Simulation of Neutron/Self-Emitted Gamma Attenuation and Effects of Silane Surface Treatment on Mechanical and Wear Resistance Properties of Sm2O3/UHMWPE Composites
Abstract
:1. Introduction
2. Experimental
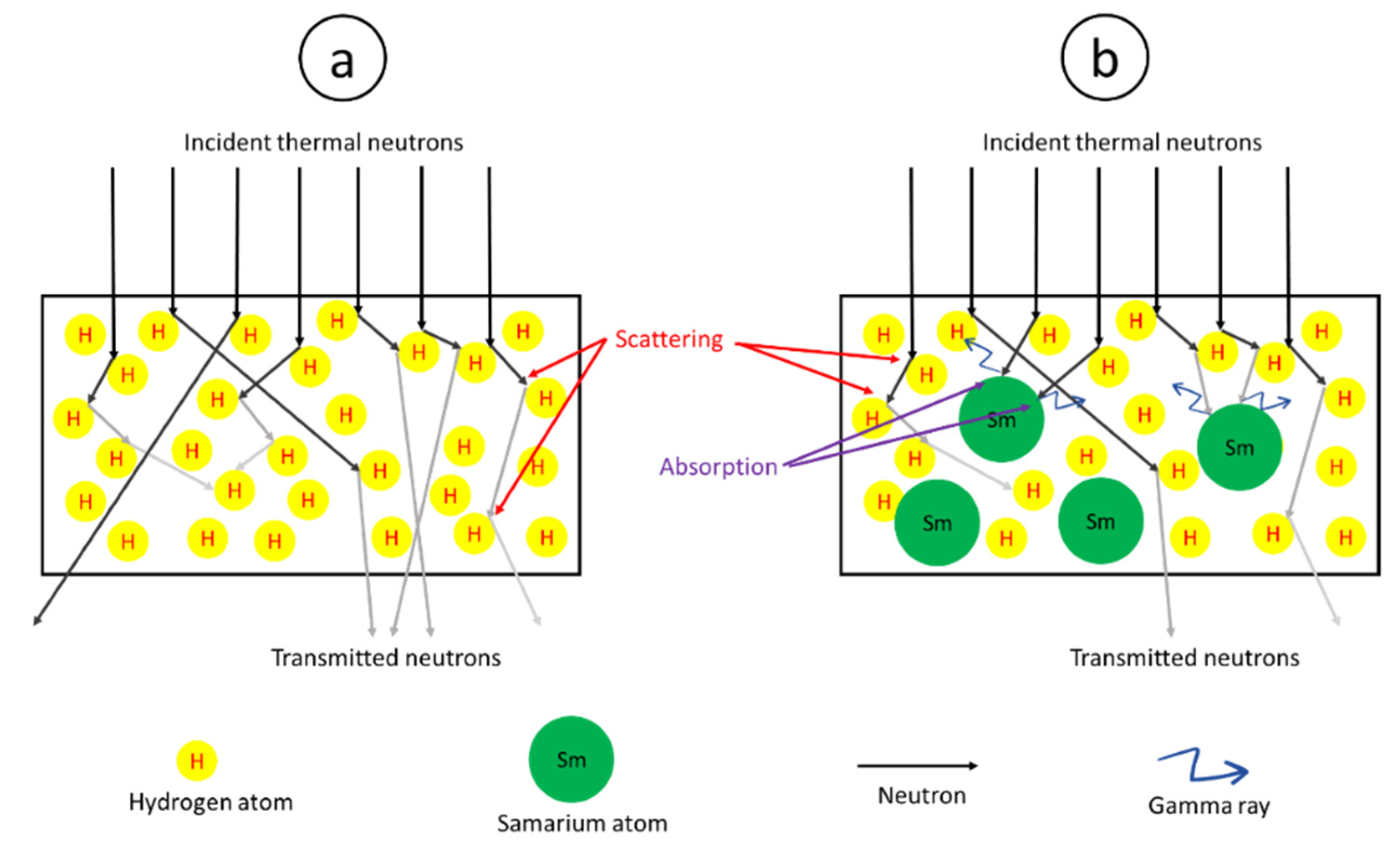
2.1. Simulation of Neutron and Self-Emitted Gamma-Shielding Properties of Sm2O3/UHMWPE Composites
2.1.1. Determination of µm, µ, and HVL
2.1.2. Determination of Recommended Sm2O3 Content
2.2. Determination of Effects of Silane Surface Treatment on Sm2O3/UHMWPE Composites
2.2.1. Materials and Chemicals
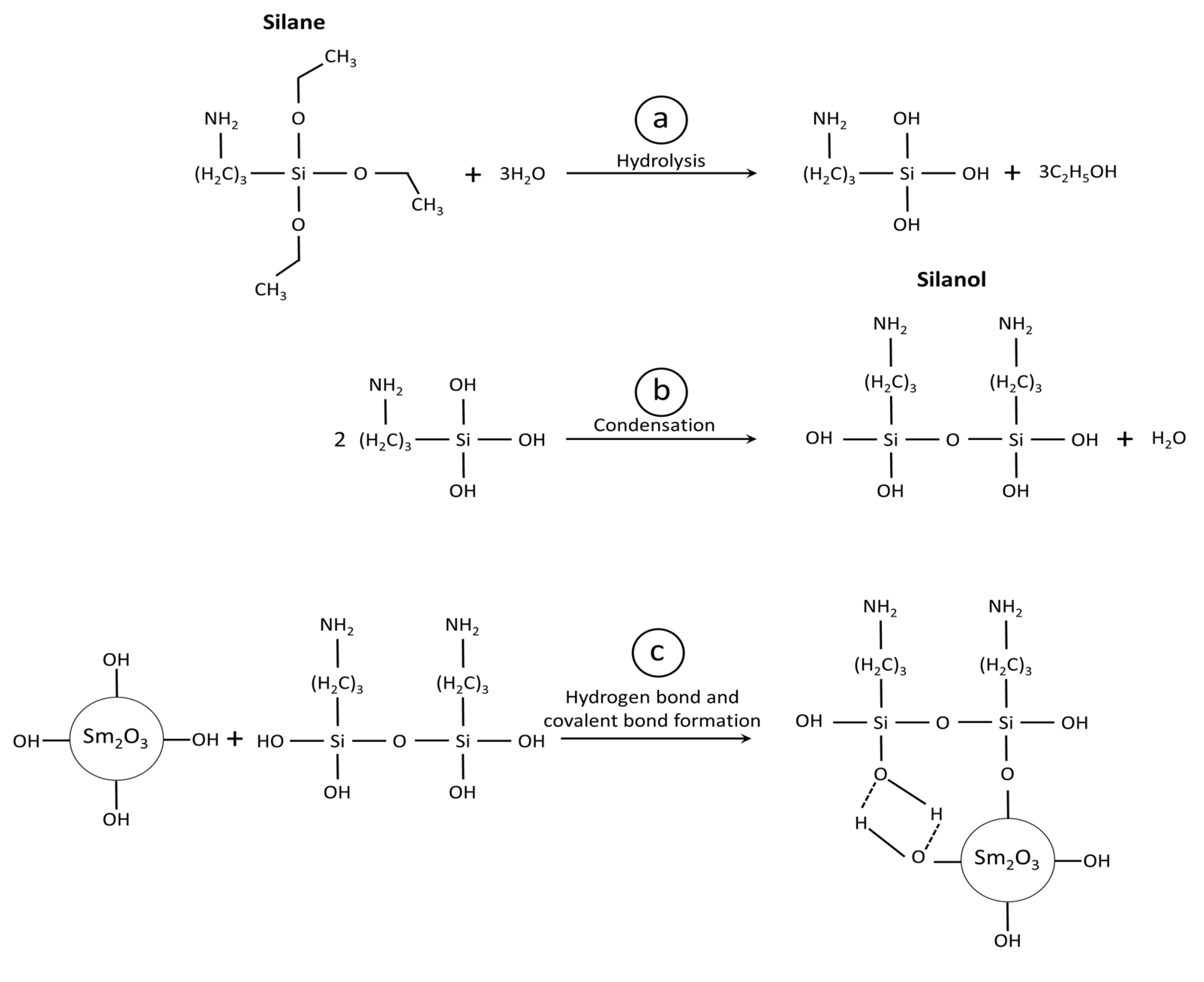
2.2.2. Silane Surface Treatment of Sm2O3 Particles
2.2.3. Preparation of Sm2O3/UHMWPE Composites
2.2.4. Characterization
Wear and Frictional Properties
Mechanical Properties
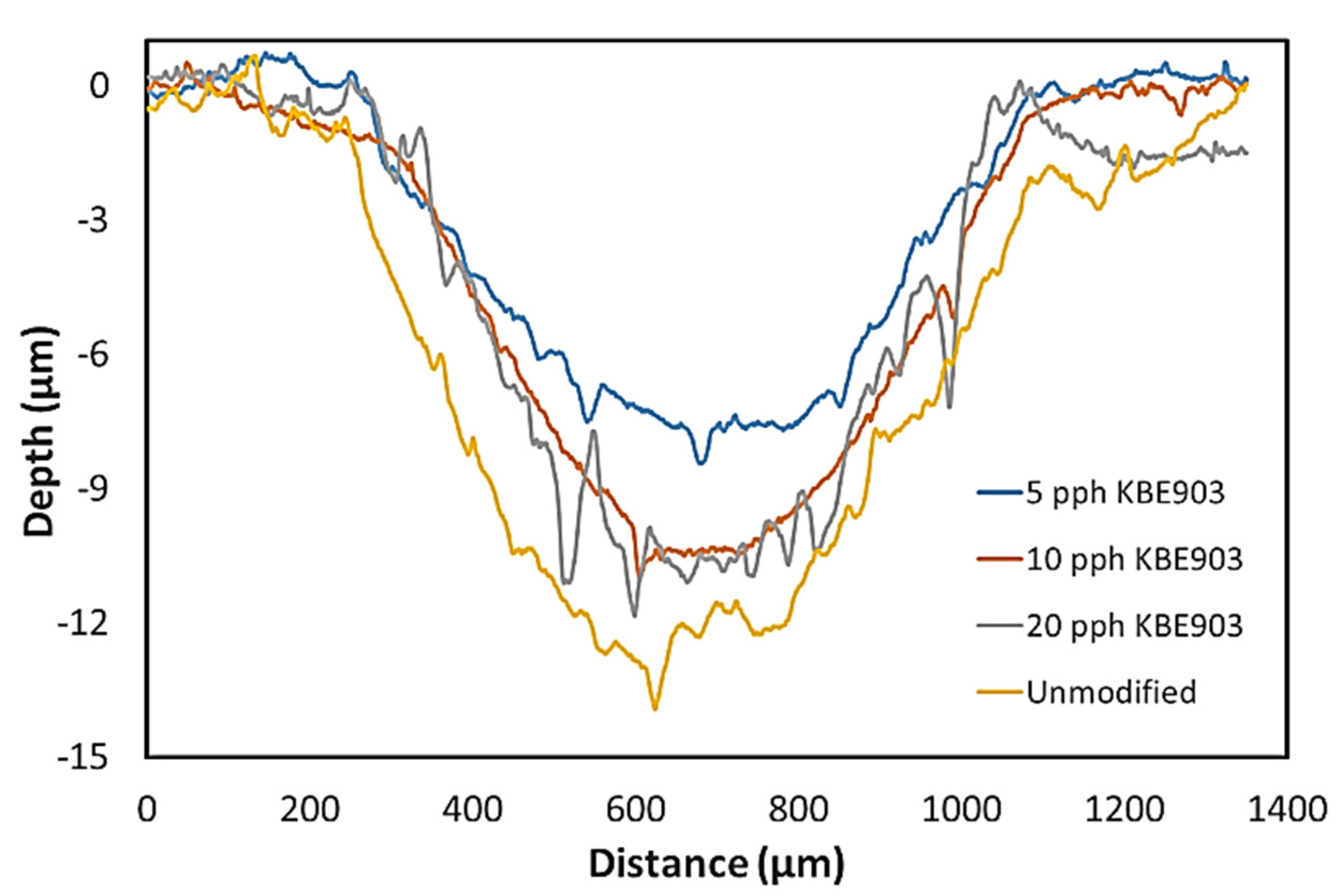
Particle Sizes, Elemental Compositions, and Morphological Properties
X-ray Diffraction (XRD) and Fourier-Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Simulated Neutron and Self-Emitted Gamma-Shielding Properties
3.2. Effects of Silane Surface Treatment on Sm2O3 Particles
3.3. Elemental Composition of Treated Sm2O3 Particles and Treated Sm2O3/UHMWPE Composites
3.4. Wear, Frictional, and Mechanical Properties of Treated Sm2O3/UHMWPE Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kardjilov, N.; Manke, I.; Hilger, A.; Strobl, M.; Banhart, J. Neutron imaging in materials science. Mater. Today 2011, 14, 248–256. [Google Scholar] [CrossRef]
- Rittirong, A.; Saenboonruang, K. Comparative measurement of metal contents in raw and cooked rice samples prepared in different rice cookers using SR-XRF and health risk assessment. J. Food Meas. Charact. 2018, 12, 2801–2808. [Google Scholar] [CrossRef]
