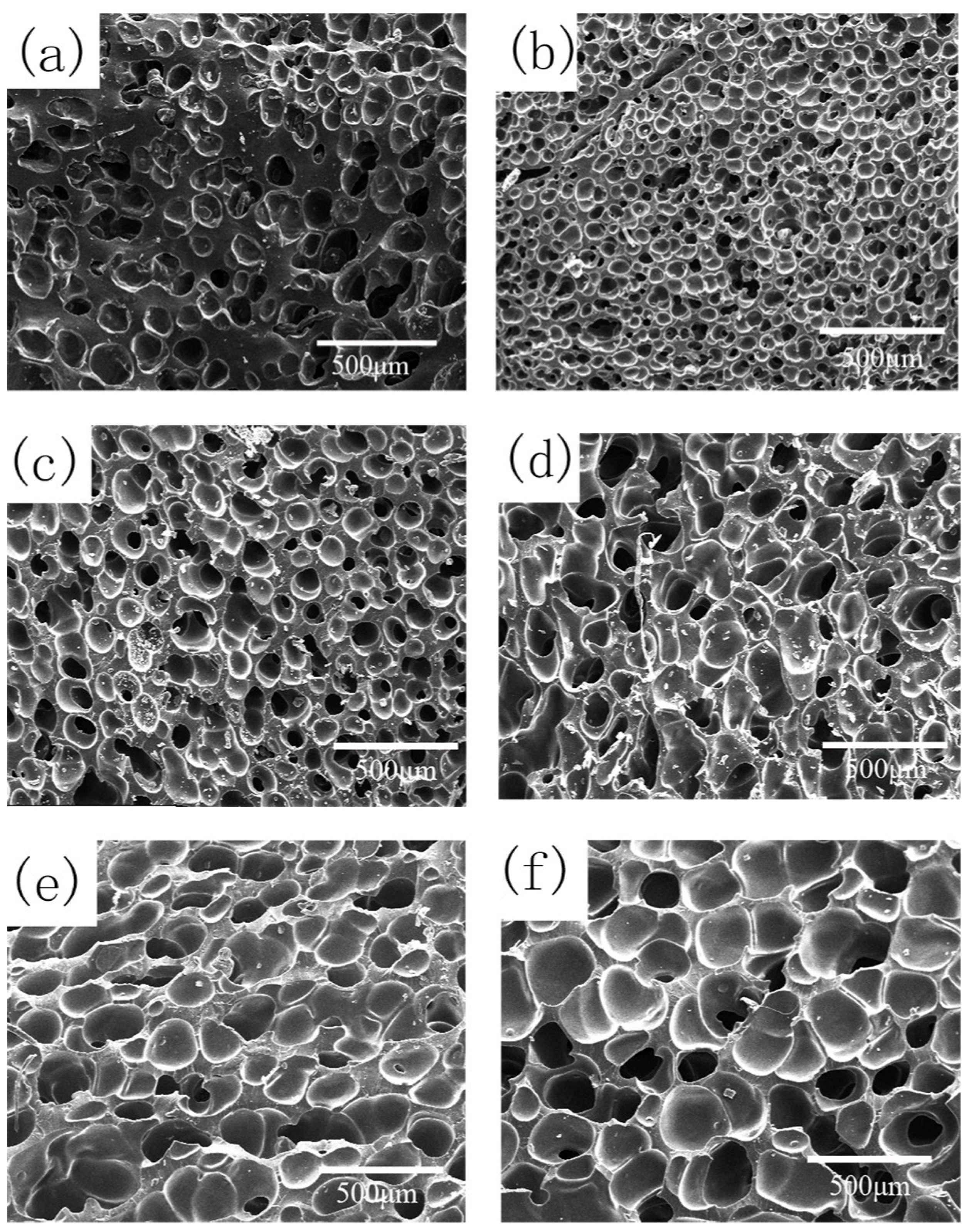
3.2.4. Effect of CO2 Saturation Pressure on Cell Morphology
3.3. Effect of Vulcanization during Bubble Nucleation and Growth
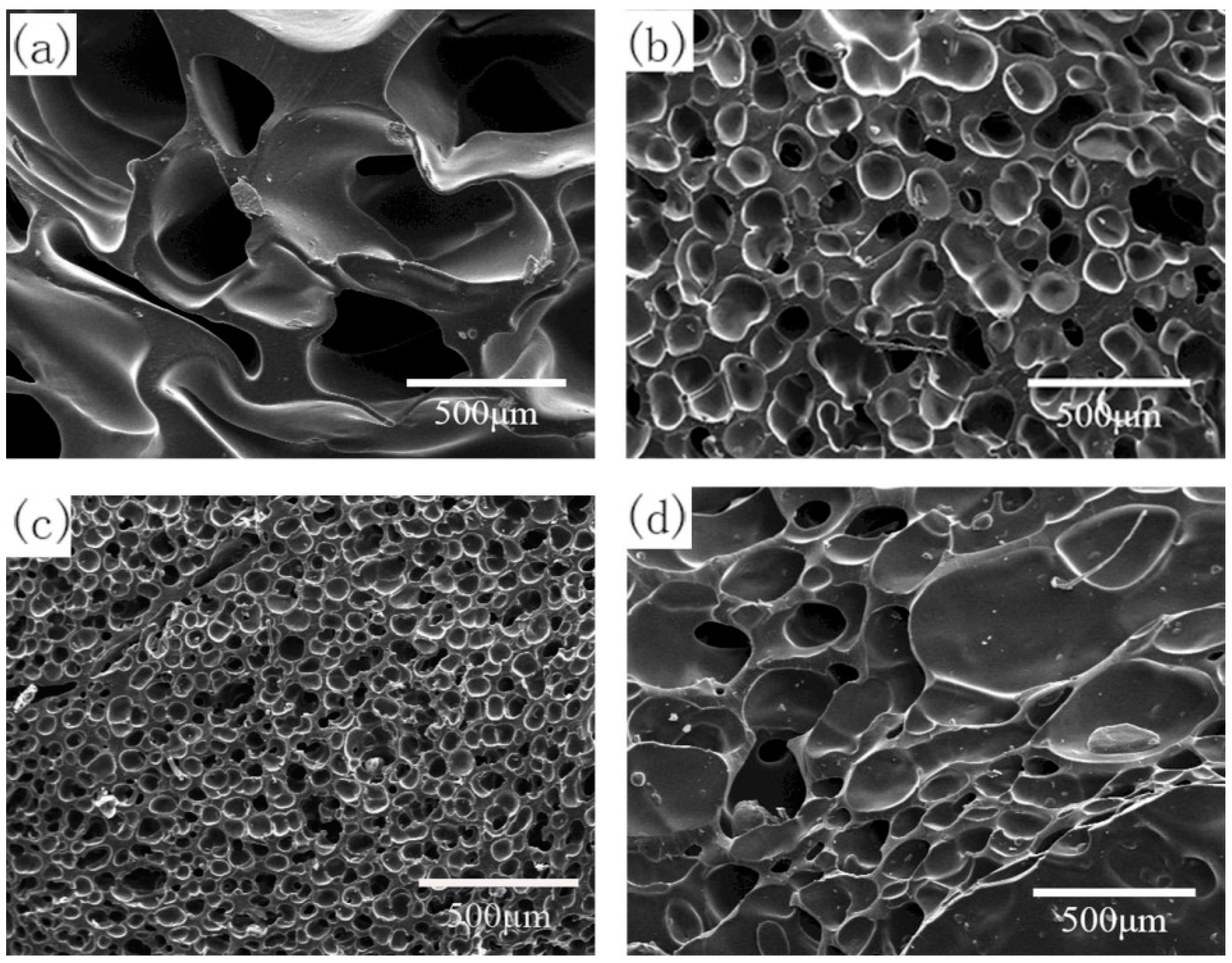
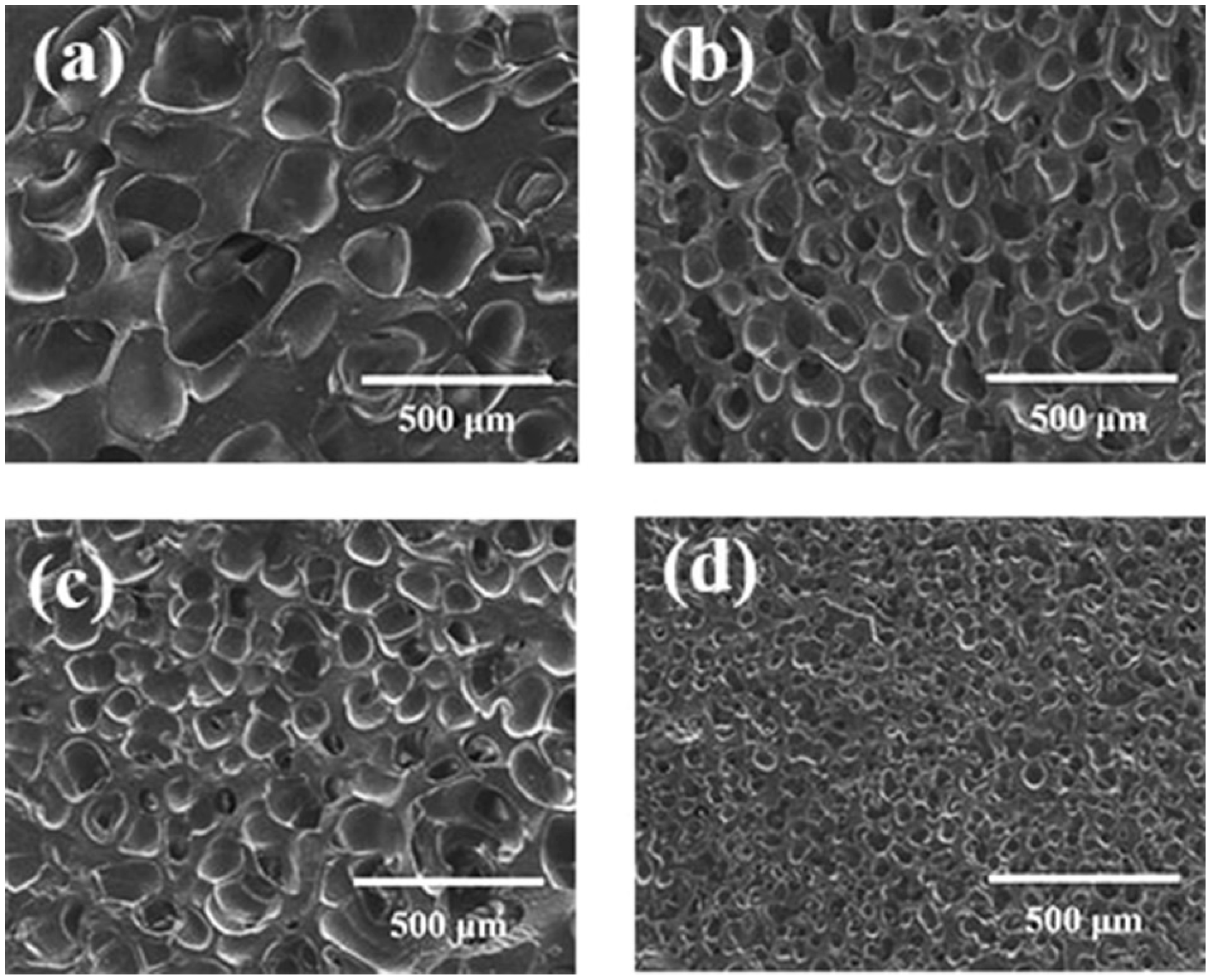
3.3.1. Effect of Foaming Temperature on Cell Morphology
3.3.2. Effect of Foaming Time on Cell Morphology
3.3.3. Effect of Temperature Rising Rate on Cell Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, W.; Jiang, J.; Park, C.B. A review on physical foaming of thermoplastic and vulcanized elastomers. Polym. Rev. 2021, 61, 1–47. [Google Scholar] [CrossRef]
- Métivier, T.; Cassagnau, P. New trends in cellular silicone: Innovations and applications. J. Cell. Plast. 2019, 55, 151–200. [Google Scholar] [CrossRef]
- Weyer, D.E. Method of Preparing Siloxane Resin Foams. U.S. Patent No. 2,833,732, 6 May 1958. [Google Scholar]
- Bruner, J.; Leonard, B. Method of Preparing Organosiloxane Elastomer Foams. U.S. Patent No. 3,070,555, 25 December 1962. [Google Scholar]
- Tomasko, D.L.; Li, H.; Liu, D.; Han, X.; Wingert, M.J.; Lee, L.J.; Koelling, K.W. A review of CO2 applications in the processing of polymers. Ind. Eng. Chem. Res. 2003, 42, 6431–6456. [Google Scholar] [CrossRef]
- Xiang, B.; Jia, Y.; Lei, Y.; Zhang, F.; He, J.; Liu, T.; Luo, S. Mechanical properties of microcellular and nanocellular silicone rubber foams obtained by supercritical carbon dioxide. Polym. J. 2019, 51, 559–568. [Google Scholar] [CrossRef]
- Shimbo, M.; Nomura, T.; Muratani, K.; Fukuruma, K. On foaming process of vulcanized rubber using physical blowing agent. In Proceedings of the Third International Conference on Axiomatic Design, Seoul, Korea, 21–24 June 2004. [Google Scholar]
