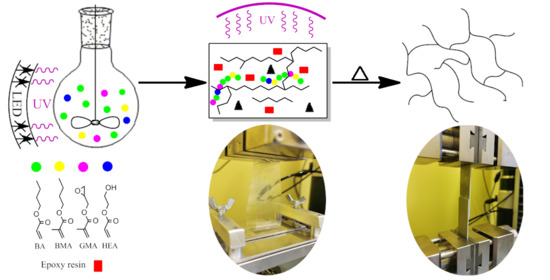
Epoxy-Based Structural Self-Adhesive Tapes Modified with Acrylic Syrups Prepared via a Free Radical Photopolymerization Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Acrylic Syrups
2.3. Characterization of Acrylic Syrups
2.4. Preparation of Structural Self-Adhesive Tapes (SATs)
2.5. Characterization of Thermally Uncured Structural Self-Adhesive Tapes (SATs)
2.6. Preparation and Characterization of Aluminum Joints with Thermally Cured SATs
3. Results
3.1. Properties of the Acrylic Syrups
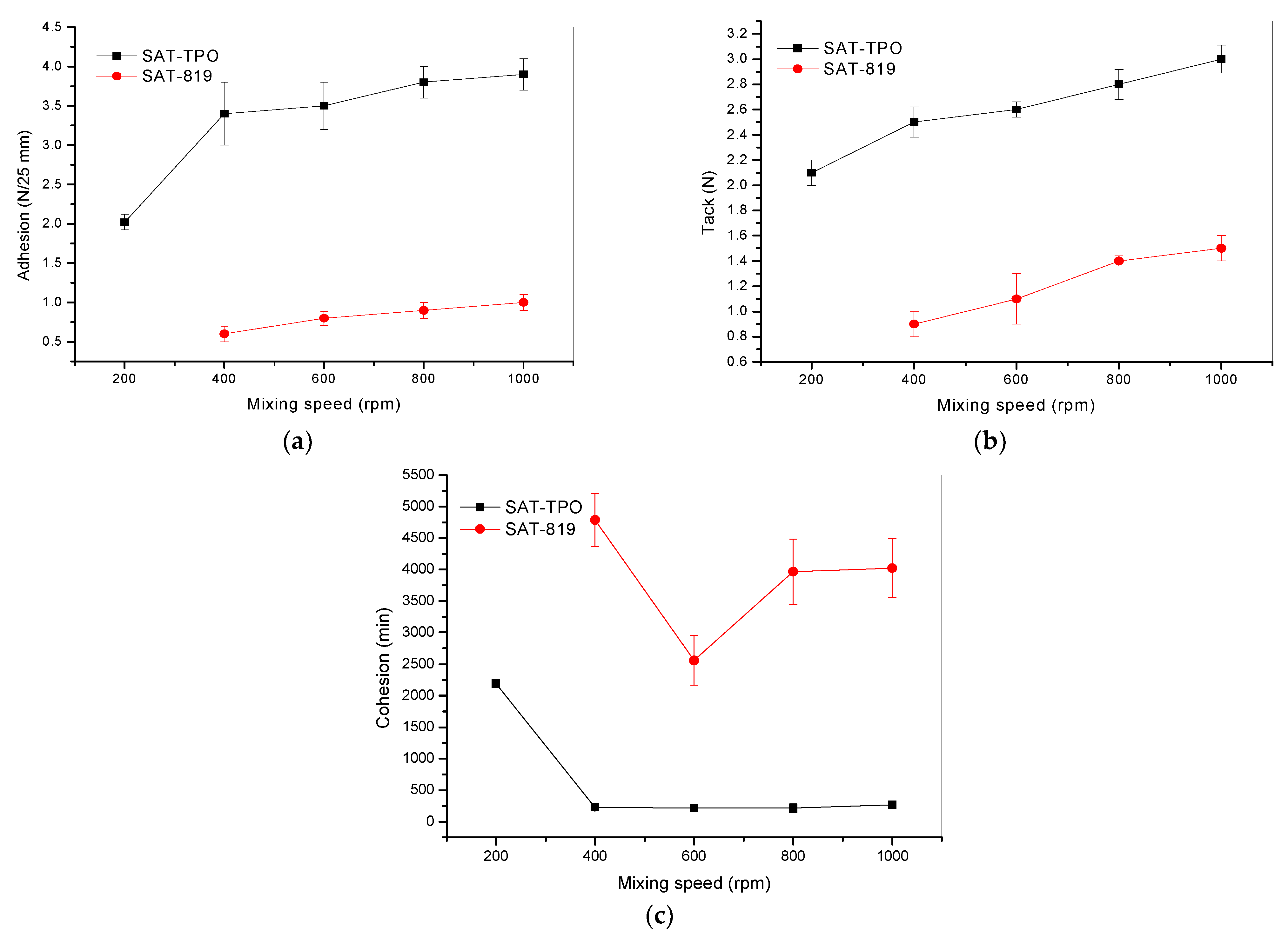
3.2. Properties of the Thermally Uncured SATs
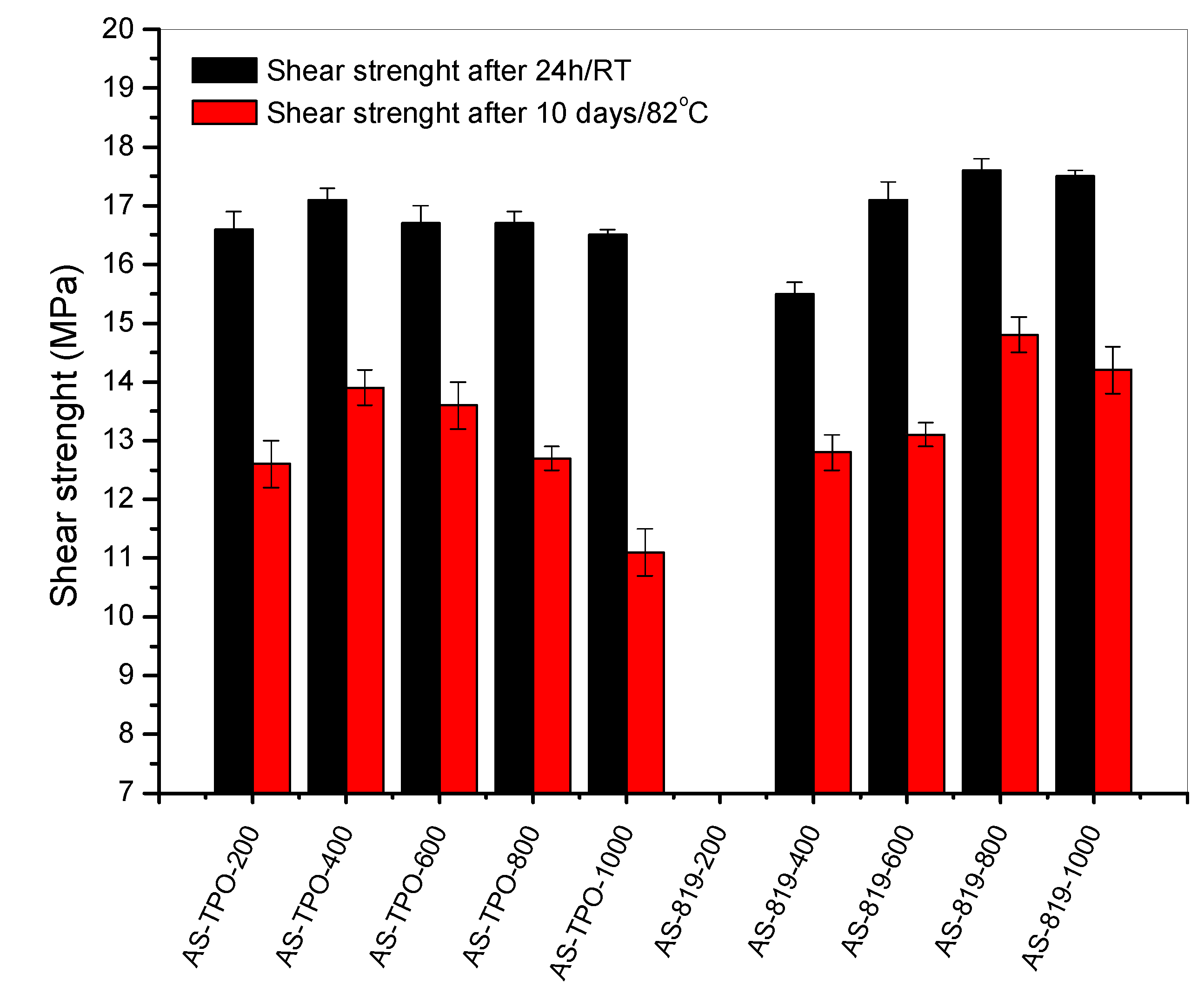
3.3. Properties of the Thermally Cured SATs and the Al/SAT/Al Joints
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudawska, A. Epoxy adhesives. In Handbook of Adhesive Technology; Pizzi, A., Mittal, K.Z., Eds.; CRS Press Taylor & Frances Group: Boca Raton, FL, USA, 2018; pp. 415–442. [Google Scholar]
- May, C.A.; Tanka, G.Y. Epoxy Resin Chemistry and Technology; Marcel Dekker: New York, NY, USA, 1973; pp. 9–20. [Google Scholar]
- Adams, R.D. Epoxy Bonding: Science, Technology and Applications; Woodhead Publishing: London, UK, 2010; pp. 221–222. [Google Scholar]
- Guangfeng, W.U.; Ji, W.; Sun, W.; Zhang, H. Effect of epoxy-functionalised core-shell particles on properties of Poly(butylenes terephthalate) (PBT). Polym. Compos. 2008, 16, 271–276. [Google Scholar]
- Zhang, F.; Sun, S.; Liu, X.; Zhang, L.; Zhang, H. Toughening of PBT/PC Blends with epoxy functionalized core-shell modifiers. E Polym. 2009, 9, 77. [Google Scholar]
- Tynys, A.; Hippi, U.; Seppala, J. Toughening liquid-crystalline polymers with polyethylenes containing epoxy functionality. J. Appl. Polym. Sci. 2002, 86, 1886–1891. [Google Scholar] [CrossRef]
