Intrinsic Self-Healing Epoxies in Polymer Matrix Composites (PMCs) for Aerospace Applications
Abstract
:1. Introduction
2. Healing Efficiency
3. Intrinsic Self-Healing Mechanisms
3.1. Physical Interactions
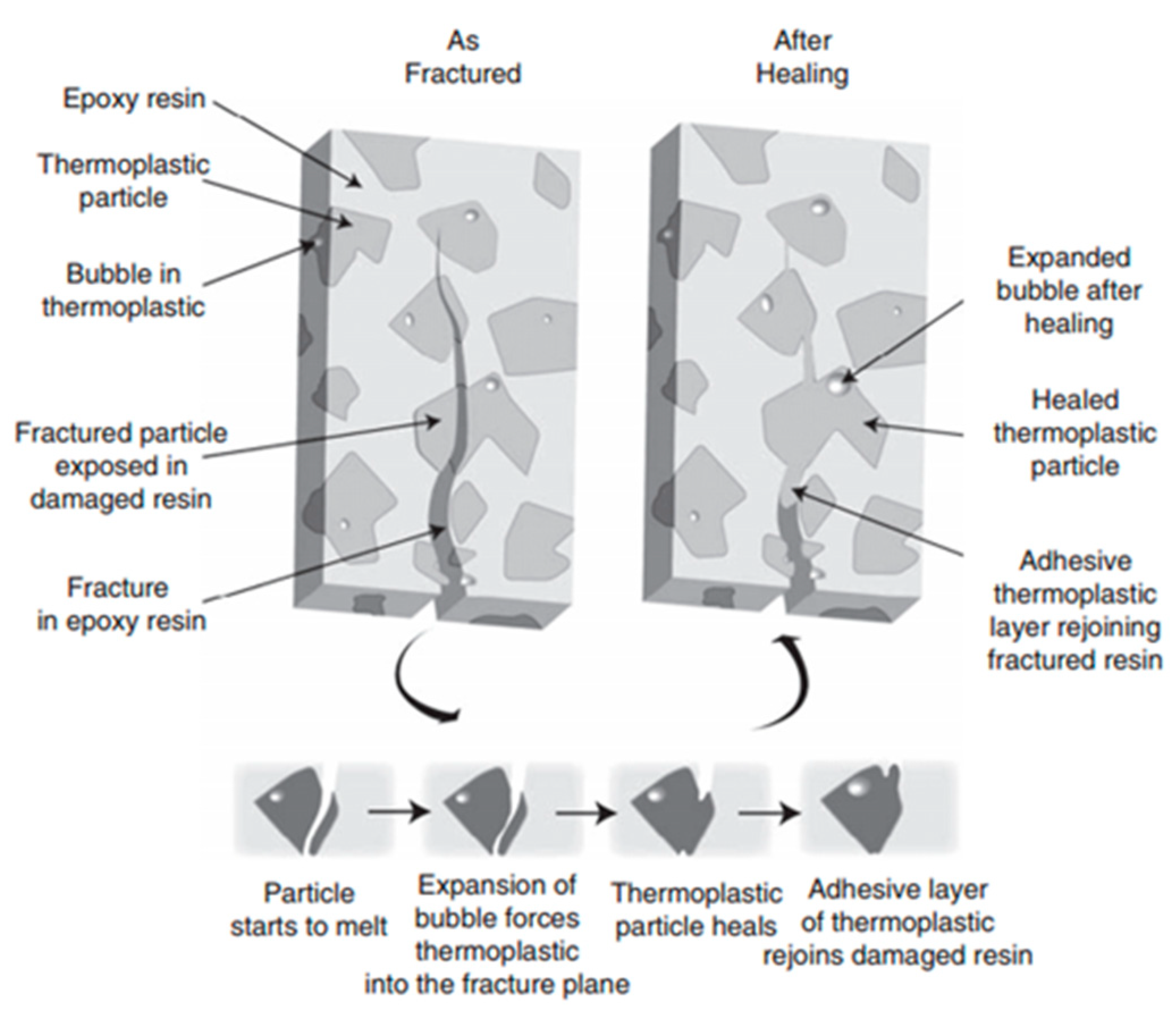
3.1.1. Miscible Polymer Blends
3.1.2. Immiscible Polymer Blends
3.2. Supramolecular Polymers
3.3. Chemical Interactions
3.3.1. DA/rDA Chemistry
3.3.2. Alkoxyamine and Imine Exchange
3.3.3. Dynamic Sulfur Chemistry
3.3.4. Aromatic Disulfide Exchange
3.3.5. Transesterification
3.3.6. Vitrimers
3.3.7. Bio-Vitrimers
4. Discussion and Application Perspective
5. Conclusions
- When dealing with some of the aforementioned healing mechanisms, fabrication of those polymers is rather complicated and costly. Tailor-made synthesis routes are unfavorable for the industry and for an upscaling of the fabrication process [135]. Thus, it is critical to implement these chemistries into well-established procedures adopted in the industry.