- Hong, I.-K.; Lee, S. Microcellular foaming of silicone rubber with supercritical carbon dioxide. Korean J. Chem. Eng. 2014, 31, 166–171. [Google Scholar] [CrossRef]
- Xiang, B.; Deng, Z.; Zhang, F.; Wen, N.; Lei, Y.; Liu, T.; Luo, S. Microcellular silicone rubber foams: The influence of reinforcing agent on cellular morphology and nucleation. Polym. Eng. Sci. 2019, 59, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Xu, H.; Li, S.; Zhou, C.; Li, G.; Park, C.B. The effects of viscoelastic properties on the cellular morphology of silicone rubber foams generated by supercritical carbon dioxide. RSC Adv. 2015, 5, 106981–106988. [Google Scholar] [CrossRef]
- Xu, H.; He, Y.; Liao, X.; Luo, T.; Li, G.; Yang, Q.; Zhou, C. A green and structure-controlled approach to the generation of silicone rubber foams by means of carbon dioxide. Cell Polym. 2016, 35, 19–32. [Google Scholar] [CrossRef]
- Jia, Y.; Xiang, B.; Zhang, W.; Liu, T.; Luo, S. Microstructure and properties of microcellular silicone rubber foams with improved surface quality. Polym. J. 2019, 52, 207–216. [Google Scholar] [CrossRef]
- Royer, J.R.; DeSimone, J.M.; Khan, S.A. Carbon dioxide-induced swelling of poly(dimethylsiloxane). Macromolecules 1999, 32, 8965–8973. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, H.; Song, L.; Lei, Y.; Zhang, F.; Lu, A.; Liu, T.; Luo, S. Solid-state microcellular high temperature vulcanized (HTV) silicone rubber foam with carbon dioxide. J. Appl. Polym. Sci. 2017, 134, 44807. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Wang, K.; Zhao, Y. Fabrication of silicone rubber foam with tailored porous structures by supercritical CO2. Macromol. Mater. Eng. 2017, 302, 1–11. [Google Scholar] [CrossRef]
