Synthesis, Characterization, and CO2/N2 Separation Performance of POEM-g-PAcAm Comb Copolymer Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
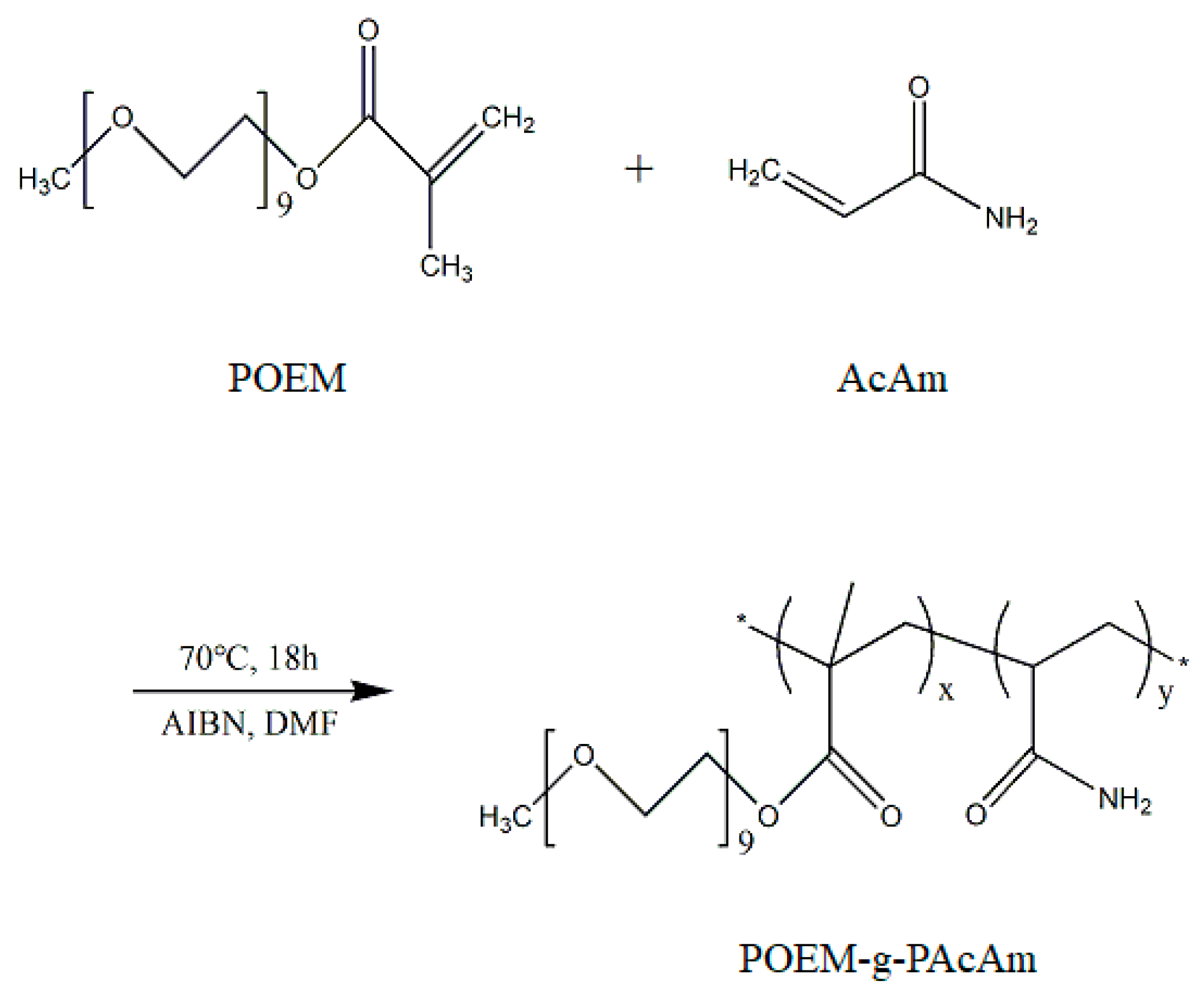
2.2. Synthesis of POEM-g-PAcAm Comb Copolymers
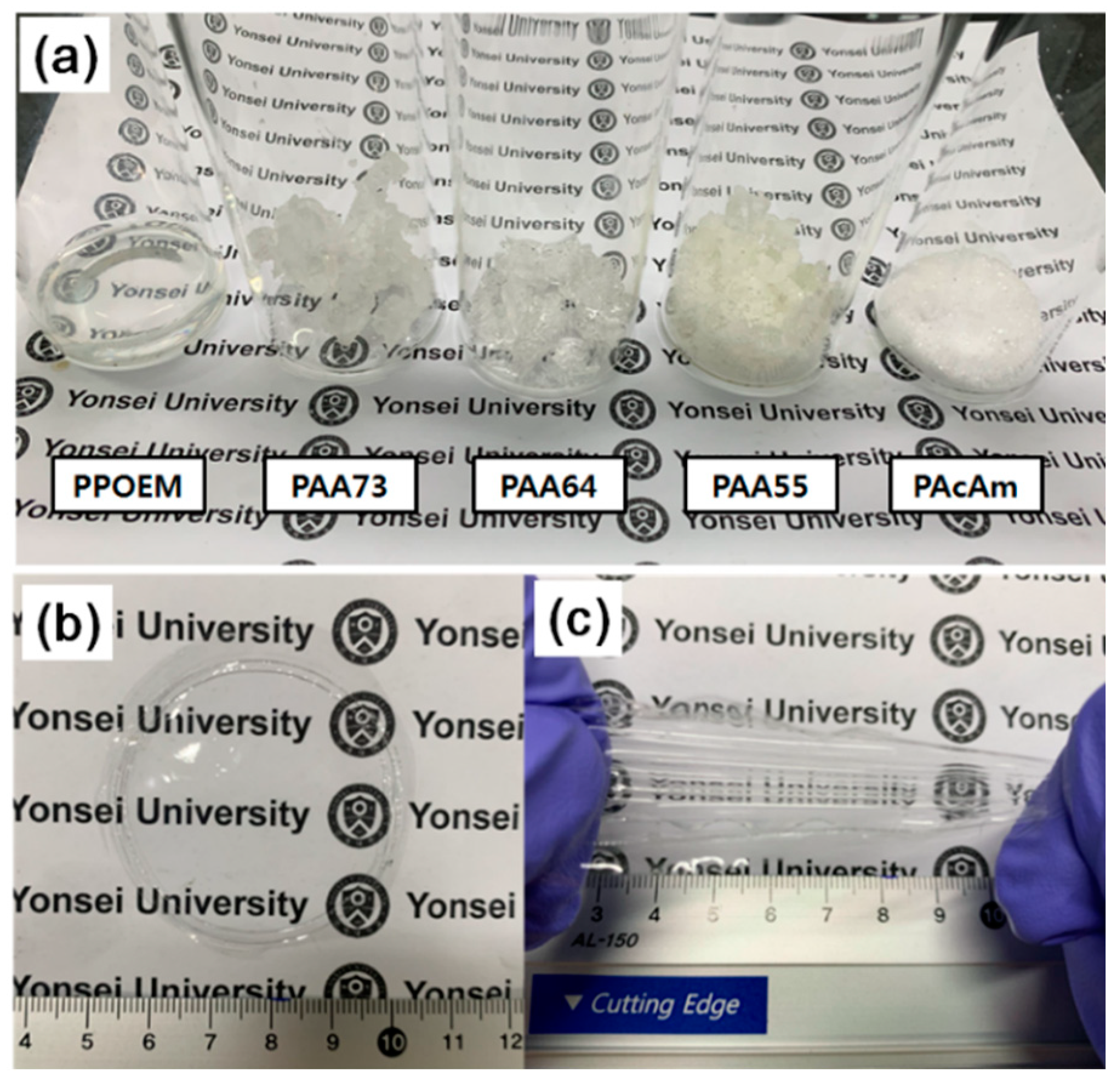
2.3. Preparation of POEM-g-PAcAm Membranes
2.4. Characterization
2.5. Gas Permeation Measurement
3. Results and Discussion
3.1. Synthesis of POEM-g-PAcAm Copolymer
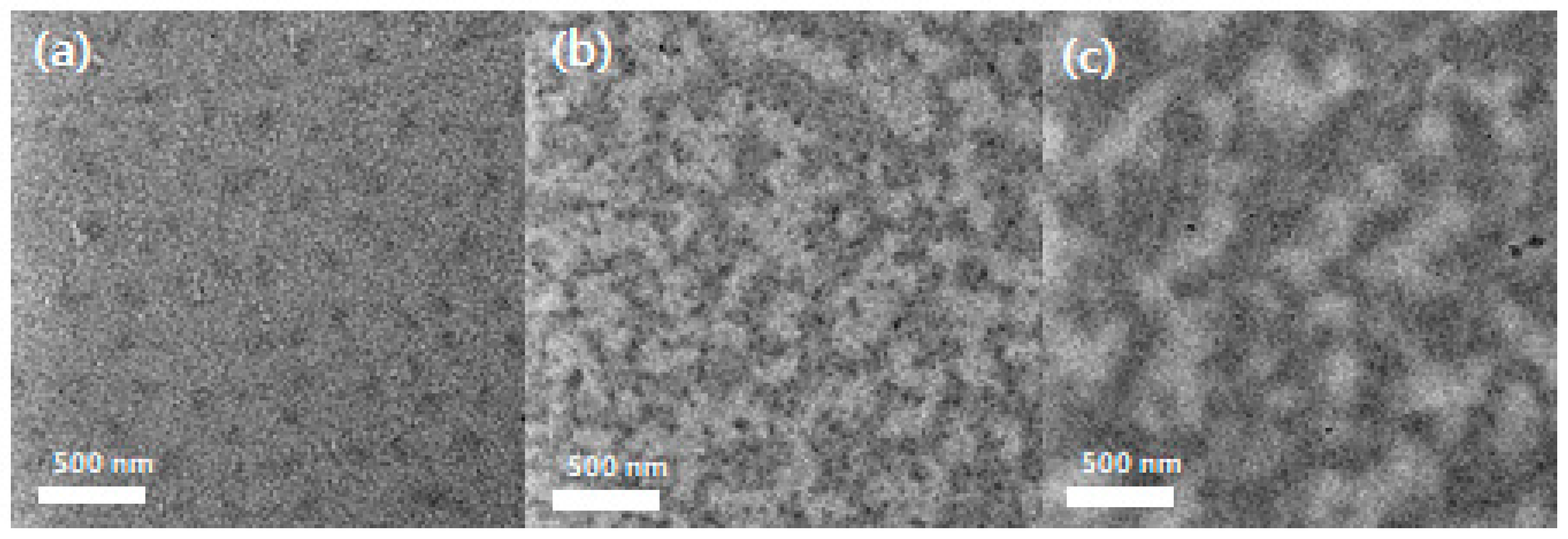
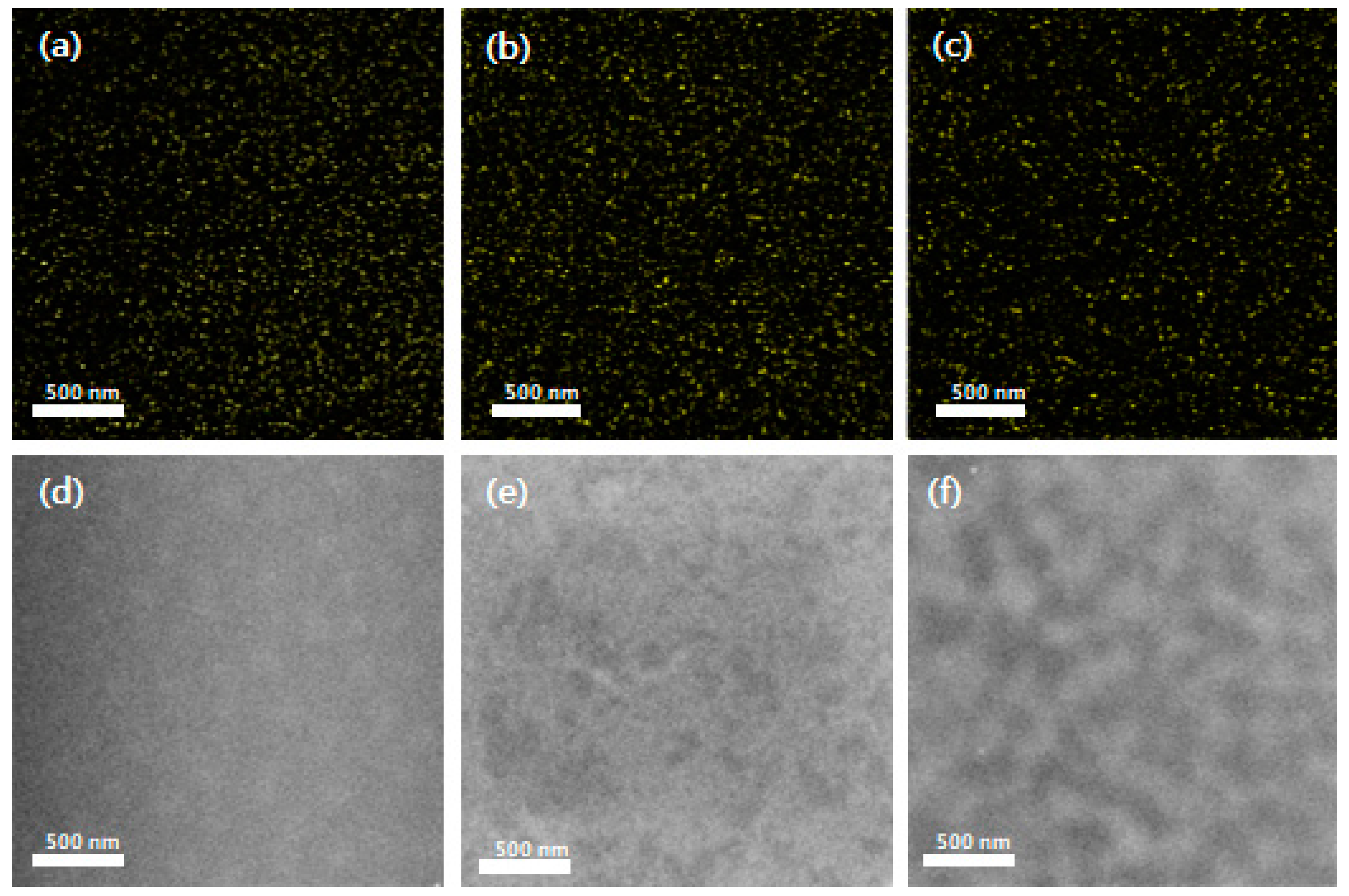
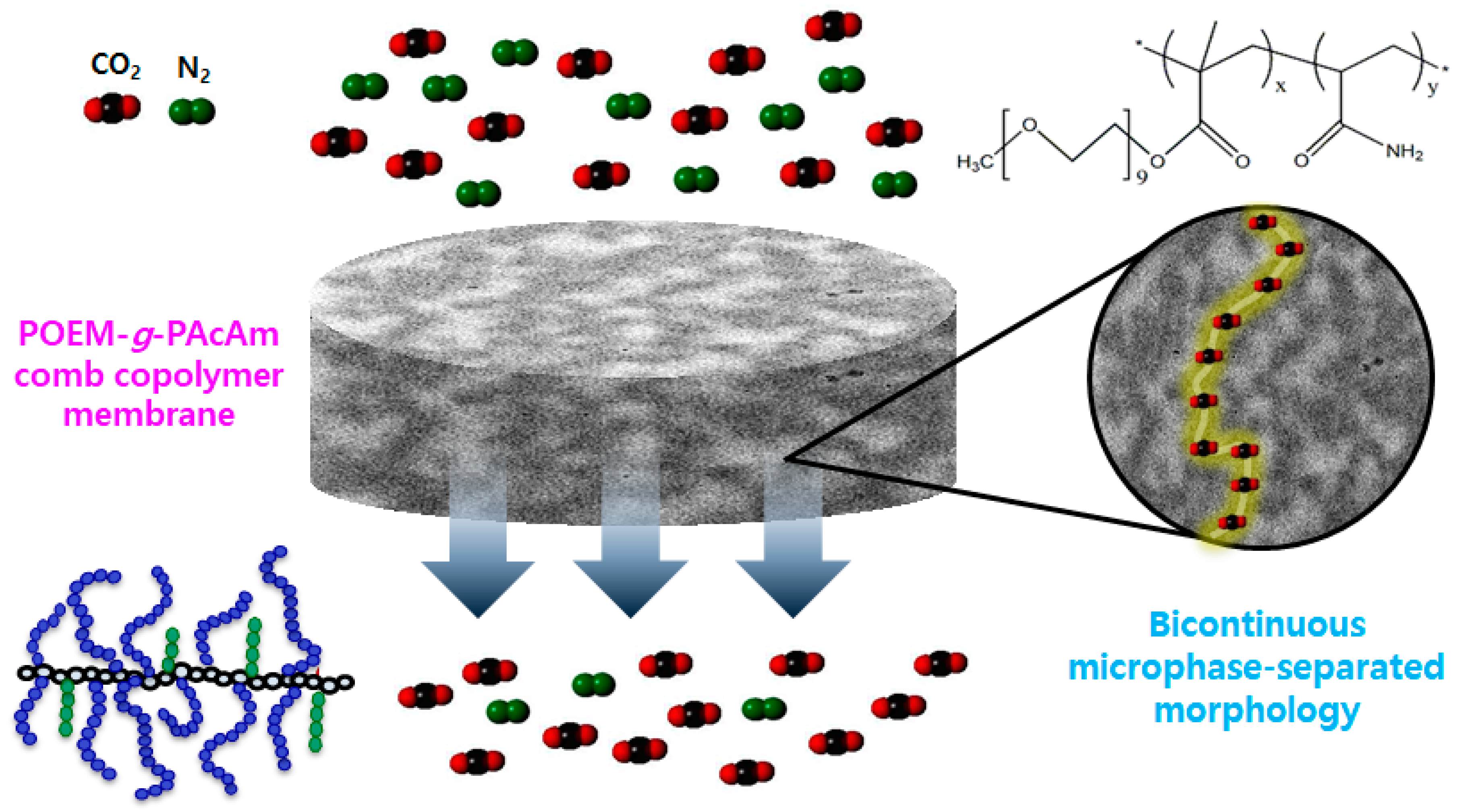
3.2. Structural and Morphological Properties
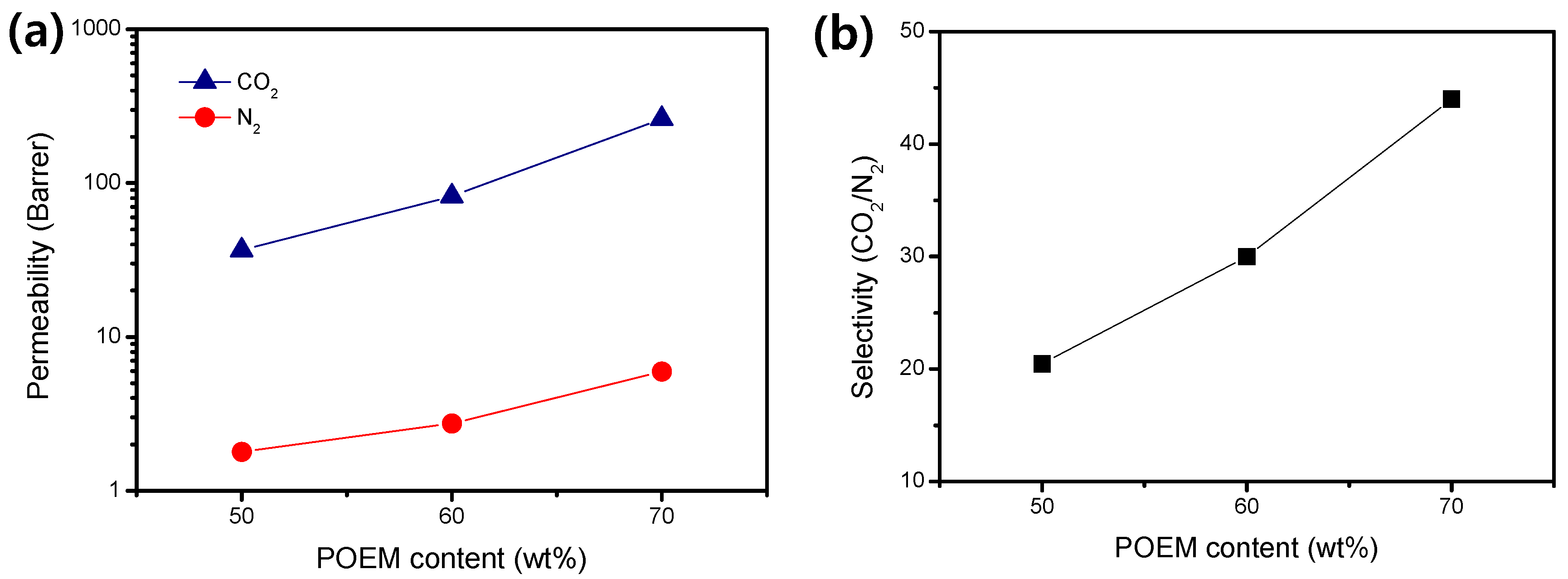
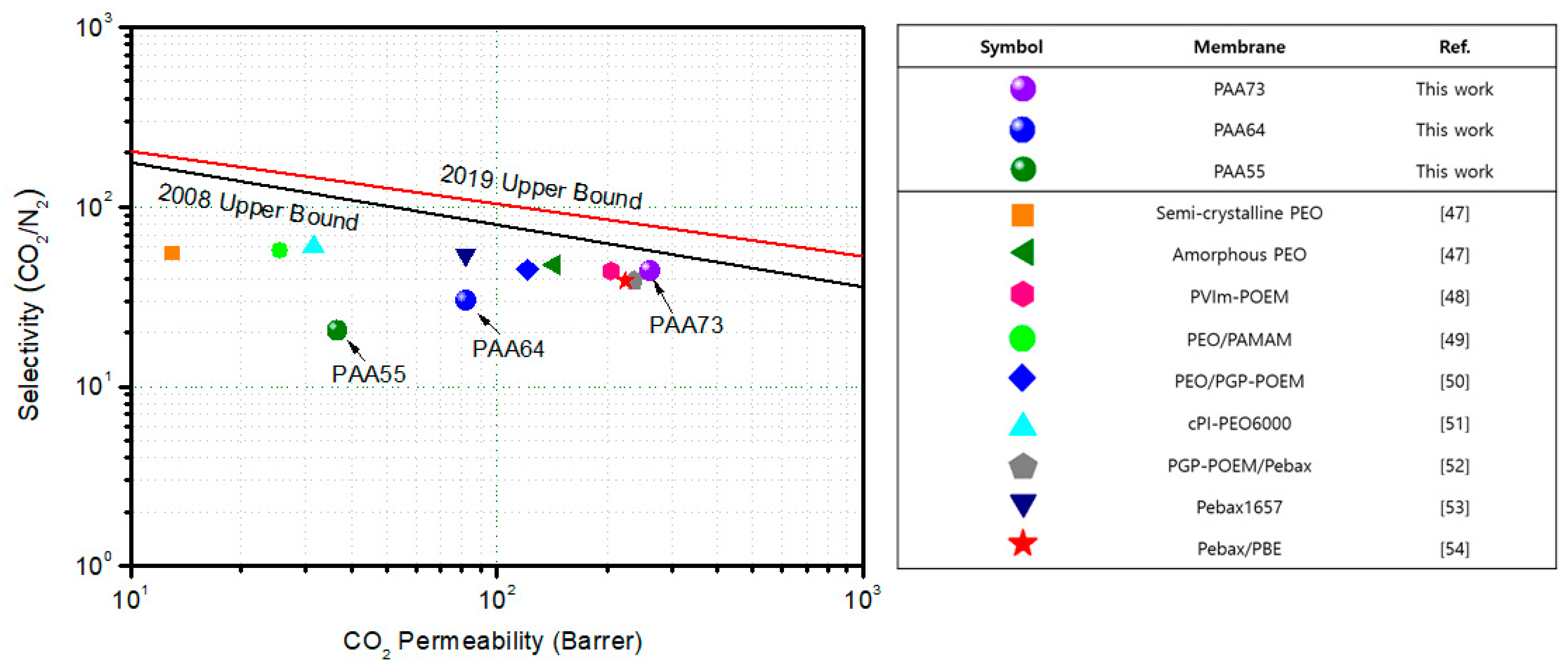
3.3. CO2/N2 Separation Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Willson, D.; Favre, E. Membrane gas separations and post-combustion carbon dioxide capture: Parametric sensitivity and process integration strategies. Chem. Eng. J. 2012, 211–212, 122–132. [Google Scholar] [CrossRef]
- Hammann, M.; Castillo, D.; Anger, C.; Rieger, B. Highly selective CO2 separation membranes through tunable poly(4-vinylphenolate)–CO2 interactions. J. Mater. Chem. A 2014, 2, 16389–16396. [Google Scholar] [CrossRef]
- Hu, T.; Dong, G.; Li, H.; Chen, V. Improved CO2 separation performance with additives of PEG and PEG–PDMS copolymer in poly(2,6-dimethyl-1,4-phenylene oxide)membranes. J. Membr. Sci. 2013, 432, 13–24. [Google Scholar] [CrossRef]
- Japip, S.; Wang, H.; Xiao, Y.; Shung Chung, T. Highly permeable zeolitic imidazolate framework (ZIF)-71 nano-particles enhanced polyimide membranes for gas separation. J. Membr. Sci. 2014, 467, 162–174. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Neumann, S.; Bolmer, S.; Khan, M.M.; Abetz, V. PEBAX® with PEG functionalized POSS as nanocomposite membranes for CO2 separation. J. Membr. Sci. 2013, 437, 286–297. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Lin, H.; Gong, K.; Ying, W.; Chen, D.; Zhang, J.; Yan, Y.; Peng, X. CO2-Philic separation membrane: Deep eutectic solvent filled graphene oxide Nanoslits. Small 2019, 15, 1904145. [Google Scholar] [CrossRef]
- Norahim, N.; Yaisanga, P.; Faungnawakij, K.; Charinpanitkul, T.; Klaysom, C. Recent membrane developments for CO2 separation and capture. Chem. Eng. Technol. 2018, 41, 211–223. [Google Scholar] [CrossRef]
- Torstensen, J.Ø.; Helberg, R.M.L.; Deng, L.; Gregersen, Ø.W.; Syverud, K. PVA/nanocellulose nanocomposite membranes for CO2 separation from flue gas. Int. J. Greenh. 2019, 81, 93–102. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Q.; Li, X.; Yun, M.; Xu, R.; Wang, S.; Li, Y.; Lin, L.; Ding, X.; Ye, H.; et al. Mixed matrix membranes comprising aminosilane-functionalized graphene oxide for enhanced CO2 separation. J. Membr. Sci. 2019, 570–571, 343–354. [Google Scholar] [CrossRef]
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent organic frameworks (COFs) functionalized mixed matrix membrane for effective CO2/N2 separation. J. Membr. Sci. 2019, 572, 588–595. [Google Scholar] [CrossRef]
- Nabais, A.R.; Martins, A.P.S.; Alves, V.D.; Crespo, J.G.; Marrucho, I.M.; Tomé, L.C.; Neves, L.A. Poly(ionic liquid)-based engineered mixed matrix membranes for CO2/H2 separation. Sep. Purif. Technol. 2019, 222, 168–176. [Google Scholar] [CrossRef]