- Koltick, D.S.; Nie, L.H. Associated Particle Neutron Imaging for Elemental Analysis in Medical Diagnostics. IEEE Trans. Nucl. Sci. 2013, 60, 824–829. [Google Scholar] [CrossRef]
- Couto, M.; Alamón, C.; García, M.F.; Kovacs, M.; Trias, E.; Nievas, S.; Pozzi, E.; Curotto, P.; Thorp, S.; Dagrosa, M.A.; et al. Closo-Carboranyl- and Metallacarboranyl [1,2,3] triazolyl-Decorated Lapatinib-Scaffold for Cancer Therapy Combining Tyrosine Kinase Inhibition and Boron Neutron Capture Therapy. Cells 2020, 9, 1408. [Google Scholar] [CrossRef]
- Köksal, E.S.; Cemek, B.; Artık, C.; Temizel, K.E.; Taşan, M. A new approach for neutron moisture meter calibration: Artificial neural network. Irrig. Sci. 2010, 29, 369–377. [Google Scholar] [CrossRef]
- Andrianova, N.N.; Borisov, A.M.; Kazakov, V.A.; Mashkova, E.S.; Popov, V.P.; Palyanov, Y.N.; Risakhanov, R.N.; Sigalaev, S.K. High-fluence ion-beam modification of a diamond surface at high temperature. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2015, 9, 346–349. [Google Scholar] [CrossRef]
- Tran, V.; Little, M.P. Dose and dose rate extrapolation factors for malignant and non-malignant health endpoints after exposure to gamma and neutron radiation. Radiat. Environ. Biophys. 2017, 56, 299–328. [Google Scholar] [CrossRef]
- Tiamduangtawan, P.; Kamkaew, C.; Kuntonwatchara, S.; Wimolmala, E.; Saenboonruang, K. Comparative mechanical, self-healing, and gamma attenuation properties of PVA hydrogels containing either nano- or micro-sized Bi2O3 for use as gamma-shielding materials. Radiat. Phys. Chem. 2020, 177, 109164. [Google Scholar] [CrossRef]
