Physical Properties and Non-Isothermal Crystallisation Kinetics of Primary Mechanically Recycled Poly(l-lactic acid) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Differential Scanning Calorimetry (DSC)
2.3. Thermogravimetric Analysis (TGA)
2.4. Rheological-Flow Properties
2.5. Mechanical Properties
2.6. Heat Resistance Testing
3. Results and Discussion
3.1. Thermal Properties and Structure of Recycled Biobased Polymers
3.2. Effect of Recycling on Non-Isothermal Crystallisation Kinetics
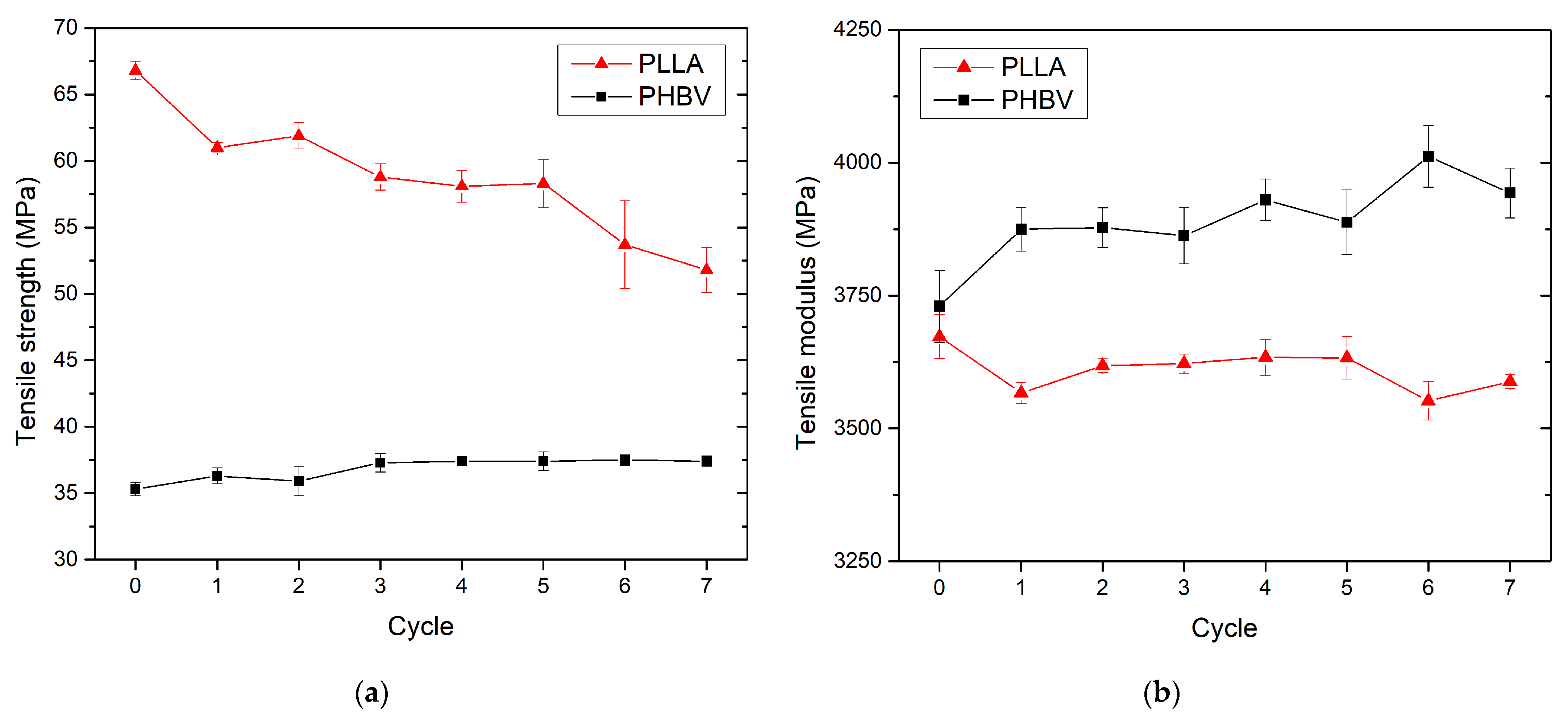
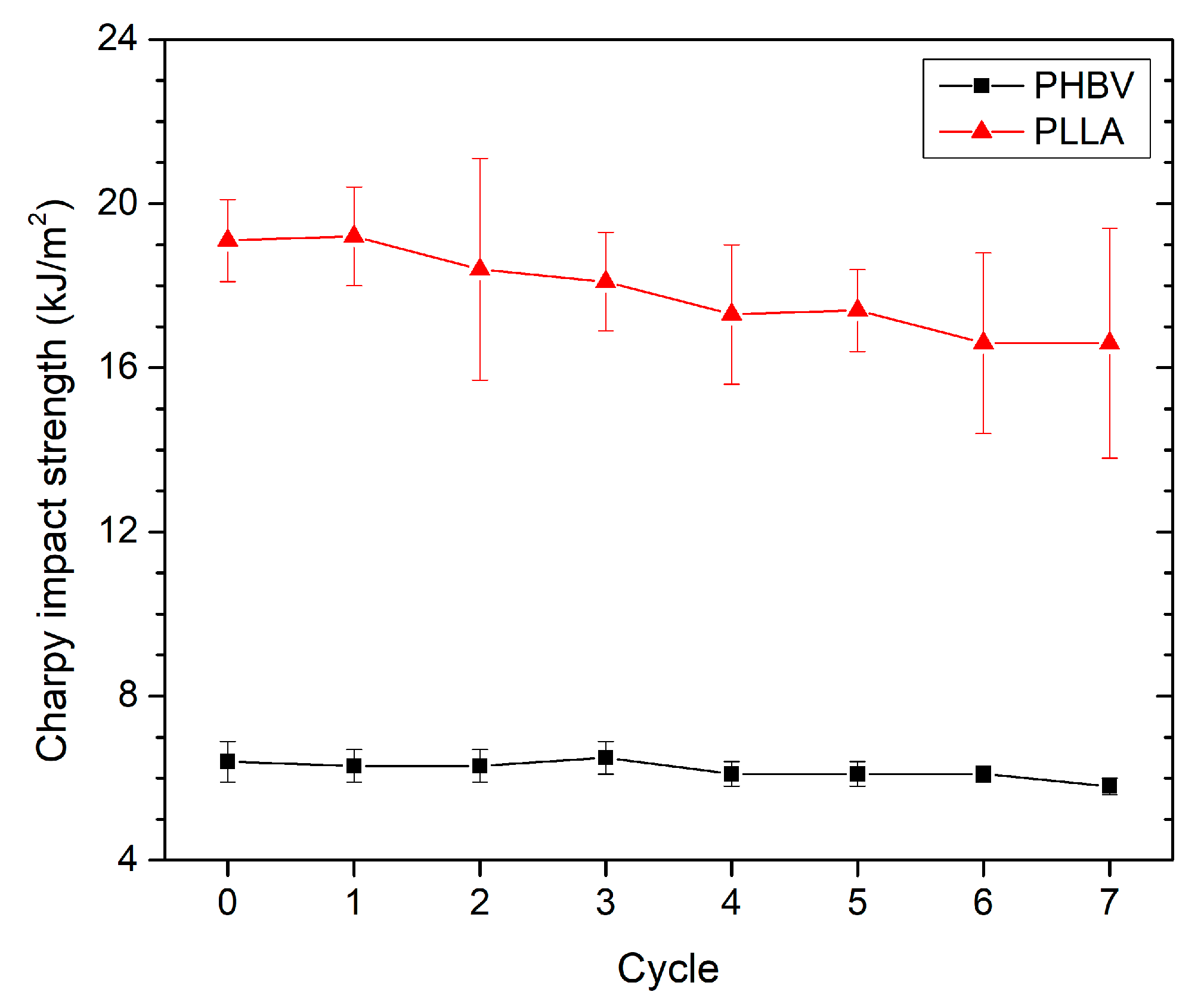
3.3. Effect of Recycling on Mechanical Properties
3.4. Effect of Recycling on Heat Resistance Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics—The Facts. 2020: An Analysis of European Plastics Production, Demand and Waste Data. Available online: www.plasticseurope.org (accessed on 4 July 2021).
- D’Ambrières, W. Plastics recycling worldwide: Current overview and desirable changes. Field Actions Sci. Rep. 2019, 19, 12–21. [Google Scholar]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased additive plasticizing Polylactic acid (PLA). Polimeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Puopolo, M. Recycling of PLA-based bioplastics: The role of chain-extenders in twin-screw extrusion compounding and cast extrusion of sheets. J. Appl. Polym. Sci. 2020, 137, 49292. [Google Scholar] [CrossRef]
- Prapruddivongs, C.; Rukrabiab, J.; Kulwongwit, N.; Wongpreedee, T. Effect of surface-modified silica on the thermal and mechanical behaviors of poly(lactic acid) and chemically crosslinked poly(lactic acid) composites. J. Thermoplast. Compos. Mater. 2019, 33, 1692–1706. [Google Scholar] [CrossRef]