- Xiang, L.; Sheng, L.; Wang, C.; Zhang, L.; Pan, Y.; Li, Y. Amino-Functionalized ZIF-7 nanocrystals: Improved intrinsic separation ability and interfacial compatibility in mixed-matrix membranes for CO2/CH4 separation. Adv. Mater. 2017, 29, 1606999. [Google Scholar] [CrossRef]
- Khan, M.M.; Halder, K.; Shishatskiy, S.; Filiz, V. Synthesis and crosslinking of polyether-based main chain benzoxazine polymers and their gas separation performance. Polymers 2018, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Kang, S.W. CO2 Separation with polymer/aniline composite membranes. Polymers 2020, 12, 1363. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, X.; Yang, P.; Li, L.; Li, X.; Zhang, Y. A novel multi-armed and star-like poly(ethylene oxide) membrane for CO2 separation. J. Membr. Sci. 2015, 489, 258–263. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Gin, D.L.; Noble, R.D.; Deng, L. Facile fabrication of CO2 separation membranes by cross-linking of poly(ethylene glycol) diglycidyl ether with a diamine and a polyamine-based ionic liquid. J. Membr. Sci. 2017, 523, 551–560. [Google Scholar] [CrossRef]
- Boroglu, M.S.; Yumru, A.B. Gas separation performance of 6FDA-DAM-ZIF-11 mixed-matrix membranes for H2/CH4 and CO2/CH4 separation. Sep. Purif. Technol. 2017, 173, 269–279. [Google Scholar] [CrossRef]
- Liu, J.; Hou, X.; Park, H.B.; Lin, H. High-Performance polymers for membrane CO2/N2 separation. Chem.–A Eur. J. 2016, 22, 15980–15990. [Google Scholar] [CrossRef] [PubMed]
- Shamsabadi, A.A.; Isfahani, A.P.; Salestan, S.K.; Rahimpour, A.; Ghalei, B.; Sivaniah, E.; Soroush, M. Pushing rubbery polymer membranes to be economic for CO2 separation: Embedment with Ti3C2Tx MXene nanosheets. ACS Appl. Mater. Interfaces 2020, 12, 3984–3992. [Google Scholar] [CrossRef]