- Tang, W.; Liao, X.; Zhang, Y.; Li, S.; Wang, G.; Yang, J.; Li, G. Cellular structure design by controlling rheological property of silicone rubber in supercritical CO2. J. Supercrit. Fluids 2020, 164, 104913. [Google Scholar] [CrossRef]
- Bai, J.; Liao, X.; Huang, E.; Luo, Y.; Yang, Q.; Li, G. Control of the cell structure of microcellular silicone rubber/nanographite foam for enhanced mechanical performance. Mater. Des. 2017, 133, 288–298. [Google Scholar] [CrossRef]
- Messinger, R.J.; Marks, T.G.; Gleiman, S.S.; Milstein, F.; Chmelka, B.F. Molecular origins of macroscopic mechanical properties of elastomeric organosiloxane foams. Macromolecules 2015, 48, 4835–4849. [Google Scholar] [CrossRef]
- Liu, T.; Lei, Y.; Zhang, F.; Guo, S.; Luo, S. Microcellular crosslinked silicone rubber foams: Influence of nucleation agent (polyhedral oligomeric silsesquioxane) on the rheological, vulcanizing, cell morphological properties. Polym. Plast. Technol. Eng. 2018, 57, 1623–1633. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Y.; Luo, Y.; Liao, X.; Tian, C.; Tang, W.; Yang, J.; Chen, J.; Li, G. Reinforcement of mechanical properties of silicone rubber foam by functionalized graphene using supercritical CO2 foaming technology. Ind. Eng. Chem. Res. 2020, 59, 22132–22143. [Google Scholar] [CrossRef]
- Pantoula, M.; Panayiotou, C. Sorption and swelling in glassy polymer/carbon dioxide systems: Part I. Sorption. J. Supercrit. Fluids 2006, 37, 254–262. [Google Scholar] [CrossRef]