- Ninyong, K.; Wimolmala, E.; Sombatsompop, N.; Saenboonruang, K. Potential use of NR and wood/NR composites as thermal neutron shielding materials. Polym. Test. 2017, 59, 336–343. [Google Scholar] [CrossRef]
- DiJulio, D.; Cooper-Jensen, C.; Perrey, H.; Fissum, K.; Rofors, E.; Scherzinger, J.; Bentley, P. A polyethylene-B 4 C based concrete for enhanced neutron shielding at neutron research facilities. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2017, 859, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Toyen, D.; Saenboonruang, K. Development of paraffin and paraffin/bitumen composites with additions of B2O3 for thermal neutron shielding applications. J. Nucl. Sci. Technol. 2017, 11, 1–7. [Google Scholar] [CrossRef]
- Tiamduangtawan, P.; Wimolmala, E.; Meesat, R.; Saenboonruang, K. Effects of Sm2O3 and Gd2O3 in poly (vinyl alcohol) hy-drogels for potential use as self-healing thermal neutron shielding materials. Radiat. Phys. Chem. 2020, 172, 108818. [Google Scholar] [CrossRef]
- Saenboonruang, K.; Poltabtim, W.; Thumwong, A.; Pianpanit, T.; Rattanapongs, C. Rare-Earth Oxides as Alternative High-Energy Photon Protective Fillers in HDPE Composites: Theoretical Aspects. Polymers 2021, 13, 1930. [Google Scholar] [CrossRef] [PubMed]
- Toyen, D.; Wimolmala, E.; Sombatsompop, N.; Markpin, T.; Saenboonruang, K. Sm2O3/UHMWPE composites for radiation shielding applications: Mechanical and dielectric properties under gamma irradiation and thermal neutron shielding. Radiat. Phys. Chem. 2019, 164, 108366. [Google Scholar] [CrossRef]