- Ji, P.; Cao, Y.; Jie, X.; Li, M.; Yuan, Q. Impacts of coating condition on composite membrane performance for CO2 separation. Sep. Purif. Technol. 2010, 71, 160–167. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G.; Clark, K.; Lin, H. Maximizing ether oxygen content in polymers for membrane CO2 removal from natural gas. ACS Appl. Mater. Interfaces 2019, 11, 10933–10940. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, Z.; Wang, M.; Qiao, Z.; Wang, J. High nanoparticles loadings mixed matrix membranes via chemical bridging-crosslinking for CO2 separation. J. Membr. Sci. 2019, 573, 455–464. [Google Scholar] [CrossRef]
- Choi, Y.; Kang, S.W. Effect of 4-hydroxybenzoic acid on CO2 separation performance of poly(ethylene oxide) membrane. Macromol. Res. 2016, 24, 1111–1114. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Funari, S.S.; Nunes, S.P.; Peinemann, K.-V. CO2-Philic polymer membrane with extremely high separation performance. Macromolecules 2010, 43, 326–333. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Peng, K.-J.; Liu, Y.-L.; Deng, L. Pebax/PEG grafted CNT hybrid membranes for enhanced CO2/N2 separation. Ind. Eng. Chem. Res. 2019, 58, 12226–12234. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53(Al) in Pebax® MH-1657 for CO2 separation. Sep. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ahmadi, R.; Amooghin, A.E.; Ghanbari, D. A novel ternary mixed matrix membrane containing glycerol-modified poly(ether-block-amide) (Pebax 1657)/copper nanoparticles for CO2 separation. J. Membr. Sci. 2019, 573, 234–246. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Wang, Y.; Qiu, Y.; Li, H.; Hua, K.; Li, X.; Ji, J.; Deng, M. High CO2 separation performance of Pebax®/CNTs/GTA mixed matrix membranes. J. Membr. Sci. 2017, 521, 104–113. [Google Scholar] [CrossRef]