- Johnsen, B.B.; Kinloch, A.J.; Mohammed, R.D.; Taylor, A.C.; Sprenger, S. Toughening mechanisms of nanoparticle-modified epoxy polymers. Polymer 2007, 48, 530–541. [Google Scholar]
- Jia, Q.M.; Zheng, M.S.; Chen, H.X.; Shen, R.J. Morphologies and properties of polyurethane/epoxy resin interpenetrating network nanocomposites modified with organoclay. Mater. Lett. 2006, 60, 1306–13099. [Google Scholar]
- Sprenger, S. Epoxy resins modified with elastomers and surface-modified silica nanoparticles. Polymers 2013, 54, 4790–4797. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Yamaguchi, A.; Iko, K.; Okubo, M.; Matsumoto, T. Internal stress of epoxy resin modified with acrylic polymers containing functional groups produced by in situ u.v. radiation polymerization. Polymers 1990, 31, 2066–2070. [Google Scholar]
- Wang, T.; Wang, J.; Chen, W. Acrylate copolymers as impact modifier for epoxy resin. J. Wuhan Univ. Technol. Mat. Sci. Ed. 2015, 30, 1210–1214. [Google Scholar] [CrossRef]
- Kamar, N.T.; Drzal, L.T.; Lee, A.; Askeland, P. Nanoscale toughening of carbon fiber reinforced/epoxy polymer composites (CFRPs) using a triblock copolymer. Polymer 2017, 111, 36–47. [Google Scholar]
- Bahrami, A.; Cordenier, F.; Van Velthem, P.; Ballout, W.; Pardoen, T.; Nysten, B.; Bailly, C. Synergistic local toughening of high performance epoxy-matrix composites using blended block copolymer-thermoplastic thin films. Compos. Appl. Sci. Manuf. 2016, 91, 1. [Google Scholar] [CrossRef]