- Florez, R.; Colorado, H.A.; Giraldo, C.H.; Alajo, A. Preparation and characterization of Portland cement pastes with Sm2O3 microparticle additions for neutron shielding applications. Constr. Build. Mater. 2018, 191, 498–506. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Tekin, H.O. The multiple characterization of gamma, neutron and proton shielding performances of xPbO-(99-x) B2O3–Sm2O3 glass system. Ceram. Int. 2019, 45, 23561–23571. [Google Scholar] [CrossRef]
- Wang, P.; Tang, X.; Chai, H.; Chen, D.; Qiu, Y. Design, fabrication, and properties of a continuous carbon-fiber reinforced Sm2O3/polyimide gamma ray/neutron shielding material. Fusion Eng. Des. 2015, 101, 218–225. [Google Scholar] [CrossRef]
- Liu, H.; Xie, D.; Qian, L.; Deng, X.; Leng, Y.X.; Huang, N. The mechanical properties of the ultrahigh molecular weight poly-ethylene (UHMWPE) modified by oxygen plasma. Surf. Coat. Technol. 2011, 8, 2697–2701. [Google Scholar] [CrossRef]
- Cao, X.; Xue, X.; Jiang, T.; Li, Z.; Ding, Y.; Li, Y.; Yang, H. Mechanical properties of UHMWPE/Sm2O3 composite shielding material. J. Rare Earths 2010, 28, 482–484. [Google Scholar] [CrossRef]
- Zhao, J.; Milanova, M.; Warmoeskerken, M.M.; Dutschk, V. Surface modification of TiO2 nanoparticles with silane coupling agents. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 273–279. [Google Scholar] [CrossRef]
- Arslan, C.; Dogan, M. The effects of silane coupling agents on the mechanical properties of basalt fiber reinforced poly(butylene terephthalate) composites. Compos. Part. B Eng. 2018, 146, 145–154. [Google Scholar] [CrossRef]