- Bisht, H.S.; Chatterjee, A.K. Living free-radical polymerization—A review. J. Macromol. Sci. Part C 2001, 41, 139–173. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, M.S.; Kim, N.U.; Ryu, D.Y.; Kim, J.H. Multifunctional amine-containing PVA-g-POEM graft copolymer membranes for CO2 capture. Macromolecules 2018, 51, 5646–5655. [Google Scholar] [CrossRef]
- Kim, K.; Kang, D.A.; Park, J.T.; Kim, K.C.; Kim, J.H. Synthesis, structure and gas separation properties of ethanol-soluble, amphiphilic POM-PBHP comb copolymers. Polymer 2019, 180, 121700. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, J.H.; Jung, J.P.; Jung, B.; Kim, J.H. A highly selective PEGBEM-g-POEM comb copolymer membrane for CO2/N2 separation. J. Membr. Sci. 2015, 492, 452–460. [Google Scholar] [CrossRef]
- Wu, L.-G.; Shen, J.-N.; Chen, H.-L.; Gao, C.-J. CO2 facilitated transport through an acrylamide and maleic anhydride copolymer membrane. Desalination 2006, 193, 313–320. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, J.P.; Jang, E.; Lee, K.B.; Kang, Y.S.; Kim, J.H. CO2-philic PBEM-g-POEM comb copolymer membranes: Synthesis, characterization and CO2/N2 separation. J. Membr. Sci. 2016, 502, 191–201. [Google Scholar] [CrossRef]
- Jones, G.R.; Li, Z.; Anastasaki, A.; Lloyd, D.J.; Wilson, P.; Zhang, Q.; Haddleton, D.M. Rapid synthesis of well-defined polyacrylamide by aqueous Cu(0)-mediated reversible-deactivation radical polymerization. Macromolecules 2016, 49, 483–489. [Google Scholar] [CrossRef]