- Song, C.; Luo, Y.; Liu, Y.; Li, S.; Xi, Z.; Zhao, L.; Cen, L.; Lu, E. Fabrication of PCL scaffolds by supercritical CO2 foaming based on the combined effects of rheological and crystallization properties. Polymers 2020, 12, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, J.; Liu, T.; Xi, Z.; Zhao, L. Effect of pre-curing process on epoxy resin foaming using carbon dioxide as blowing agent. J. Cell. Plast. 2016, 53, 181–197. [Google Scholar] [CrossRef]
- Goel, S.K.; Beckman, E.J. Nucleation and growth in microcellular materials: Supercritical CO2 as foaming agent. AIChE J. 1995, 41, 357–367. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Yao, S.; Zhang, H.; Zhao, L. Insight into the influence of properties of poly(ethylene-co-octene) with different chain structures on their cell morphology and dimensional stability foamed by supercritical CO2. Polymers 2021, 13, 1494. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Hu, D.; Liu, T.; Zhao, L. Non-isothermal kinetics of epoxy resin curing reaction under compressed CO2. J. Therm. Anal. Calorim. 2017, 131, 1499–1507. [Google Scholar] [CrossRef]
- Yang, Z.; Dongdong, H.U.; Liu, T.; Cao, K.; Zhao, L. Non-isothermal curing kinetics of polyurethane under high-pressure gas atmosphere. CIESC J. 2018, 69, 4728–4736. [Google Scholar] [CrossRef]
- Hu, D.-d.; Lyu, J.-x.; Liu, T.; Lang, M.-d.; Zhao, L. Solvation effect of CO2 on accelerating the curing reaction process of epoxy resin. Chem. Eng. Process. 2018, 127, 159–167. [Google Scholar] [CrossRef]