- The healing processes described in this review are not fully autonomous, meaning that they requires an external energy source to trigger the healing mechanisms. In most cases, the trigger for the drop in viscosity is driven by an increase in temperature, which may not be seen as an issue in the academic domain, as testing is done on small samples of material. Nevertheless, it is foreseen to be quite cumbersome and expensive for large aerospace structures, however local heating strategies can be adopted to overcame this issue. A more thoughtful means of the temperature trigger could be envisaged with devices that supply heat, e.g., via IR, microwaves or ultrasounds, in a localized fashion. These methods could speed up even more the repair process, yielding even shorter maintenance times of aircrafts and spacecraft. Autonomous repair in intrinsic self-healing could be theoretically achievable by controlling the healing rate through combining energy delivery control and sensing [26].
- Intrinsic self-healing is typically restricted to a small damage zone. Even if these materials can heal microcracks before any crack growth leading to catastrophic failure, thus de facto increasing the fatigue life of components. However, following high-energy impact, the material cannot heal when large damages are produced. When it does, it is generally because its size is relatively large (on the order of cm2). In the aerospace scenario, this may represent a major setback, since self-healing polymer matrices have always been associated with the idea of counteracting the deficiency of composites regarding impact resistance. This issue can be overcome by including into self-healing polymers a shape memory capability so that the shape memory will bring the fractured surfaces in contact and the intrinsic healing mechanisms will occur [136]. This so-called close-then-heal (CTH) strategy could also be an incredible help in bringing in contact fracture surfaces of load-carrying real-world structures [112].
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-ME | 2-mercaptoethanol |
| 2MF | 2-methyl furan |
| 4AFD | 4-aminophenyl disulfide |
| 4SH | Pentaerythritol tetrakis(3-mercaptopropionate) |
| APTS | (3-Aminopropyl)trimethoxysilane |
| BDMA | benzyl dimethyl amine |
| BDS | Bis [3-(triethoxysilyl)propyl]disulfide |
| BDSER | bis-disulfide bond dynamic epoxy resin |
| B-GNP | bismaleimide-grafted graphite nanoplatelet |
| BMI | bismaleimide |
| CAI | compression after impact |
| CAN | covalent adaptable network |
| CBMI | chain-extended bismaleimide |
| CNF | carbon nanofiber |
| CT | compact tension |
| D230 | polyether amine (commercial name) |
| DDS | 4,4′-diaminodiphenylsulfone |
| DENT | double edge notched tension |
| DETA | diethylenetriamine |
| DGEBA | diglycidyl ether bisphenol A |
| DGEBF | diglycidyl ether bisphenol F |
| diEP | alkoxyamine-containing epoxy |
| DMA | dynamic mechanical analysis |
| DMAP | 4-dimethylaminopyridine |
| DMF | dimethylformamide |
| DMTA | dynamic mechanical thermal analysis |
| DTDA | 4,4′-dithiodianiline (4AFD synonym) |
| E | Young’s modulus |
| Ea | activation energy |
| ECH | epichlorohydrin |
| EMA | poly(ethylene-co-methyl acrylate) |
| EMAA | poly(ethylene-co-methacrylic acid) |
| ENF | end-notched flexure |
| EN-VAN-AP | epoxy network w/vanillin and aminophenol |
| EpF | furan functionalized epoxy |
| EPS | epoxidized polysulfides (commercial name) |
| ER | epoxy resin |
| ESO | epoxy soybean oil |
| EVA | ethylene vinyl acetate |
| FA | furfurylamine |
| FDB | 2,20-(Methylenebis(4,1-phenylene))bis(4-((oxiran-2-ylmethoxy)methyl)-3a,4,7,7a-tetrahydro-1H-4,7-epoxyisoin-dole-1,3(2H)-dione) |