- Chong, H.; Taylor, A. The microstructure and fracture performance of styrene–butadiene–methylmethacrylate block copolymer-modified epoxy polymers. J. Mater. Sci. 2013, 48, 6762–6777. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H. Design of highly adhesive and water-resistant UV/heat dual-curable epoxy–acrylate composite for narrow bezel display based on reactive organic–inorganic hybrid nanoparticles. Polymers 2020, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Kowalczyk, K.; Czech, Z. Synthesis and properties of solid structural adhesives modified in-situ using 1D and 2D-type microfillers. Int. J. Adhes. Adhes. 2012, 32, 76–81. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kowalczyk, K.; Weisbrodt, M. Influence of phosphorus-based methacrylate monomer on features of thermally curable self-adhesive structural tapes. Int. J. Adhes. Adhes. 2018, 85, 286–292. [Google Scholar]
- Kowalczyk, A.; Kowalczyk, K.; Gziut, K.; Nowakowski, D.; Sałaciński, M. Influence of a wollastonite microfiller and a halloysite nanofiller on properites of thermally curable pressure-sensitive structural adhesives. Int. J. Adhes. Adhes. 2019, 95, 102397. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kowalczyk, K.; Gziut, K. Synthesis of monoacryloxypropyl-POSS-based hybrid epoxyacrylate copolymers and their application in thermally curable structural self-adhesive tapes. Polymers 2019, 11, 2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gziut, K.; Kowalczyk, A.; Schmidt, B. Free-radical bulk-photopolymerization process as a method of obtaining thermally curable structural self-adhesive tapes and effect of used type I photoinitiators. Polymers 2020, 12, 2191. [Google Scholar]
- Higgins, A. Adhesive bonding of aircraft structures. Int. J. Adhes. Adhes. 2000, 20, 367–376. [Google Scholar]
- Weglewski, J.; Pastirik, D. Stuctural Bonding Tapes and Articles Containing the Same. Patent Application WO 079337, 10 November 2002. [Google Scholar]