- Fox, T.G.; Loshaek, S.J. Influence of molecular weight and degree of crosslinking of the specific volume and glass temperature of polymers. J. Polym. Sci. 1955, 15, 371–390. [Google Scholar] [CrossRef]
- Zhang, J.; Han, B.; Li, J.; Zhao, Y.; Yang, G. Carbon dioxide in ionic liquid microemulsions. Angew. Chem. Int. Ed. 2011, 50, 9911–9915. [Google Scholar] [CrossRef]
- Tuminello, W.H.; Dee, G.T.; McHugh, M.A. Dissolving perfluoropolymers in supercritical carbon dioxide. Macromolecules 1995, 28, 1506–1510. [Google Scholar] [CrossRef]
- Şerbescu, A.; Saalwächter, K. Particle-induced network formation in linear PDMS filled with silica. Polymer 2009, 50, 5434–5442. [Google Scholar] [CrossRef]




| Pre-Vulcanization Time (min) | Pre-Vulcanization Degree (%) | CO2 Content (g CO2/g SR) | Foam Density (g·cm−3) | Average Cell Diameter (μm) | Cell Density (×105 Cells·cm−3) | Expansion Ratio |
|---|---|---|---|---|---|---|
| 0 | 0 | - | 0.43 ± 0.04 | 465.2 ± 93.0 | 0.1 | 2.4 |
| 10 | 12.7 | - | 0.41 ± 0.02 | 307.2 ± 61.4 | 0.3 | 2.5 |
| 20 | 23.9 | 0.086 | 0.45 ± 0.03 | 157.7 ± 35.1 | 2.2 | 2.3 |
| 30 | 36.0 | 0.070 | 0.51 ± 0.03 | 51.6 ± 8.6 | 72.1 | 2.0 |
| 40 | 43.8 | 0.063 | 0.40 ± 0.04 | 115.0 ± 23.0 | 7.9 | 2.5 |
| 50 | 50.7 | 0.015 | 1.03 ± 0.05 | - | - | - |
| 60 | 56.6 | 0.008 | 1.05 ± 0.05 | - | - | - |
| CO2 Saturation Time | c | λ | η0 | R2 |
|---|---|---|---|---|
| 0 min | 0.76 | 83.34 | 2,608,843.0 | 0.9999 |
| 30 min | 0.76 | 80.96 | 2,598,144.4 | 0.9999 |
| 60 min | 0.81 | 75.18 | 2,557,879.8 | 0.9999 |
| 90 min | 0.71 | 61.03 | 1,272,419.6 | 0.9994 |
| 120 min | 0.72 | 59.53 | 1,128,299.6 | 0.9999 |
| 150 min | 0.73 | 32.90 | 618,824.5 | 0.9995 |
| 180 min | 0.67 | 26.08 | 474,267.6 | 0.9993 |