| FGE | furan-epoxy monomer |
| FPA | fumaropimaric acid |
| FRP | fiber-reinforced polymer |
| FTIR | Fourier transform infrared spectroscopy |
| GIC | fracture energy (mode I) |
| GNS | graphite nanosheet |
| GR | graphene |
| HNO3 | nitric acid |
| IPD | isophorone diamine |
| IR | infrared |
| IS | isosorbide |
| KIC | fracture toughness (Mode I) |
| MHHPA | 4-methylcyclohexane-1,2-dicarboxylic anhydride |
| MWCNTs | multiwalled carbon nanotubes |
| NMA | nadic methylanhydride |
| NMP | N-Methyl-2-pyrrolidone |
| OGE | octanediol glycidyl ether |
| OMSA | organically modified silicone alkoxides |
| P | load |
| PACM | 4,4′-methylene biscyclohexanamine |
| PC | load at failure/fracture |
| PCL | poly(ε-caprolactone) |
| PEGMA | poly (ethylene-co-glycidyl)-methacrylate |
| PETMP | pentaerythritoltetra(3-mercaptopropionate) |
| PMC | polymer matrix composite |
| PPy | polypyrrole |
| RT | room temperature |
| SEM | scanning electron microscopy/microscope |
| SENB | single-edge notched bending |
| SENT | single-edge notched tension |
| TBD | Triazabicyclodecene |
| TDCB | tapered double cantilever beam |
| TEA | triethylamine |
| TEP | triepoxy (vanillin + guaiacol + triphenol) |
| TEPA | tetraethylenepentamine |
| TETA | triethyltetramine |
| TF | tetra-furan monomer |
| Tg | glass transition temperature |
| TGDDM | tetraglycidyl diamino diphenyl methane |
| TPB | three-point bending |
| U | strain energy |
| US | ultrasounds |
| VARIM | vacuum assisted resin infusion molding |
| WOF | work-of-fracture |
| Zn(acac)2 | Zinc acetylacetonate hydrate |
| Zn(OAc)2 | zinc acetate |
| Δl | displacement |
| η | healing efficiency |
| σX | strength (T = tensile, C = compressive, I = impact, F = flexural) |
References
- Bond, I.P.; Trask, R.S.; Williams, H.R. Self-healing fiber- reinforced polymer composites. MRS Bull. 2008, 8, 770–774. [Google Scholar] [CrossRef]
- Soutis, C. Fibre reinforced composites in aircraft construction. Prog. Aerosp. Sci. 2005, 41, 143–151. [Google Scholar] [CrossRef]
- Mauldin, T.C.; Kessler, M.R. Self-healing polymers and composites. Int. Mater. Rev. 2010, 55, 317–346. [Google Scholar] [CrossRef]
- Van der Zwaag, S. An introduction to material design principles: Damage prevention versus damage management. In Self Healing Materials: An Alternative Approach to 20 Centuries of Materials Science; van der Zwaag, S., Ed.; Springer: Amsterdam, The Netherlands, 2007; pp. 1–18. [Google Scholar]
- Garcia, S.J. Effect of polymer architecture on the intrinsic self-healing character of polymers. Eur. Polym. J. 2014, 53, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Garcia, S.J.; Fischer, H.R. Self-healing polymer systems: Properties, synthesis and applications. In Smart Polymers and Their Applications; Aguilar, M.R., San Román, J., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2014; ISBN 9780857096951. [Google Scholar]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.W.C.; Bond, I.P. A hollow fibre reinforced polymer composite encompassing self-healing and enhanced damage visibility. Compos. Sci. Technol. 2005, 65, 1791–1799. [Google Scholar] [CrossRef]
- Binder, W.H. Self-Healing Polymers: From Principles to Applications; Wiley-VCH: Weinheim, Germany, 2013; ISBN 9783527670185. [Google Scholar]
- Fiore, V.; Valenza, A. Epoxy Resins as a Matrix Material in Advanced Fiber-Reinforced Polymer (FRP) Composites; Woodhead Publishing: Cambridge, UK, 2013; pp. 88–121. ISBN 9780857094186. [Google Scholar]