- Bernardus, J.; Sikkel, F.; Brandys, A.; Chen, P. Adhesive Tape for Structural Bonding. Patent Application WO 2005073330, 11 August 2005. [Google Scholar]
- Sohaib, E.; Cura, E. Structural Adhesive Film. Patent Application WO 2005073330, 23 August 2012. [Google Scholar]
- Cura, E.; Elgimiabi, S.; Luebbe, S.; Shinozaki, K.; Ueda, S. Multilayer Structural Adhesive Film. Patent Application US 10632707, 28 April 2020. [Google Scholar]
- Jang, S.; Baek, S.; Kim, J.; Hwang, S. Preparation and adhesion performance of transparent pressure-sensitive adhesives for touch screen panel. J. Adhes. Sci. Technol. 2014, 28, 1990–2000. [Google Scholar]
- Beak, S.; Hwang, S. Eco-friendly UV-curable pressure sensitive adhesives containing acryloyl derivatives of monosaccharides and their adhesive performances. Int. J. Adhes. Adhes. 2016, 70, 110–113. [Google Scholar]
- Beak, S.; Hwang, S. Preparation and adhesion performance of transparent acrylic pressure-sensitive adhesives containing menthyl acrylate. Polym. Bull. 2016, 73, 687–701. [Google Scholar] [CrossRef]
- Baek, S.; Jang, S.; Hwang, S. Preparation and adhesion performance of transparent acrylic pressure sensitive adhesives: Effects of substituent structure of acrylate monomer. Int. J. Adhes. Adhes. 2016, 64, 72–77. [Google Scholar] [CrossRef]
- Baek, S.; Jang, S.; Hwang, S. Sustainable isosorbide-based transparent pressure-sensitive adhesives for optically clear adhesive and their adhesion performance. Polym. Int. 2017, 66, 1834–1840. [Google Scholar] [CrossRef]
- Baek, S.; Jang, S.; Hwang, S. Construction and adhesion performance of biomass tetrahydro-geraniol-based sustainable/transparent pressure sensitive adhesives. J. Ind. Eng. Chem. 2017, 53, 429–434. [Google Scholar] [CrossRef]