- Sato, T.; Iwamoto, Y.; Hashimoto, S.; Ogawa, T.; Furuta, T.; Abe, S.-I.; Kai, T.; Tsai, P.-E.; Matsuda, N.; Iwase, H.; et al. Features of Particle and Heavy Ion Transport code System (PHITS) version 3. J. Nucl. Sci. Technol. 2018, 55, 684–690. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.J.; Hubbell, J.H.; Seltzer, S.M.; Chang, J.; Coursey, J.S.; Sukumar, R.; Zucker, D.S.; Olsen, K. XCOM: Photon Cross Section Database (version 1.5); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010.
- Toyen, D.; Saenboonruang, K. Comparative X-ray shielding properties of bismuth oxide/natural rubber composites using a Monte Carlo code of PHITS. IOP Conf. Ser. Mater. Sci. Eng. 2020, 773, 12024. [Google Scholar] [CrossRef]
- Poltabtim, W.; Toyen, D.; Saenboonruang, K. Comparative neutron-shielding properties of metal oxide/HDPE composites using a Monte Carlo Code of PHITS. IOP Conf. Ser. Mater. Sci. Eng. 2019, 526, 12013. [Google Scholar] [CrossRef]
- Poltabtim, W.; Toyen, D.; Saenboonruang, K. Theoretical Determination of High-Energy Photon Attenuation and Recommended Protective Filler Contents for Flexible and Enhanced Dimensionally Stable Wood/NR and NR Composites. Polymers 2021, 13, 869. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Ferrari, F.; Buccoliero, M.G.; Trono, G. Thermal and Mechanical Analysis of Polyethylene Homo-Composites Processed by Rotational Molding. Polymers 2019, 11, 528. [Google Scholar] [CrossRef] [Green Version]
- Azhan, H.; Azman, K.; Hassan, O.H.; Osman, N.; Abd-Shukor, R.; Deraman, M.; Kong, W.; Halimah, M.K.; Azis, R.S.; Sahar, M.R.; et al. Effect of Sm3+ concentration on physical properties of magnesium tellurite glasses containing silver nanoparticles. Mater. Sci. Forum 2016, 846, 107–114. [Google Scholar]
- Shieldwerx. Available online: http://www.shieldwerx.com/poly-based-shielding.html#swx201hd (accessed on 24 August 2021).