- Jia, S.; Yu, D.; Zhu, Y.; Wang, Z.; Chen, L.; Fu, L. Morphology, Crystallization and Thermal Behaviors of PLA-Based Composites: Wonderful Effects of Hybrid GO/PEG via Dynamic Impregnating. Polymers 2017, 9, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallo, E.; McPhee, D.J.; Luzi, F.; Dominici, F.; Cerrutti, P.; Bernal, C.; Foresti, M.L.; Torre, L.; Puglia, D. UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles. Molecules 2020, 26, 126. [Google Scholar] [CrossRef]
- Vu, D.; Åkesson, D.; Taherzadeh, M.J.; Ferreira, J.A. Recycling strategies for polyhydroxyalkanoate-based waste materials: An overview. Bioresour. Technol. 2019, 298, 122393. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K.; Kessler, M.R. Handbook of composites from renewable materials. In Structure and Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 1. [Google Scholar]
- Wang, B.; Zhang, Y.; Zhang, J.; Gou, Q.; Wang, Z.; Chen, P.; Gu, Q. Crystallization behavior, thermal and mechanical prop-erties of PHBV/graphene nanosheet composites. Chin. J. Polym. Sci. 2013, 31, 670–678. [Google Scholar] [CrossRef]
- Magnani, C.; Idström, A.; Nordstierna, L.; Müller, A.J.; Dubois, P.; Raquez, J.-M.; Re, G.L. Interphase Design of Cellulose Nanocrystals/Poly(hydroxybutyrate-ran-valerate) Bionanocomposites for Mechanical and Thermal Properties Tuning. Biomacromolecules 2020, 21, 1892–1901. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Haufe, J.; Patel, M.K. Product Overview and Market Projection of Emerging Bio-Based Plastics; PRO-BIP Final Report; Utrecht University Commissioned by European Polysaccharide Network of Excellence and European Bioplastics: Ultrecht, The Netherlands, 2009. [Google Scholar]
- Guo, M.; Stuckey, D.; Murphy, R. Is it possible to develop biopolymer production systems independent of fossil fuels? Case study in energy profiling of polyhydroxybutyrate-valerate (PHBV). Green Chem. 2013, 15, 706–717. [Google Scholar] [CrossRef]
- McKeown, P.; Jones, M.D. The Chemical Recycling of PLA: A Review. Sustain. Chem. 2020, 1, 1. [Google Scholar] [CrossRef]