| Solution Time (Min) | CO2 Content (g CO2/g SR) | Foam Density (g·cm−3) | Average Cell Diameter (μm) | Cell Density (×105 Cells·cm−3) | Expansion Ratio |
|---|---|---|---|---|---|
| 30 | 0.010 | 0.88 ± 0.06 | - | - | 1.2 |
| 60 | 0.025 | 0.76 ± 0.05 | - | - | 1.4 |
| 90 | 0.058 | 0.48 ± 0.03 | 68.7 ± 13.7 | 13.5 | 2.1 |
| 120 | 0.070 | 0.51 ± 0.03 | 51.6 ± 8.6 | 72.1 | 2.0 |
| 150 | 0.071 | 0.48 ± 0.03 | 75.6 ± 12.6 | 10.2 | 2.1 |
| 180 | 0.072 | 0.41 ± 0.01 | 214.0 ± 35.7 | 2.1 | 2.5 |
| P (MPa) | CO2 Content (g CO2/g SR) | Foam Density (g·cm−3) | Average Cell Diameter (μm) | Cell Density (×105 Cells·cm−3) | Expansion Ratio |
|---|---|---|---|---|---|
| 8 | 0.064 | 0.40 ± 0.03 | 102.6 ± 20.5 | 10.4 | 2.6 |
| 10 | 0.070 | 0.51 ± 0.03 | 51.6 ± 8.6 | 72.1 | 2.0 |
| 12 | 0.075 | 0.43 ± 0.02 | 94.5 ± 15.8 | 14.4 | 2.4 |
| 14 | 0.081 | 0.40 ± 0.03 | 124.7 ± 18.6 | 7.2 | 2.6 |
| 16 | 0.087 | 0.37 ± 0.02 | 132.9 ± 20.8 | 7.1 | 2.8 |
| 18 | 0.091 | 0.31 ± 0.01 | 201.2 ± 40.2 | 4.0 | 3.3 |
| Temperature (°C) | Vulcanization Degree (%) | Foam Density (g·cm−3) | Average Cell Diameter (μm) | Cell Density (×105 Cells·cm−3) | Expansion Ratio |
|---|---|---|---|---|---|
| 150 | 67.3 | 0.46 ± 0.03 | - | - | 2.2 |
| 160 | 70.2 | 0.45 ± 0.02 | 120.9 ± 15.1 | 7.5 | 2.3 |
| 170 | 94.4 | 0.51 ± 0.03 | 51.6 ± 8.6 | 72.1 | 2.0 |
| 180 | 100.0 | 0.63 ± 0.03 | 152.0 ± 31.6 | 1.8 | 1.6 |
| Time (Min) | Vulcanization Degree (%) | Foam Density (g·cm−3) | Average Cell Diameter (μm) | Cell Density (×105 Cells·cm−3) | Expansion Ratio |
|---|---|---|---|---|---|
| 20 | 74.6 | 0.43 ± 0.01 | 324.4 ± 54.1 | 0.6 | 2.4 |
| 40 | 86.9 | 0.47 ± 0.03 | 202.2 ± 33.7 | 2.2 | 2.2 |