- Jansinak, S.; Markpin, T.; Wimolmala, E.; Mahathanabodee, S.; Sombatsompop, N. Tribological properties of carbon nanotube as co-reinforcing additive in carbon black/acrylonitrile butadiene rubber composites for hydraulic seal applications. J. Reinf. Plast. Compos. 2018, 37, 1255–1266. [Google Scholar] [CrossRef]
- NIST Center of Neutron Research, Neutron Scattering Lengths and Cross Sections. Available online: https://www.ncnr.nist.gov/resources/n-lengths/ (accessed on 25 August 2021).
- Hirayama, H. Lecture Note on Photon Interactions and Cross Sections. Available online: http://rcwww.kek.jp/research/shield/photon_r.pdf (accessed on 1 February 2021).
- Chang, B.P.; Akil, H.M.; Nasir, R.M.; Nurdijati, S. Abrasive wear performance and antibacterial assessment of untreated and treated ZnO-reinforced polymer composite. Polym. Compos. 2013, 34, 1020–1032. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ren, J.; Zhang, C. TiO2-KH550 Nanoparticle-Reinforced PVA/xylan Composite Films with Multifunctional Properties. Materials 2018, 11, 1589. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Yang, Y.; Via, B.K.; Liu, Y.; Guo, H.; Zhang, F. Modification and characterization of nano-Ag/TiO2 antimold agent for wood materials. For. Prod. J. 2018, 68, 70–77. [Google Scholar]
- Chuang, W.; Geng-Sheng, J.; Lei, P.; Bao-Lin, Z.; Ke-Zhi, L.; Jun-Long, W. Influences of surface modification of nano-silica by silane coupling agents on the thermal and frictional properties of cyanate ester resin. Results Phys. 2018, 9, 886–896. [Google Scholar] [CrossRef]
- Park, J.; Rhee, K.; Park, S. Silane treatment of Fe3O4 and its effect on the magnetic and wear properties of Fe3O4/epoxy nanocomposites. Appl. Surf. Sci. 2010, 256, 6945–6950. [Google Scholar] [CrossRef]
- Song, J.; Dai, Z.; Li, J.; Zhao, H.; Wang, L. Silane coupling agent modified BN–OH as reinforcing filler for epoxy nanocomposite. High Perform. Polym. 2017, 31, 116–123. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilemany, J.; Miguel, J.; Vizcaino, S.; Climent, F. Role of three-body abrasion wear in the sliding wear behaviour of WC–Co coatings obtained by thermal spraying. Surf. Coat. Technol. 2001, 140, 141–146. [Google Scholar] [CrossRef]
- Sheykh, M.J.; Tarmian, A.; Doosthoseini, K.; Abdulkhani, A. Wear resistance and friction coefficient of nano-SiO2 and ash-filled HDPE/lignocellulosic fiber composites. Polym. Bull. 2017, 74, 4537–4547. [Google Scholar] [CrossRef]
- Smooth-On, Shore Hardness Scales. Available online: https://www.smooth-on.com/assets/pdf/durometer_chart.pdf (accessed on 19 September 2021).
- Teclock, Durometer and IRHD Hardness Tester. Available online: http://teclock.co.jp/wordpress/wp-content/uploads/2017/05/10Durometer.pdf (accessed on 19 September 2021).