- Kim, J.; Shim, G.; Baek, D.; Back, J.; Jang, S.; Kim, H.; Choi, J.; Yeom, J. UV/UV step-curing of optically clear acrylate adhesives for mobile devices. Express Polym. Lett. 2019, 13, 794–805. [Google Scholar] [CrossRef]
- Fouassier, J.; Lalevée, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency; Wiley-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Boodhoo, K.; Dunk, W.; Jassim, M.; Jachuck, R. Thin film solvent-free photopolymerization of n-butyl acrylate. I. static film studies. J. Appl. Polym. Sci. 2004, 91, 2079–2095. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassiera, J.; Lalevée, J. Photopolymerization processes of thick films and in shadow areas: A review for the access to composites. Polym. Chem. 2017, 8, 7088–7101. [Google Scholar] [CrossRef]
- Ming, L.; Schlüter, D.; Sakamoto, J. Solid-state photopolymerization of a shape-persistent macrocycle with two 1,8-Diazaanthracene units in a single crystal. J. Am. Chem. Soc. 2012, 134, 11721–11725. [Google Scholar]
- Eckhardt, H.; Prusik, T.; Chance, R. Solid-state photopolymerization of diacetylenes. In Polydiacetylenes; Bloor, D., Chance, R.R., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 25–39. [Google Scholar]
- Wang, J.; Jian, Y.; Nie, J.; He, Y. Solid photopolymerization and polymer properties of octadecyl vinyl ether. J. Photochem. Photobiol. A Chem. 2013, 271, 105–110. [Google Scholar] [CrossRef]
- Yemin, L.; Hill, F.B. Measurement of effects of mixing of chain centers in a nonuniformly initiated photopolymerization. Ind. Eng. Chem. Fundam. 1969, 8, 210–215. [Google Scholar] [CrossRef]