| 60 | 94.4 | 0.51 ± 0.03 | 51.6 ± 8.6 | 72.1 | 2.0 |
| 80 | 100.0 | 0.53 ± 0.02 | 98.3 ± 16.4 | 11.5 | 1.9 |
| 100 | 100.0 | 0.52 ± 0.02 | 97.2 ± 15.8 | 11.4 | 2.0 |
| 120 | 100.0 | 0.53 ± 0.03 | 101.6 ± 16.9 | 11.3 | 1.9 |
| Temperature Rising Rate (°C/min) | Foam Density (g·cm−3) | Average Cell Diameter (μm) | Cell Density (×105 Cells·cm−3) | Expansion Ratio |
|---|---|---|---|---|
| 5 | 0.62 ± 0.03 | 182.0 ± 36.4 | 1.6 | 1.6 |
| 10 | 0.56 ± 0.02 | 90.7 ± 18.1 | 10.7 | 1.8 |
| 15 | 0.52 ± 0.03 | 78.0 ± 15.6 | 19.1 | 2.0 |
| 20 | 0.57 ± 0.03 | 37.5 ± 7.5 | 109.1 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Yao, S.; Wang, L.; Zhen, W.; Zhao, L. Effect of Vulcanization and CO2 Plasticization on Cell Morphology of Silicone Rubber in Temperature Rise Foaming Process. Polymers 2021, 13, 3384. https://doi.org/10.3390/polym13193384
Zhang T, Yao S, Wang L, Zhen W, Zhao L. Effect of Vulcanization and CO2 Plasticization on Cell Morphology of Silicone Rubber in Temperature Rise Foaming Process. Polymers. 2021; 13(19):3384. https://doi.org/10.3390/polym13193384
Chicago/Turabian StyleZhang, Tianping, Shun Yao, Lu Wang, Weijun Zhen, and Ling Zhao. 2021. "Effect of Vulcanization and CO2 Plasticization on Cell Morphology of Silicone Rubber in Temperature Rise Foaming Process" Polymers 13, no. 19: 3384. https://doi.org/10.3390/polym13193384
APA StyleZhang, T., Yao, S., Wang, L., Zhen, W., & Zhao, L. (2021). Effect of Vulcanization and CO2 Plasticization on Cell Morphology of Silicone Rubber in Temperature Rise Foaming Process. Polymers, 13(19), 3384. https://doi.org/10.3390/polym13193384