- Shaban, M.; Saadatabadi, A.R.; Ahadian, M.M.; Tamsilian, Y.; Weber, A.P. Facile synthesis of cauliflower-like hydrophobically modified polyacrylamide nanospheres by aerosol-photopolymerization. Eur. Polym. J. 2016, 83, 323–336. [Google Scholar] [CrossRef]
- Koh, J.H.; Lee, K.J.; Seo, J.A.; Kim, J.H. Amphiphilic polymer electrolytes consisting of PVC-g-POEM comb-like copolymer and LiCF3SO3. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1443–1451. [Google Scholar] [CrossRef]
- El-Sharif, H.F.; Yapati, H.; Kalluru, S.; Reddy, S.M. Highly selective BSA imprinted polyacrylamide hydrogels facilitated by a metal-coding MIP approach. Acta Biomater. 2015, 28, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, T.; Yin, H.; Easton, C.D.; Seeber, A.; Hao, X.; Huang, C.; Zeng, R. New strategy of improving the dispersibility of acrylamide-functionalized graphene oxide in aqueous solution by RAFT copolymerization of acrylamide and acrylic acid. Eur. Polym. J. 2019, 117, 148–158. [Google Scholar] [CrossRef]
- Yu, X.; Huang, X.; Bai, C.; Xiong, X. Modification of microcrystalline cellulose with acrylamide under microwave irradiation and its application as flocculant. Environ. Sci. Pollut. Res. Int. 2019, 26, 32859–32865. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Chen, Y.; Zhang, C.; Cheng, J.; Wu, D.; Ma, W.; Liu, C.; Fu, Z. Preparation of near-infrared laser responsive hydrogels with enhanced laser marking performance. Soft Matter 2019, 15, 2950–2959. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, N.U.; Park, J.T.; Kim, J.H. Imidazole-functionalized hydrophilic rubbery comb copolymers: Microphase-separation and good gas separation properties. Sep. Purif. Technol. 