- Mendiratta, S.K.; Felder, R.M.; Hill, F.B. Benzoin- and benzoin methyl ether-sensitized photopolymerization of styrene and methyl methacrylate: Quantum yields and mixing effects. AIChE J. 1975, 21, 1115–1123. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Xue, X. Morphology and properties of UV-curing epoxy acrylate coatings modified with methacryl-POSS. Prog. Org. Coat. 2015, 78, 404–410. [Google Scholar] [CrossRef]
- Glöckner, P. Radiation Curing. Coatings and Proniting Inks; Vincentz Network: Hannover, Germany, 2008. [Google Scholar]




| AS Symbol | PI (0.01 mol%) | Mixing Speed (rpm) | Monomers (mol%) | |||
|---|---|---|---|---|---|---|
| BA | BMA | GMA | HEA | |||
| AS-TPO-200 | Omnirad TPO | 200 | 60.8 | 19.6 | 9.8 | 9.8 |
| AS-819-200 | Omnirad 819 | |||||
| AS-TPO-400 | Omnirad TPO | 400 | ||||
| AS-819-400 | Omnirad 819 | |||||
| AS-TPO-600 | Omnirad TPO | 600 | ||||
| AS-819-600 | Omnirad 819 | 60.8 | 19.6 | 9.8 | 9.8 | |
| AS-TPO-800 | Omnirad TPO | 800 | ||||
| AS-819-800 | Omnirad 819 | |||||
| AS-TPO-1000 | Omnirad TPO | 1000 | ||||
| AS-819-1000 | Omnirad 819 | |||||
| AS Symbol | Tmax (°C) | η (Pa∙s) | SC (wt%) | Mn (g/mol) | Mw (g/mol) | PDI |
|---|---|---|---|---|---|---|
| AS-TPO-200 | 40 | 0.2 | 24 | 19,840 | 48,835 | 2.46 |
| AS-TPO-400 | 38 | 0.1 | 21 | 17,955 | 37,570 | 2.09 |
| AS-TPO-600 | 26 | <0.1 | 10 | 12,530 | 23,020 | 1.84 |
| AS-TPO-800 | 30 | <0.1 | 12 | 13,580 | 26,545 | 1.95 |
| AS-TPO-1000 | 31 | <0.1 | 12 | 14,120 | 26,760 | 1.89 |
| AS-819-200 | 100 | gel | 68 | 43,515 | 359,085 | 8.25 |
| AS-819-400 | 51 | 13 | 49 | 28,670 | 103,285 | 3.60 |
| AS-819-600 | 38 | 0.4 | 31 | 22,380 | 54,985 | 2.46 |
| AS-819-800 | 42 | 1.2 | 37 | 24,865 | 63,370 | 2.55 |
| AS-819-1000 | 39 | 0.7 | 34 | 22,700 | 55,090 | 2.43 |
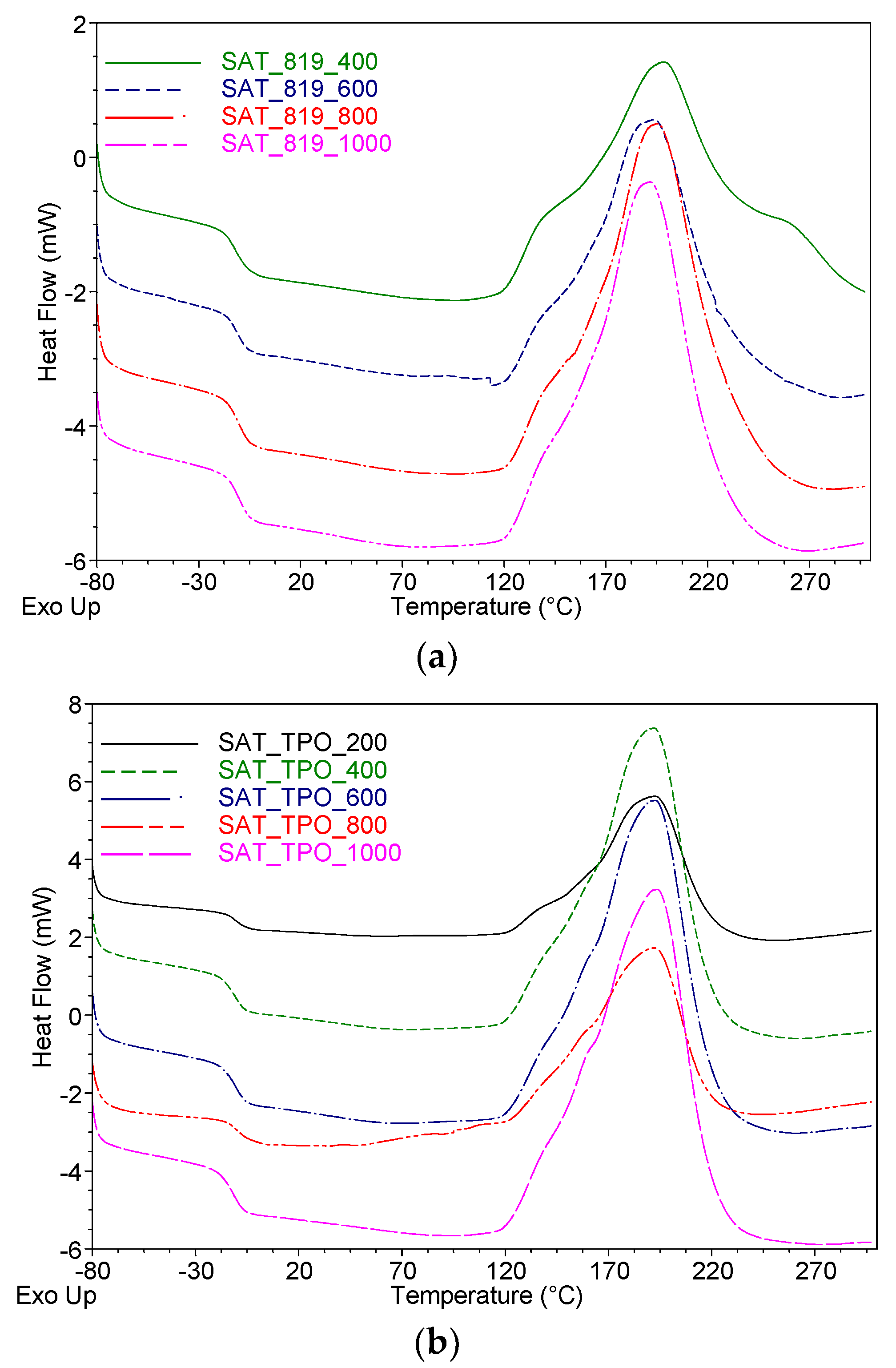
| SAT Symbol | Tg (°C) | Ti (°C) | Tp (°C) | ΔH (J/g) | α (a.u.) | Tg * (°C) |