- Soroudi, A.; Jakubowicz, I. Recycling of bioplastics, their blends and biocomposites: A review. Eur. Polym. J. 2013, 49, 2839–2858. [Google Scholar] [CrossRef]
- Aitor, L.; Erlantz, L. A review on the thermomechanical properties and biodegradation behaviour of polyester. Eur. Polym. J. 2019, 121, 1–31. [Google Scholar]
- Ndazi, B.; Karlsson, S. Characterization of hydrolytic degradation of polylactic acid/rice hulls composites in water at different temperatures. Express Polym. Lett. 2011, 5, 119–131. [Google Scholar] [CrossRef]
- Kolstad, J.J.; Vink, E.T.H.; De Wilde, B.; Debeer, L. Assessment of anaerobic degradation of IngeoTM polylactides under accelerated landfill conditions. Polym. Degrad. Stab. 2012, 97, 1131–1141. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Rehovsky, C.; Bajwa, S.G.; Vahidi, G. Deterioration in the Physico-Mechanical and Thermal Properties of Biopolymers Due to Reprocessing. Polymers 2019, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Åkesson, D.; Vrignaud, T.; Tissot, C.; Skrifvars, M. Mechanical Recycling of PLA Filled with a High Level of Cellulose Fibres. J. Polym. Environ. 2016, 24, 185–195. [Google Scholar] [CrossRef]
- Piemonte, V.; Sabatini, S.; Gironi, F. Chemical recycling of PLA: A great opportunity towards the sustainable development? J. Polym. Environ. 2013, 21, 640–647. [Google Scholar] [CrossRef]
- De Andrade, M.F.C.; Souza, P.M.S.; Cavalett, Ó.; Morales, A.R. Life cycle assessment of poly (lactic acid) (PLA): Comparison between chemical recycling, mechanical recycling and composting. J. Polym. Environ. 2016, 24, 372–384. [Google Scholar] [CrossRef]
- Badia, J.; Ribes-Greus, A. Mechanical recycling of polylactide, upgrading trends and combination of valorization techniques. Eur. Polym. J. 2016, 84, 22–39. [Google Scholar] [CrossRef] [Green Version]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Riebes-Greus, A. Material valorisation of amorphous polylactide. Influence of thermomechanical degradation on the morphology, segmental dynamics, thermal and mechanical performance. Polym. Degrad. Stab. 2012, 97, 670–678. [Google Scholar] [CrossRef]