| Sm2O3 Content (wt.%) | Density (g/cm3) |
|---|---|
| 0 | 0.940 |
| 5 | 0.994 |
| 10 | 1.042 |
| 15 | 1.096 |
| 20 | 1.155 |
| 25 | 1.220 |
| 30 | 1.294 |
| 35 | 1.377 |
| 40 | 1.472 |
| 45 | 1.580 |
| 50 | 1.706 |
| Chemical | Content (wt.%) | Function | Supplier |
|---|---|---|---|
| UHMWPE | 75 | Main matrix | IRPC Public Co., Ltd. (Bangkok, Thailand) |
| Samarium oxide (Sm2O3) | 25 | Radiation protective filler | Richest Group (Shanghai, China) |
| Paraffinic oil * | 10% of UHMWPE | Processing aid | Facobis Co., Ltd. (Bangkok, Thailand) |
| Chemical | Content (pph) | Supplier |
|---|---|---|
| Samarium oxide (Sm2O3) | 100 | Richest Group (Shanghai, China) |
| Silane coupling agent (KBE903) | 5, 10, and 20 | Kisco(T) Ltd. (Bangkok, Thailand) |
| 99% Ethanol | 92 − x * | Gammaco Co., Ltd. (Bangkok, Thailand) |
| Distilled water | 8 | Kasetsart University (Bangkok, Thailand) |
| Filler | Filler Content (wt.%) | Linear Attenuation Coefficient (cm−1) | |||
|---|---|---|---|---|---|
| Thermal Neutron | Gamma Rays | ||||
| 0.334 MeV | 0.712 MeV | 0.737 MeV | |||
| Sm | 0 | 2.7025 | 0.1129 | 0.0819 | 0.0805 |
| 5 | 3.6516 | 0.1206 | 0.0852 | 0.0838 | |
| 10 | 4.7053 | 0.1296 | 0.0894 | 0.0877 | |
| 15 | 5.9034 | 0.1403 | 0.0940 | 0.0924 | |
| 20 | 7.1626 | 0.1503 | 0.0977 | 0.0961 | |
| 25 | 8.6581 | 0.1632 | 0.1031 | 0.1011 | |
| 30 | 10.2055 | 0.1765 | 0.1087 | 0.1066 | |
| 35 | 12.0982 | 0.1924 | 0.1155 | 0.1133 | |
| 40 | 14.0712 | 0.2098 | 0.1227 | 0.1202 | |
| 45 | 16.2235 | 0.2298 | 0.1310 | 0.1282 | |
| 50 | 19.1070 | 0.2543 | 0.1409 | 0.1381 | |
| B | 5 | 4.8062 | 0.1162 | 0.0848 | 0.0833 |
| Filler | Filler Content (wt.%) | Half-Value Layer (cm) | |||
|---|---|---|---|---|---|
| Thermal Neutron | Gamma Rays | ||||
| 0.334 MeV | 0.712 MeV | 0.737 MeV | |||
| Sm | 0 | 0.2565 | 6.1419 | 8.4671 | 8.6140 |
| 5 | 0.1898 | 5.7481 | 8.1378 | 8.2705 | |
| 10 | 0.1473 | 5.3486 | 7.7539 | 7.8995 | |
| 15 | 0.1174 | 4.9389 | 7.3760 | 7.5054 | |
| 20 | 0.0968 | 4.6129 | 7.0941 | 7.2142 | |
| 25 | 0.0801 | 4.2473 | 6.7214 | 6.8531 | |
| 30 | 0.0679 | 3.9274 | 6.3738 | 6.5014 | |
| 35 | 0.0573 | 3.6035 | 6.0033 | 6.1171 | |
| 40 | 0.0493 | 3.3045 | 5.6500 | 5.7667 | |
| 45 | 0.0427 | 3.0159 | 5.2902 | 5.4082 | |
| 50 | 0.0363 | 2.7259 | 4.9186 | 5.0189 | |
| B | 5 | 0.1442 | 5.9632 | 8.1772 | 8.3248 |
| Filler | Filler Content (wt.%) | Mass Attenuation Coefficient (cm2/g) | |||
|---|---|---|---|---|---|
| Thermal Neutron | Gamma Rays | ||||
| 0.334 MeV | 0.712 MeV | 0.737 MeV | |||
| Sm | 0 | 2.8447 | 0.1187 | 0.0861 | 0.0847 |
| 5 | 3.6735 | 0.1213 | 0.0856 | 0.0843 | |
| 10 | 4.5140 | 0.1243 | 0.0857 | 0.0841 | |
| 15 | 5.3880 | 0.1280 | 0.0857 | 0.0842 | |
| 20 | 6.2032 | 0.1301 | 0.0846 | 0.0832 | |
| 25 | 7.0946 | 0.1337 | 0.0845 | 0.0828 | |
| 30 | 7.8865 | 0.1363 | 0.0840 | 0.0823 | |
| 35 | 8.7848 | 0.1396 | 0.0838 | 0.0822 | |
| 40 | 9.5611 | 0.1425 | 0.0833 | 0.0816 | |
| 45 | 10.2668 | 0.1454 | 0.0829 | 0.0811 | |