- Trask, R.S.; Williams, H.R.; Bond, I.P. Self-healing polymer composites: Mimicking nature to enhance performance. Bioinspir. Biomim. 2007, 2, 1–9. [Google Scholar] [CrossRef]
- Kessler, M.R. Self-healing: A new paradigm in materials design. Proc. Inst. Mech. Eng. Part. G J. Aerosp. Eng. 2007, 221, 479–495. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.Y.; Meure, S.; Solomon, D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. [Google Scholar] [CrossRef]
- Wool, R.P. Self-healing materials: A review. Soft Matter 2008, 4, 400–418. [Google Scholar] [CrossRef]
- Bergman, S.D.; Wudl, F. Mendable polymers. J. Mater. Chem. 2008, 18, 41–62. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Yin, T.; Rong, M.Z.; Zhang, M.Q. Self-healing in polymers and polymer composites. Concepts, realization and outlook: A review. Express Polym. Lett. 2008, 2, 238–250. [Google Scholar] [CrossRef]
- Van Der Zwaag, S.; Van Dijk, N.H.; Jonkers, H.M.; Mookhoek, S.D.; Sloof, W.G. Self-healing behaviour in Man-made engineering materials: Bioinspired but taking into account their intrinsic character. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1689–1704. [Google Scholar] [CrossRef]
- White, S.R.; Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R. Self-healing polymers and composites. Am. Sci. 2011, 99, 392–399. [Google Scholar] [CrossRef]
- García, S.J.; Fischer, H.R.; Van Der Zwaag, S. A critical appraisal of the potential of self healing polymeric coatings. Prog. Org. Coat. 2011, 72, 211–221. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chuo, T.W. Self-healing polymers based on thermally reversible Diels-Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Billiet, S.; Hillewaere, X.K.D.; Teixeira, R.F.A.; Du Prez, F.E. Chemistry of crosslinking processes for self-healing polymers. Macromol. Rapid Commun. 2013, 34, 290–309. [Google Scholar] [CrossRef]
- Herbst, F.; Döhler, D.; Michael, P.; Binder, W.H. Self-healing polymers via supramolecular forces. Macromol. Rapid Commun. 2013, 34, 203–220. [Google Scholar] [CrossRef]
- Van Der Zwaag, S.; Grande, A.M.; Post, W.; Garcia, S.J.; Bor, T.C. Review of current strategies to induce self-healing behaviour in fibre reinforced polymer based composites. Mater. Sci. Technol. 2014, 30, 1633–1641. [Google Scholar] [CrossRef]
- Zhong, N.; Post, W. Self-repair of structural and functional composites with intrinsically self-healing polymer matrices: A review. Compos. Part. A Appl. Sci. Manuf. 2015, 69, 226–239. [Google Scholar] [CrossRef] [Green Version]
- Diesendruck, C.E.; Sottos, N.R.; Moore, J.S.; White, S.R. Biomimetic Self-Healing. Angew. Chem. Int. Ed. 2015, 54, 10428–10447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pham, D.T.; Ji, C. Self-healing composites: A review. Cogent Eng. 2015, 2, 1075686. [Google Scholar] [CrossRef] [Green Version]
- An, S.Y.; Arunbabu, D.; Noh, S.M.; Song, Y.K.; Oh, J.K. Recent strategies to develop self-healable crosslinked polymeric networks. Chem. Commun. 2015, 51, 13058–13070. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Bruchmann, B.; Lehn, J.M. DYNAMERS: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ding, X.; Urban, M.W. Chemical and physical aspects of self-healing materials. Prog. Polym. Sci. 2015, 49, 34–59. [Google Scholar] [CrossRef] [Green Version]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2015, 7, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, M.; Dickens, T.J.; Okoli, O. Progress towards self-healing polymers for composite structural applications. Polymer 2016, 83, 260–282. [Google Scholar] [CrossRef]