- Abe, H. Thermal Degradation of Environmentally Degradable Poly(hydroxyalkanoic acid)s. Macromol. Biosci. 2006, 6, 469–486. [Google Scholar] [CrossRef]
- Pillin, I.; Montrelay, N.; Bourmaud, A.; Grohens, Y. Effect of thermo-mechanical cycles on the physico-chemical properties of poly(lactic acid). Polym. Degrad. Stab. 2008, 93, 321–328. [Google Scholar] [CrossRef]
- Dai, X.; Cao, X.Y.; Shi, X.; Wang, X. Non-isothermal crystallization kinetics, thermal degradation behavior and mechanical properties of poly (lactic acid)/MOF composites prepared by melt-blending methods. RSC Adv. 2016, 6, 71461–71471. [Google Scholar] [CrossRef]
- Nicolae, C.A.; Grigorescu, M.A.; Gabor, R.A. An investigation of thermal degradation of poly(lactic acid). Eng. Lett. 2008, 16, 568–571. [Google Scholar]
- Zaverl, M.; Seydibeyoğlu, M.; Misra, M.; Mohanty, A. Studies on recyclability of polyhydroxybutyrate-co-valerate bioplastic: Multiple melt processing and performance evaluations. J. Appl. Polym. Sci. 2012, 125, E324–E331. [Google Scholar] [CrossRef]
- Lagazzo, A.; Moliner, C.; Bosio, B.; Botter, R.; Arato, E. Evaluation of the Mechanical and Thermal Properties Decay of PHBV/Sisal and PLA/Sisal Biocomposites at Different Recycle Steps. Polymers 2019, 11, 1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, R.; Mohanty, A.K.; Drzal, L.T.; Pourboghrat, F.; Misra, M. Renewable resource-based green composites from recycled cellulose fiber and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic. Biomacromolecules 2006, 7, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Kratochvíl, J.; Kelnar, I. A simple method of evaluating non-isothermal crystallization kinetics in multicomponent polymer systems. Polym. Test. 2015, 47, 79–86. [Google Scholar] [CrossRef]