2020, 242, 116780. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.Y.; Park, M.S.; Kim, J.H. Improvement in the CO2 permeation properties of high-molecular-weight poly(ethylene oxide): Use of amine-branched poly(amidoamine) dendrimer. Macromolecules 2018, 51, 8800–8807. [Google Scholar] [CrossRef]
- Kim, N.U.; Park, B.J.; Guiver, M.D.; Kim, J.H. Use of non-selective, high-molecular-weight poly(ethylene oxide) membrane for CO2 separation by incorporation of comb copolymer. J. Membr. Sci. 2020, 605, 118092. [Google Scholar] [CrossRef]
- Tena, A.; Marcos-Fernández, A.; Lozano, A.E.; de Abajo, J.; Palacio, L.; Prádanos, P.; Hernández, A. Influence of the PEO length in gas separation properties of segregating aromatic–aliphatic copoly(ether-imide)s. Chem. Eng. Sci. 2013, 104, 574–585. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.U.; Park, B.J.; Park, M.S.; Park, J.T.; Kim, J.H. Semi-interpenetrating polymer network membranes based on a self-crosslinkable comb copolymer for CO2 capture. Chem. Eng. J. 2019, 360, 1468–1476. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z.; Wu, Y.; Zhang, H.; Cheng, Y.; Guo, R.; Wu, H. High-performance composite membranes incorporated with carboxylic acid nanogels for CO2 separation. J. Membr. Sci. 2015, 495, 72–80. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, C.H.; Jung, J.P.; Kim, J.-H.; Kim, J.H. Dual-phase all-polymeric membranes with graft copolymer filler for CO2 capture. Chem. Eng. J. 2018, 334, 939–947. [Google Scholar] [CrossRef]


| Sample | Feed Ratio | Determined by 1H NMR | ||
|---|---|---|---|---|
| POEM (wt%) | AcAm (wt%) | POEM (wt%) | AcAm (wt%) | |
| PAA73 | 70 | 30 | 71.7 | 28.3 |