- Das, R.; Melchior, C.; Karumbaiah, K.M. Self-healing composites for aerospace applications. In Advanced Composite Materials for Aerospace Engineering; Rana, S., Fangueiro, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 333–364. [Google Scholar]
- Hia, I.L.; Vahedi, V.; Pasbakhsh, P. Self-Healing Polymer Composites: Prospects, Challenges, and Applications. Polym. Rev. 2016, 56, 225–261. [Google Scholar] [CrossRef]
- Azcune, I.; Odriozola, I. Aromatic disulfide crosslinks in polymer systems: Self-healing, reprocessability, recyclability and more. Eur. Polym. J. 2016, 84, 147–160. [Google Scholar] [CrossRef]
- Urdl, K.; Kandelbauer, A.; Kern, W.; Müller, U.; Thebault, M.; Zikulnig-Rusch, E. Self-healing of densely crosslinked thermoset polymers—A critical review. Prog. Org. Coat. 2017, 104, 232–249. [Google Scholar] [CrossRef]
- Imato, K.; Otsuka, H. Self-healing Polymers through Dynamic Covalent Chemistry. In Dynamic Covalent Chemistries: Principles, Reactions and Applications; Zhang, W., Jin, Y., Eds.; John Wiley & Sons: Chichester, UK, 2017; pp. 359–387. [Google Scholar]
- Kissounko, D.A.; Taynton, P.; Kaffer, C. New material: Vitrimers promise to impact composites. Reinf. Plast. 2018, 62, 162–166. [Google Scholar] [CrossRef]
- Campanella, A.; Döhler, D.; Binder, W.H. Self-Healing in Supramolecular Polymers. Macromol. Rapid Commun. 2018, 39, 1700739. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.I.; Halder, S.; Gunjan, S.B.; Prasad, T. A review on Diels-Alder based self-healing polymer composites. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Sikkim, India, 8–10 December 2017; Volume 377. [Google Scholar]
- Du, Y.; Li, D.; Liu, L.; Gai, G. Recent achievements of self-healing graphene/polymer composites. Polymers 2018, 10, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohades, A.; Branfoot, C.; Rae, S.; Bond, I.; Michaud, V. Progress in Self-Healing Fiber-Reinforced Polymer Composites. Adv. Mater. Interfaces 2018, 5, 1800177. [Google Scholar] [CrossRef]
- Chung, D.D.L. A review of multifunctional polymer-matrix structural composites. Compos. Part. B Eng. 2019, 160, 644–660. [Google Scholar] [CrossRef]
- Kanu, N.J.; Gupta, E.; Vates, U.K.; Singh, G.K. Self-healing composites: A state-of-the-art review. Compos. Part. A Appl. Sci. Manuf. 2019, 121, 474–486. [Google Scholar] [CrossRef]
- Bode, S.; Enke, M.; Hernandez, M.; Bose, R.K.; Grande, A.M.; van der Zwaag, S.; Schubert, U.S.; Garcia, S.J.; Hager, M.D. Characterization of self-healing polymers: From macroscopic healing tests to the molecular mechanism. In Self-Healing Materials. Advances in Polymer Science; Hager, M., Van Der Zwaag, S., Schubert, U.S., Eds.; Springer: Cham, Switzerland, 2016; pp. 113–142. [Google Scholar]
- Wool, R.P.; O’Connor, K.M. A theory of crack healing in polymers. J. Appl. Phys. 1981, 52, 5953–5963. [Google Scholar] [CrossRef]
- Outwater, J.O.; Gerry, D.J. On the Fracture Energy, Rehealing Velocity and Refracture Energy of Cast Epoxy Resin. J. Adhes. 1969, 1, 290–298. [Google Scholar] [CrossRef]
- Rahmathullah, M.A.M.; Palmese, G.R. Crack-healing behavior of epoxy-amine thermosets. J. Appl. Polym. Sci. 2009, 113, 2191–2201. [Google Scholar] [CrossRef]
- Hayes, S.A.; Jones, F.R.; Marshiya, K.; Zhang, W. A self-healing thermosetting composite material. Compos. Part. A Appl. Sci. Manuf. 2007, 4, 1116–1120. [Google Scholar] [CrossRef]