- Sarasua, J.R.; Prud’Homme, R.E.; Wisniewski, M.; Le Borgne, A.; Spassky, N. Crystallization and melting behavior of polylactides. Macromolecules 1998, 31, 3895–3905. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Mubarak, Y.; Harkin-Jones, E.M.A.; Martin, P.J.; Ahmad, M. Modeling of non-isothermal crystallization kinetics of isotactic polypropylene. Polymer 2001, 42, 3171–3182. [Google Scholar] [CrossRef]
- Coburn, N.; Douglas, P.; Kaya, D.; Gupta, J.; McNally, T. Isothermal and non-isothermal crystallization kinetics of composites of poly(propylene) and MWCNTs. Adv. Ind. Eng. Polym. Res. 2018, 1, 99–110. [Google Scholar] [CrossRef]
- Pantani, R.; De Santis, F.; Sorrentino, A.; De Maio, F.; Titomanlio, G. Crystallization kinetics of virgin and processed poly(lactic acid). Polym. Degrad. Stab. 2010, 95, 1148–1159. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Richert, J.; Rztlewsku, P.; Moraczewski, K.; Stepczyńska, M.; Karasiewicz, T. Characterisation of multi-extruded poly(lactic acid). Polym. Test. 2009, 28, 412–418. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Oswald, H.J.; Turi, E.A.; Harget, P.J.; Khanna, Y.P. Development of a middle endotherm in DSC thermograms of thermally treated drawn PET yarns and its structural and mechanistic interpretation. J. Macromol. Sci. Part B 1977, 13, 231–254. [Google Scholar] [CrossRef]
- Righetti, M.C.; Di Lorenzo, M.L. Melting process of poly(butylene terephthalate) analyzed by temperature-modulated differential scanning calorimetry. J. Polym. Sci. B Polym. Phys. 2004, 42, 2191–2201. [Google Scholar] [CrossRef]