| 50 | 11.2005 | 0.1490 | 0.0821 | 0.0809 | |
| B | 5 | 5.0592 | 0.1223 | 0.0892 | 0.0876 |
| Quantity | Mathematical Form | Mathematical Coefficient | |
|---|---|---|---|
| A | B | ||
| µ | 3.1232 | 0.0379 | |
| HVL | 0.2219 | −0.038 | |
| µm | x + B | 0.1669 | 2.8666 |
| Element | Elemental Composition (by Weight) (%) | |||||
|---|---|---|---|---|---|---|
| Treated Sm2O3 Particles | Treated Sm2O3/UHMWPE Composites | |||||
| Silane Content (pph) | ||||||
| 5 | 10 | 20 | 5 | 10 | 20 | |
| C | - | - | - | 69.69 ± 1.55 | 68.20 ± 1.72 | 67.85 ± 1.71 |
| O | 25.77 ± 1.89 | 26.69 ± 0.16 | 24.90 ± 2.19 | 6.78 ± 0.38 | 7.43 ± 0.67 | 7.43 ± 0.92 |
| Sm | 73.76 ± 2.52 | 72.83 ± 2.42 | 74.34 ± 2.11 | 23.21 ± 1.33 | 23.81 ± 1.14 | 24.04 ± 0.69 |
| Si | 0.47 ± 0.20 | 0.48 ± 0.05 | 0.75 ± 0.10 | 0.33 ± 0.03 | 0.55 ± 0.08 | 0.67 ± 0.10 |
| Silane Content (pph) | Specific Wear Rate (×10−5 mm3/Nm) | Average Coefficient of Friction | Roughness (µm) | Crystallinity (%) |
|---|---|---|---|---|
| 5 | 1.78 | 0.32 ± 0.03 | 0.27 ± 0.05 | 71.10 ± 6.24 |
| 10 | 4.17 | 0.19 ± 0.01 | 0.20 ± 0.03 | 86.73 ± 2.06 |
| 20 | 4.25 | 0.25 ± 0.03 | 0.31 ± 0.14 | 78.87 ± 2.80 |
| Silane Content (wt.%) | Tensile Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Hardness (Shore D) |
|---|---|---|---|---|
| 5 | 136.7 ± 28.4 | 22.0 ± 1.5 | 364 ± 42 | 63 ± 1 |
| 10 | 156.8 ± 20.9 | 24.9 ± 0.6 | 497 ± 27 | 68 ± 1 |
| 20 | 158.8 ± 35.2 | 21.1 ± 0.8 | 361 ± 86 | 62 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyen, D.; Paopun, Y.; Changjan, D.; Wimolmala, E.; Mahathanabodee, S.; Pianpanit, T.; Anekratmontree, T.; Saenboonruang, K. Simulation of Neutron/Self-Emitted Gamma Attenuation and Effects of Silane Surface Treatment on Mechanical and Wear Resistance Properties of Sm2O3/UHMWPE Composites. Polymers 2021, 13, 3390. https://doi.org/10.3390/polym13193390
Toyen D, Paopun Y, Changjan D, Wimolmala E, Mahathanabodee S, Pianpanit T, Anekratmontree T, Saenboonruang K. Simulation of Neutron/Self-Emitted Gamma Attenuation and Effects of Silane Surface Treatment on Mechanical and Wear Resistance Properties of Sm2O3/UHMWPE Composites. Polymers. 2021; 13(19):3390. https://doi.org/10.3390/polym13193390
Chicago/Turabian StyleToyen, Donruedee, Yupadee Paopun, Dararat Changjan, Ekachai Wimolmala, Sithipong Mahathanabodee, Theerasarn Pianpanit, Thitisorn Anekratmontree, and Kiadtisak Saenboonruang. 2021. "Simulation of Neutron/Self-Emitted Gamma Attenuation and Effects of Silane Surface Treatment on Mechanical and Wear Resistance Properties of Sm2O3/UHMWPE Composites" Polymers 13, no. 19: 3390. https://doi.org/10.3390/polym13193390
APA StyleToyen, D., Paopun, Y., Changjan, D., Wimolmala, E., Mahathanabodee, S., Pianpanit, T., Anekratmontree, T., & Saenboonruang, K. (2021). Simulation of Neutron/Self-Emitted Gamma Attenuation and Effects of Silane Surface Treatment on Mechanical and Wear Resistance Properties of Sm2O3/UHMWPE Composites. Polymers, 13(19), 3390. https://doi.org/10.3390/polym13193390