- Hayes, S.A.; Zhang, W.; Branthwaite, M.; Jones, F.R. Self-healing of damage in fibre-reinforced polymer-matrix composites. J. R. Soc. Interface 2007, 4, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Ou, R.; Eberly, D.E.; Singhal, A.; Viratyaporn, W.; Mather, P.T. A thermoplastic/thermoset blend exhibiting thermal mending and reversible adhesion. ACS Appl. Mater. Interfaces 2009, 1, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Cohades, A.; Manfredi, E.; Plummer, C.J.G.; Michaud, V. Thermal mending in immiscible poly(ϵ-caprolactone)/epoxy blends. Eur. Polym. J. 2016, 81, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Cohades, A.; Michaud, V. Thermal mending in E-glass reinforced poly(ε-caprolactone)/epoxy blends. Compos. Part. A Appl. Sci. Manuf. 2017, 99, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Cohades, A.; Michaud, V. Damage recovery after impact in E-glass reinforced poly(ε-caprolactone)/epoxy blends. Compos. Struct. 2017, 180, 439–447. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, T.; Cheung, S.C.P.; Wang, C.H. The effect of carbon nanofibres on self-healing epoxy/poly(ε-caprolactone) blends. Compos. Sci. Technol. 2012, 72, 1952–1959. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.H.; Niu, H.; Gestos, A.; Lin, T.; Wang, X. Thermally mendable epoxy resin strengthened with carbon nanofibres. Compos. Part. A Appl. Sci. Manuf. 2013, 55, 45–52. [Google Scholar] [CrossRef]
- Zako, M.; Takano, N. Intelligent material systems using epoxy particles to repair microcracks and delamination damage in GFRP. J. Intell. Mater. Syst. Struct. 1999, 10, 836–841. [Google Scholar] [CrossRef]
- Meure, S.; Wu, D.Y.; Furman, S. Polyethylene-co-methacrylic acid healing agents for mendable epoxy resins. Acta Mater. 2009, 57, 4312–4320. [Google Scholar] [CrossRef]
- Meure, S.; Varley, R.J.; Wu, D.Y.; Mayo, S.; Nairn, K.; Furman, S. Confirmation of the healing mechanism in a mendable EMAA-epoxy resin. Eur. Polym. J. 2012, 48, 524–531. [Google Scholar] [CrossRef]
- Varley, R.J.; Parn, G.P. Thermally activated healing in a mendable resin using a non woven EMAA fabric. Compos. Sci. Technol. 2012, 72, 453–460. [Google Scholar] [CrossRef]
- Pingkarawat, K.; Dell’Olio, C.; Varley, R.J.; Mouritz, A.P. An efficient healing agent for high temperature epoxy composites based upon tetra-glycidyl diamino diphenyl methane. Compos. Part. A Appl. Sci. Manuf. 2015, 78, 201–210. [Google Scholar] [CrossRef]
- Pingkarawat, K.; Dell’Olio, C.; Varley, R.J.; Mouritz, A.P. Poly(ethylene-co-methacrylic acid) (EMAA) as an efficient healing agent for high performance epoxy networks using diglycidyl ether of bisphenol A (DGEBA). Polymer 2016, 92, 153–163. [Google Scholar] [CrossRef]
- Hargou, K.; Pingkarawat, K.; Mouritz, A.P.; Wang, C.H. Ultrasonic activation of mendable polymer for self-healing carbon-epoxy laminates. Compos. Part. B Eng. 2013, 45, 1031–1039. [Google Scholar] [CrossRef]
- Varley, R.J.; Craze, D.A.; Mouritz, A.P.; Wang, C.H. Thermoplastic healing in epoxy networks: Exploring performance and mechanism of alternative healing agents. Macromol. Mater. Eng. 2013, 298, 1232–1242. [Google Scholar] [CrossRef]
- Pingkarawat, K.; Bhat, T.; Craze, D.A.; Wang, C.H.; Varley, R.J.; Mouritz, A.P. Healing of carbon fibre-epoxy composites using thermoplastic additives. Polym. Chem. 2013, 4, 5007–5015. [Google Scholar] [CrossRef]
- Wang, C.H.; Sidhu, K.; Yang, T.; Zhang, J.; Shanks, R. Interlayer self-healing and toughening of carbon fibre/epoxy composites using copolymer films. Compos. Part. A Appl. Sci. Manuf. 2012, 43, 512–518. [Google Scholar] [CrossRef]
- Montarnal, D.; Tournilhac, F.; Hidalgo, M.; Leibler, L. Epoxy-based networks combining chemical and supramolecular hydrogen-bonding crosslinks. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 1133–1141. [Google Scholar] [CrossRef]