- Monnier, X.; Cavallo, D.; Righetti, M.C.; Di Lorenzo, M.L.; Marina, S.; Martin, J.; Cangialosi, D. Physical Aging and Glass Transition of the Rigid Amorphous Fraction in Poly(l-lactic acid). Macromolecules 2020, 53, 8741–8750. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L. The Crystallization and Melting Processes of Poly(l-lactic acid). Macromol. Symp. 2006, 234, 176–183. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Crompton, T.R. Thermal Stability of Polymers; Smithers Rapra: Akron, OH, USA, 2012. [Google Scholar]
- Tarani, E.; Pušnik Črešnar, K.; Zemljič, L.F.; Chrissafis, K.; Papageorgiou, G.Z.; Lambropoulou, D.; Zamboulis, A.; Bikiaris, N.; Terzopoulou, Z. Cold Crystallization Kinetics and Thermal Degradation of PLA Composites with Metal Oxide Nanofillers. Appl. Sci. 2021, 11, 3004. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L. Crystallization behavior of poly(l-lactic acid). Eur. Polym. J. 2005, 41, 569–575. [Google Scholar] [CrossRef]
- Paukszta, D.; Borysiak, S. Influence of reprocessing on the crystallization of polypropylene in PP/PA-6 blends. Polimery 2009, 54, 126–131. [Google Scholar] [CrossRef] [Green Version]
- El-Taweel, S.H.; Stoll, B.; Höhne, G.W.H.; Mansour, A.A.; Seliger, H. Stress–strain behavior of blends of bacterial polyhydroxybutyrate. J. Appl. Polym. Sci. 2004, 94, 2528–2537. [Google Scholar] [CrossRef]



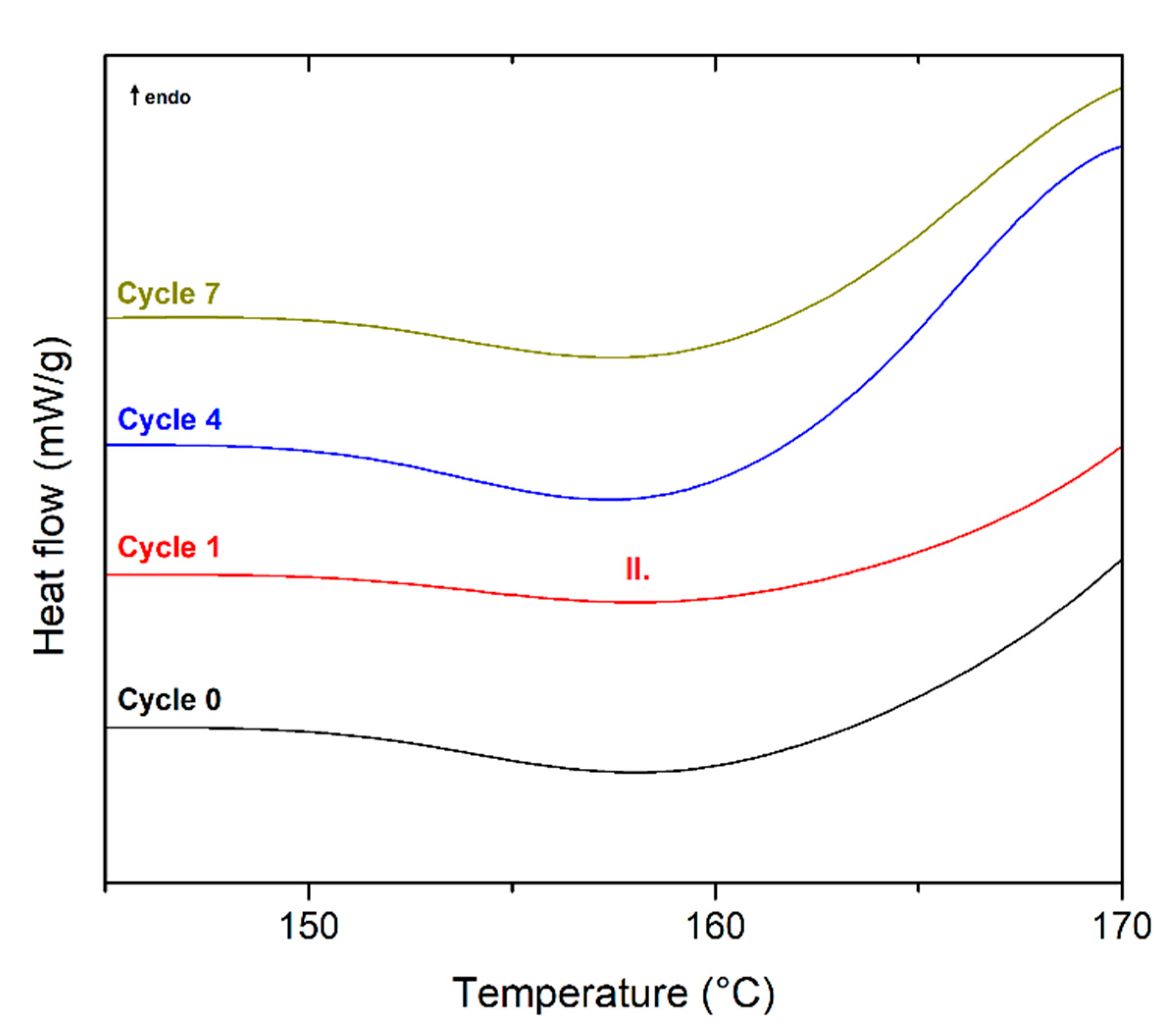




| Recycling Cycle | First Heating | Cooling | Second Heating | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔHcc (J/g) | ΔHpc (J/g) | ΔHm (J/g) | Xc (%) | Tp,c (°C) | ΔHc (J/g) | Tp,cc (°C) | ΔHcc (J/g) | Tp,pc (°C) | ΔHpc (J/g) | Tp,m (°C) | ΔHm (J/g) | Xc (%) | |
| 0 | 31.3 | 6.6 | 51.1 | 12.5 | 100 | 4.4 | 97.8 | 27.1 | 158 | 6.0 | 174 | 51.3 | 17.2 |
| 1 | 29.7 | 5.8 | 52.8 | 16.3 | 101 | 38.1 | - | - | 163 | 1.4 | 175 | 49.0 | 44.9 |
| 2 | 30.6 | 5.4 | 53.7 | 16.7 | 107 | 39.6 | - | - | 163 | 0.6 | 174 | 48.9 | 45.6 |
| 3 | 28.7 | 5.2 | 54.1 | 19.1 | 107 | 39.4 | - | - | 163 | 0.9 | 174 | 49.3 | 45.6 |