| PAA64 | 60 | 40 | 61.1 | 38.9 |
| PAA55 | 50 | 50 | 50.8 | 49.2 |
| # | Polymer | CO2 (Barrer) | N2 (Barrer) | Selectivity (CO2/N2) | Reference |
|---|---|---|---|---|---|
| 1 | PAA73 | 261.7 | 5.9 | 44.0 | This work |
| 2 | PAA64 | 82.2 | 2.7 | 30.0 | This work |
| 3 | PAA55 | 36.7 | 1.8 | 20.5 | This work |
| 4 | Semi-Crystalline PEO | 13 | 0.24 | 55 | [47] |
| 5 | Amorphous PEO a | 143 | 3.0 | 47.7 | [47] |
| 6 | PVIm-POEM | 204.5 | 4.7 | 43.9 | [48] |
| 7 | PEO/PAMAM | 25.5 | 0.45 | 56.7 | [49] |
| 8 | PEO/PGP-POEM | 120.9 | 2.69 | 44.9 | [50] |
| 9 | cPI-PEO6000 | 31.5 | 0.53 | 59.9 | [51] |
| 10 | PGP-POEM/Pebax | 236.6 | 6.1 | 38.8 | [52] |
| 11 | Pebax1657 | 82 | 1.5 | 54 | [53] |
| 12 | Pebax/PBE | 224.4 | 5.79 | 38.7 | [54] |
| # | Polymer | CO2 Diffusivity (×10−7) | CO2 Solubility (×10−2) | CO2 Permeability * (Barrer) |
|---|---|---|---|---|
| 1 | PAA73 | 9.25 | 2.83 | 261.7 |
| 2 | PAA64 | 3.73 | 2.20 | 82.2 |
| 3 | PAA55 | 1.88 | 1.95 | 36.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.J.; Kim, N.U.; Lee, C.S.; Kim, J.H. Synthesis, Characterization, and CO2/N2 Separation Performance of POEM-g-PAcAm Comb Copolymer Membranes. Polymers 2021, 13, 177. https://doi.org/10.3390/polym13020177
Park BJ, Kim NU, Lee CS, Kim JH. Synthesis, Characterization, and CO2/N2 Separation Performance of POEM-g-PAcAm Comb Copolymer Membranes. Polymers. 2021; 13(2):177. https://doi.org/10.3390/polym13020177
Chicago/Turabian StylePark, Byeong Ju, Na Un Kim, Chang Soo Lee, and Jong Hak Kim. 2021. "Synthesis, Characterization, and CO2/N2 Separation Performance of POEM-g-PAcAm Comb Copolymer Membranes" Polymers 13, no. 2: 177. https://doi.org/10.3390/polym13020177