- Sordo, F.; Mougnier, S.J.; Loureiro, N.; Tournilhac, F.; Michaud, V. Design of Self-Healing Supramolecular Rubbers with a Tunable Number of Chemical Cross-Links. Macromolecules 2015, 48, 4394–4402. [Google Scholar] [CrossRef]
- Sordo, F.; Michaud, V. Processing and damage recovery of intrinsic self-healing glass fiber reinforced composites. Smart Mater. Struct. 2016, 25, 084012. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Sorrentino, A.; Binder, W.H.; Kadlec, M. Development of self-healing multifunctional materials. Compos. Part. B Eng. 2017, 128, 30–38. [Google Scholar] [CrossRef]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Calabrese, E.; Barra, G.; Raimondo, M.; Sorrentino, A.; Binder, W.H.; Michael, P.; Rana, S. Self-healing epoxy nanocomposites via reversible hydrogen bonding. Compos. Part. B Eng. 2019, 157, 1–13. [Google Scholar] [CrossRef]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic Covalent Chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Spanswick, J. Controlled/living radical polymerization. Mater. Today 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent adaptable networks (CANs): A unique paradigm in cross-linked polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef] [Green Version]
- Bowman, C.N.; Kloxin, C.J. Covalent adaptable networks: Reversible bond structures incorporated in polymer networks. Angew. Chem. Int. Ed. 2012, 51, 4272–4274. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [Green Version]
- Craven, J.M. Cross-Linked Thermally Reversible Polymers Produced from Condensation Polymers with Pendant Furan Groups Cross-Linked with Maleimides. U.S. Patent US554300A, 25 March 1969. [Google Scholar]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Chen, X.; Wudl, F.; Mal, A.K.; Shen, H.; Nutt, S.R. New thermally remendable highly cross-linked polymeric materials. Macromolecules 2003, 36, 1802–1807. [Google Scholar] [CrossRef]
- Peterson, A.M.; Jensen, R.E.; Palmese, G.R. Reversibly cross-linked polymer gels as healing agents for epoxy-amine thermosets. ACS Appl. Mater. Interfaces 2009, 1, 992–995. [Google Scholar] [CrossRef]
- Peterson, A.M.; Jensen, R.E.; Palmese, G.R. Room-temperature healing of a thermosetting polymer using the diels-alder reaction. ACS Appl. Mater. Interfaces 2010, 2, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Saito, K.; Simon, G.P. Synthesis of a diamine cross-linker containing Diels-Alder adducts to produce self-healing thermosetting epoxy polymer from a widely used epoxy monomer. Polym. Chem. 2013, 4, 724–730. [Google Scholar] [CrossRef]
- Turkenburg, D.H.; Fischer, H.R. Diels-Alder based, thermo-reversible cross-linked epoxies for use in self-healing composites. Polymer 2015, 79, 187–194. [Google Scholar] [CrossRef]
- Coope, T.S.; Turkenburg, D.H.; Fischer, H.R.; Luterbacher, R.; Van Bracht, H.; Bond, I.P. Novel Diels-Alder based self-healing epoxies for aerospace composites. Smart Mater. Struct. 2016, 25, 084010. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.M.; Jensen, R.E.; Palmese, G.R. Thermoreversible and remendable glass-polymer interface for fiber-reinforced composites. Compos. Sci. Technol. 2011, 71, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Martone, A.; Iacono, S.D.; Xiao, S.; Xu, F.; Amendola, E. An integrated strategy to promote self-healing at interface in composites based on Diels-Alder epoxy. In Proceedings of the AIP Conference Proceedings, Ischia, Italy, 17–21 June 2018; Volume 1981. [Google Scholar]