| 4 | 29.8 | 4.9 | 54.3 | 18.5 | 108 | 41.1 | - | - | - | - | 174 | 50.4 | 47.6 |
| 5 | 30.3 | 4.7 | 53.6 | 17.5 | 111 | 41.6 | - | - | - | - | 175 | 54.0 | 51.0 |
| 6 | 30.2 | 4.6 | 54.6 | 18.7 | 110 | 42.5 | - | - | - | - | 175 | 54.9 | 51.7 |
| 7 | 30.7 | 4.4 | 54.8 | 18.5 | 110 | 42.4 | - | - | - | - | 174 | 55.5 | 52.3 |
| Recycling Cycle | First Heating | Cooling | Second Heating | ||||
|---|---|---|---|---|---|---|---|
| ΔHm (J/g) | Xc (%) | Tp,c (°C) | ΔHc (J/g) | Tp,m (°C) | ΔHm (J/g) | Xc (%) | |
| 0 | 97.2 | 60.1 | 122 | 85.7 | 172 | 97.2 | 66.6 |
| 1 | 97.7 | 60.0 | 123 | 86.8 | 170 | 97.7 | 66.9 |
| 2 | 96.3 | 60.0 | 123 | 86.7 | 172 | 96.3 | 66.0 |
| 3 | 87.2 | 60.0 | 123 | 86.7 | 171 | 87.2 | 66.6 |
| 4 | 97.5 | 60.7 | 123 | 86.8 | 170 | 97.5 | 66.8 |
| 5 | 97.0 | 60.9 | 124 | 86.8 | 171 | 97.0 | 66.4 |
| 6 | 97.0 | 60.4 | 124 | 86.8 | 170 | 97.0 | 66.4 |
| 7 | 98.2 | 61.2 | 124 | 87.8 | 171 | 98.2 | 67.2 |
| Polymer | Degradation Temperature | Recycling Cycle | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| PLLA | T5 (°C) | 343 | 332 | 324 | 272 | 263 | 327 | 269 | 272 |
| Tmax (°C) | 370 | 368 | 367 | 349 | 348 | 366 | 345 | 346 | |
| PHBV | T5 (°C) | 281 | 281 | 278 | 279 | 278 | 271 | 273 | 275 |
| Tmax (°C) | 298 | 298 | 296 | 297 | 296 | 292 | 294 | 293 | |
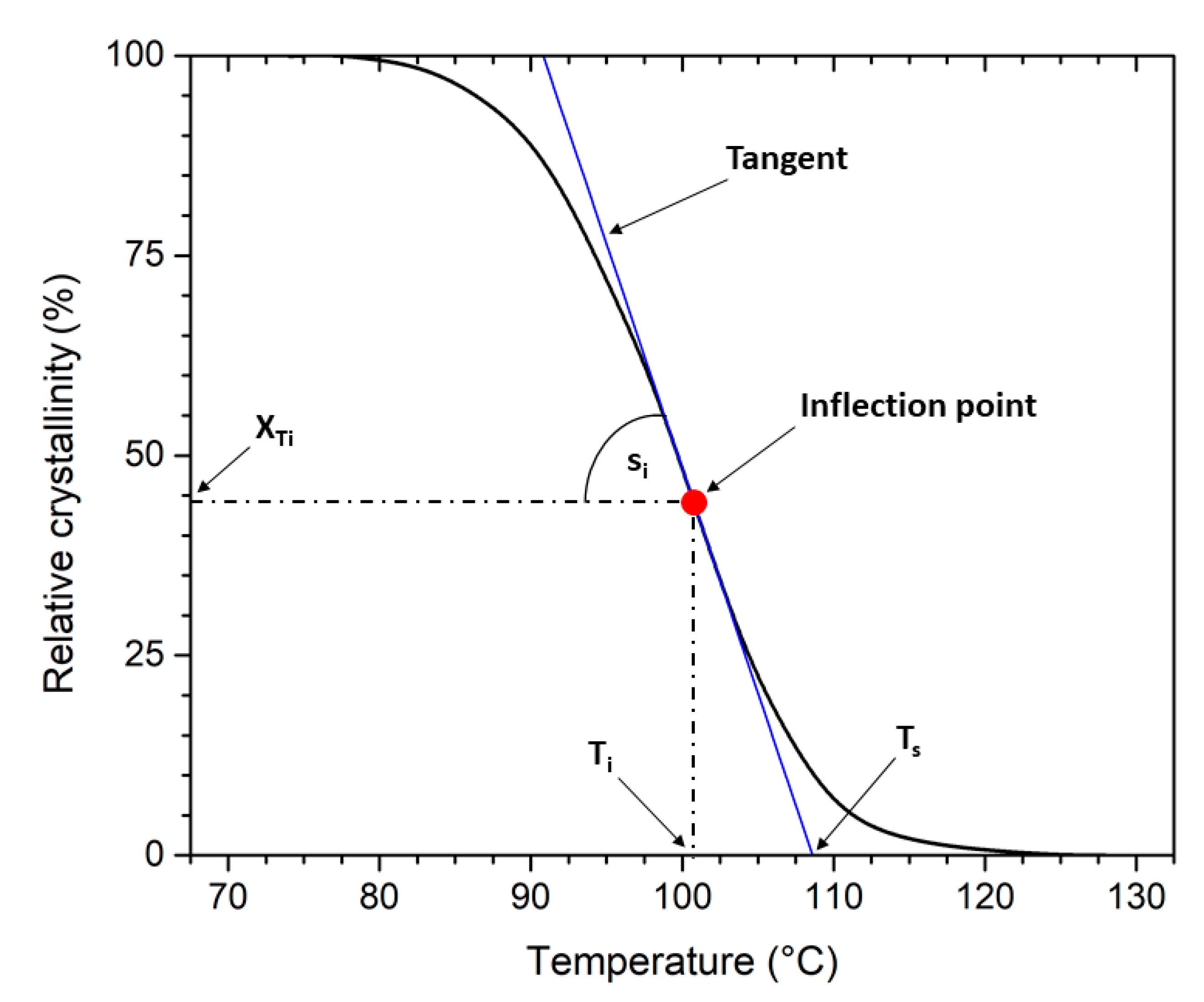
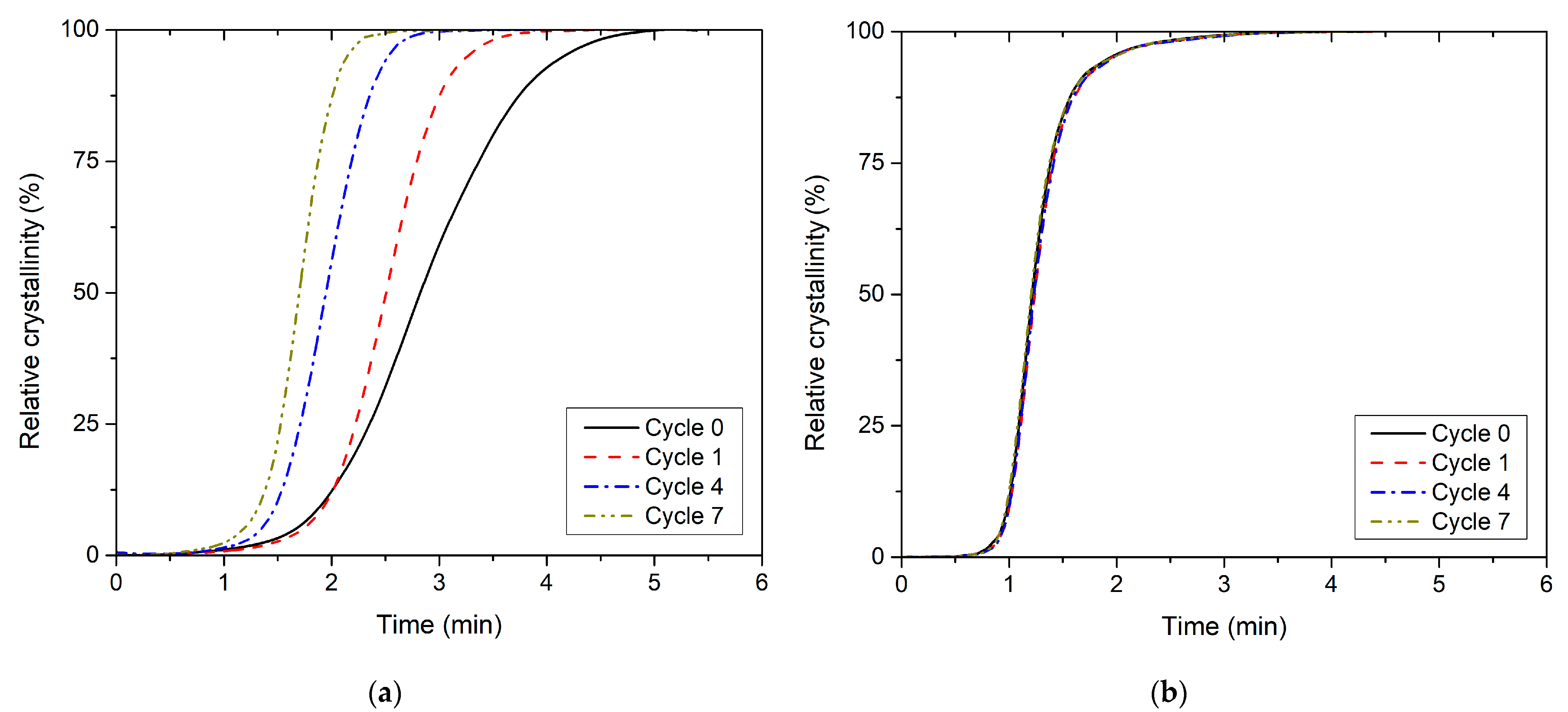
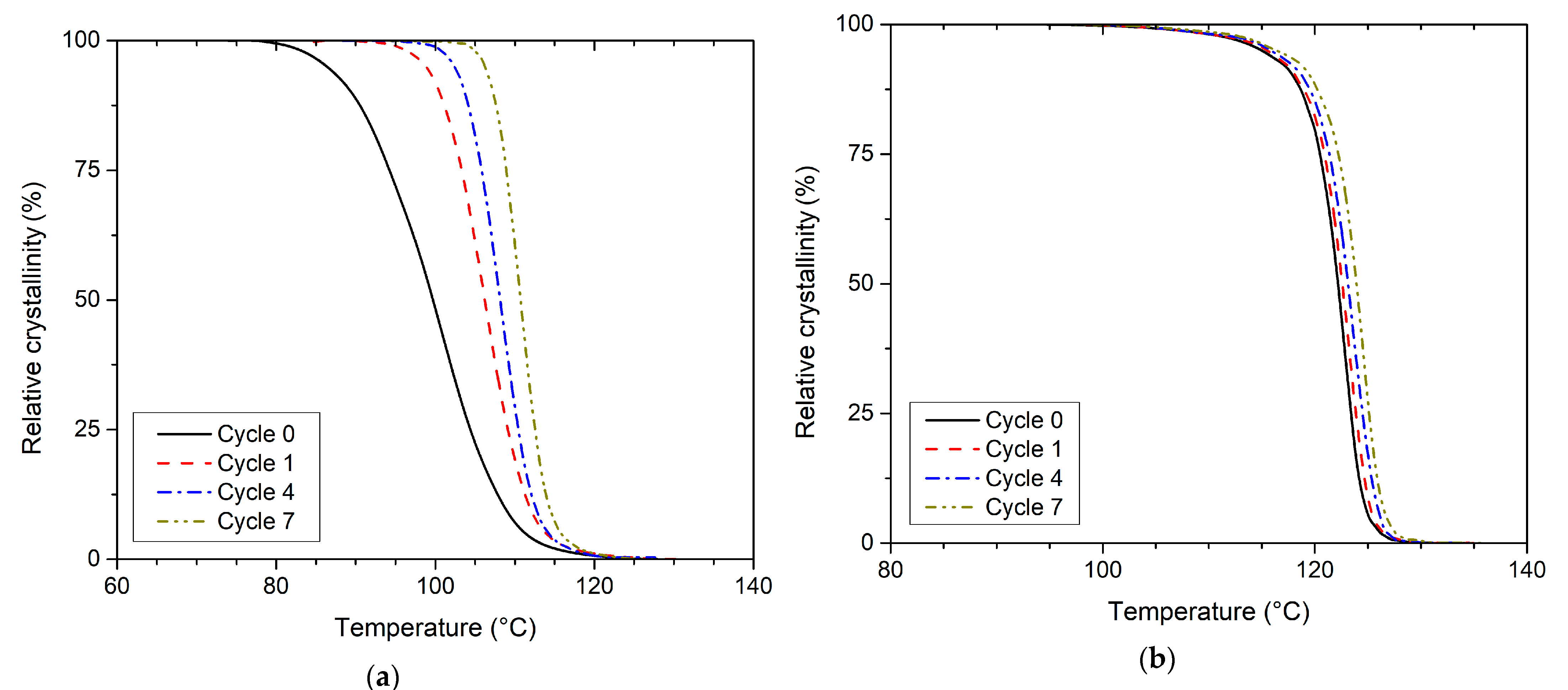
| Recycling Cycle | Melt Crystallisation of PLLA | Melt Crystallisation of PHBV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t0.5 (min) | Ts (°C) | Ti (°C) | XTi (°C) | si (1/°C) | t0.5 (min) | Ts (°C) | Ti (°C) | XTi (°C) | si (1/°C) | |
| 0 | 2.81 | 108.6 | 100.8 | 44.1 | 5.6 | 1.21 | 124.6 | 122.7 | 39.9 | 21.0 |
| 1 | 2.50 | 111.5 | 106.3 | 48.9 | 9.4 | 1.24 | 125.0 | 123.3 | 36.2 | 20.5 |
| 2 | 2.26 | 112.3 | 107.3 | 49.9 | 10.3 | 1.24 | 125.6 | 123.3 | 42.7 | 19.2 |