|---|---|---|---|---|---|---|
| SAT-TPO-200 | −11 | 114 | 193 | 211 | 0.78 | 46 |
| SAT-TPO-400 | −11 | 113 | 193 | 232 | 0.82 | 47 |
| SAT-TPO-600 | −11 | 114 | 192 | 231 | 0.90 | 51 |
| SAT-TPO-800 | −10 | 110 | 191 | 232 | 0.90 | 51 |
| SAT-TPO-1000 | −12 | 111 | 193 | 235 | 0.90 | 51 |
| SAT-819-200 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| SAT-819-400 | −12 | 107 | 198 | 217 | 0.75 | 15 |
| SAT-819-600 | −12 | 116 | 196 | 224 | 0.78 | 29 |
| SAT-819-800 | −11 | 121 | 194 | 230 | 0.78 | 27 |
| SAT-819-1000 | −11 | 121 | 192 | 238 | 0.84 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gziut, K.; Kowalczyk, A.; Schmidt, B.; Kowalczyk, K.; Weisbrodt, M. Epoxy-Based Structural Self-Adhesive Tapes Modified with Acrylic Syrups Prepared via a Free Radical Photopolymerization Process. Polymers 2021, 13, 189. https://doi.org/10.3390/polym13020189
Gziut K, Kowalczyk A, Schmidt B, Kowalczyk K, Weisbrodt M. Epoxy-Based Structural Self-Adhesive Tapes Modified with Acrylic Syrups Prepared via a Free Radical Photopolymerization Process. Polymers. 2021; 13(2):189. https://doi.org/10.3390/polym13020189
Chicago/Turabian StyleGziut, Konrad, Agnieszka Kowalczyk, Beata Schmidt, Krzysztof Kowalczyk, and Mateusz Weisbrodt. 2021. "Epoxy-Based Structural Self-Adhesive Tapes Modified with Acrylic Syrups Prepared via a Free Radical Photopolymerization Process" Polymers 13, no. 2: 189. https://doi.org/10.3390/polym13020189
APA StyleGziut, K., Kowalczyk, A., Schmidt, B., Kowalczyk, K., & Weisbrodt, M. (2021). Epoxy-Based Structural Self-Adhesive Tapes Modified with Acrylic Syrups Prepared via a Free Radical Photopolymerization Process. Polymers, 13(2), 189. https://doi.org/10.3390/polym13020189