| 3 | 2.17 | 111.7 | 106.3 | 54.4 | 10.0 | 1.21 | 125.5 | 123.5 | 41.7 | 20.9 |
| 4 | 1.94 | 112.4 | 108.4 | 47.1 | 11.7 | 1.23 | 125.8 | 123.5 | 43.4 | 18.5 |
| 5 | 1.61 | 114.5 | 111.5 | 47.6 | 15.7 | 1.24 | 125.9 | 123.8 | 40.6 | 19.6 |
| 6 | 1.68 | 113.9 | 110.6 | 49.3 | 15.0 | 1.27 | 126.3 | 124.5 | 37.1 | 20.4 |
| 7 | 1.70 | 114.0 | 110.9 | 46.8 | 15.3 | 1.21 | 126.2 | 124.6 | 36.8 | 22.1 |
| Recycling Cycle | Cold Crystallisation of PLLA—First Heating | ||||
|---|---|---|---|---|---|
| Tp,cc (min) | Ts,cc (°C) | Ti,cc (°C) | XTi,cc (°C) | si,cc (1/°C) | |
| 0 | 97.5 | 101.0 | 98.0 | 49.9 | 16.7 |
| 1 | 95.4 | 98.1 | 95.2 | 49.1 | 16.9 |
| 2 | 94.6 | 97.5 | 94.7 | 49.1 | 17.6 |
| 3 | 94.1 | 97.2 | 94.4 | 47.3 | 17.1 |
| 4 | 93.2 | 96.2 | 93.4 | 49.9 | 17.5 |
| 5 | 93.4 | 96.6 | 93.6 | 50.6 | 16.8 |
| 6 | 92.4 | 95.6 | 92.5 | 49.0 | 16.0 |
| 7 | 92.5 | 95.4 | 92.6 | 48.1 | 16.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Běhálek, L.; Novák, J.; Brdlík, P.; Borůvka, M.; Habr, J.; Lenfeld, P. Physical Properties and Non-Isothermal Crystallisation Kinetics of Primary Mechanically Recycled Poly(l-lactic acid) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polymers 2021, 13, 3396. https://doi.org/10.3390/polym13193396
Běhálek L, Novák J, Brdlík P, Borůvka M, Habr J, Lenfeld P. Physical Properties and Non-Isothermal Crystallisation Kinetics of Primary Mechanically Recycled Poly(l-lactic acid) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polymers. 2021; 13(19):3396. https://doi.org/10.3390/polym13193396
Chicago/Turabian StyleBěhálek, Luboš, Jan Novák, Pavel Brdlík, Martin Borůvka, Jiří Habr, and Petr Lenfeld. 2021. "Physical Properties and Non-Isothermal Crystallisation Kinetics of Primary Mechanically Recycled Poly(l-lactic acid) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)" Polymers 13, no. 19: 3396. https://doi.org/10.3390/polym13193396