- Zhang, W.; Duchet, J.; Gérard, J.F. Self-healable interfaces based on thermo-reversible Diels-Alder reactions in carbon fiber reinforced composites. J. Colloid Interface Sci. 2014, 430, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhang, Y.; Zou, X.; Zhang, R.; Wang, X.; Wu, Q.; Sun, P. Rapid self-healing and recycling of multiple-responsive mechanically enhanced epoxy resin/graphene nanocomposites. RSC Adv. 2017, 7, 46336–46343. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zou, D.; Zhu, W.; Yang, X.; Zhao, P.; Chen, C.; Shuai, M. Infrared induced repeatable self-healing and removability of mechanically enhanced graphene-epoxy flexible materials. RSC Adv. 2019, 9, 14024–14032. [Google Scholar] [CrossRef] [Green Version]
- Handique, J.; Dolui, S.K. A thermally remendable multiwalled carbon nanotube/epoxy composites via Diels-Alder bonding. J. Polym. Res. 2019, 26, 163. [Google Scholar] [CrossRef]
- Khan, N.I.; Halder, S.; Wang, J. Diels-Alder based epoxy matrix and interfacial healing of bismaleimide grafted GNP infused hybrid nanocomposites. Polym. Test. 2019, 74, 138–151. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, M.Q.; Rong, M.Z. Application of alkoxyamine in self-healing of epoxy. J. Mater. Chem. A 2014, 2, 6558–6566. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Room temperature self-healable epoxy elastomer with reversible alkoxyamines as crosslinkages. Polymer 2014, 55, 3936–3943. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Recyclable and Malleable Epoxy Thermoset Bearing Aromatic Imine Bonds. Macromolecules 2018, 51, 9816–9824. [Google Scholar] [CrossRef]
- Martin, R.; Rekondo, A.; De Luzuriaga, A.R.; Casuso, P.; Dupin, D.; Cabañero, G.; Grande, H.J.; Odriozola, I. Dynamic sulfur chemistry as a key tool in the design of self-healing polymers. Smart Mater. Struct. 2016, 25, 0841017. [Google Scholar] [CrossRef]
- Park, I.; Sheiko, S.S.; Nese, A.; Matyjaszewski, K. Molecular tensile testing machines: Breaking a specific covalent bond by adsorption-induced tension in brushlike macromolecules. Macromolecules 2009, 42, 1805–1807. [Google Scholar] [CrossRef]
- Larsson, R.; Ramström, O. Dynamic combinatorial thiolester libraries for efficient catalytic self-screening of hydrolase substrates. Eur. J. Org. Chem. 2006, 1, 285–291. [Google Scholar] [CrossRef]
- Sutton, L.R.; Donaubauer, W.A.; Hampel, F.; Hirsch, A. Tris(thioacetals) from benzene hexathiol: Towards covalent self-assembly. Chem. Commun. 2004, 4, 1758–1759. [Google Scholar] [CrossRef]
- Shi, B.; Greaney, M.F. Reversible Michael addition of thiols as a new tool for dynamic combinatorial chemistry. Chem. Commun. 2005, 7, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Pepels, M.; Filot, I.; Klumperman, B.; Goossens, H. Self-healing systems based on disulfide-thiol exchange reactions. Polym. Chem. 2013, 4, 4955–4965. [Google Scholar] [CrossRef]
- Lafont, U.; Van Zeijl, H.; Van Der Zwaag, S. Influence of cross-linkers on the cohesive and adhesive self-healing ability of polysulfide-based thermosets. ACS Appl. Mater. Interfaces 2012, 4, 6280–6288. [Google Scholar] [CrossRef]
- Abdolahzadeh, M.; Esteves, A.C.; Van Der Zwaag, S.; Garcia, S.J. Healable dual organic-inorganic crosslinked sol-gel based polymers: Crosslinking density and tetrasulfide content effect. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1953–1961. [Google Scholar] [CrossRef]
- Abdolah Zadeh, M.; Grande, A.M.; Van Der Zwaag, S.; Garcia, S.J. Effect of curing on the mechanical and healing behaviour of a hybrid dual network: A time resolved evaluation. RSC Adv. 2016, 6, 91806–91814. [Google Scholar] [CrossRef] [Green Version]
- Post, W.; Cohades, A.; Michaud, V.; van der Zwaag, S.; Garcia, S.J. Healing of a glass fibre reinforced composite with a disulphide containing organic-inorganic epoxy matrix. Compos. Sci. Technol. 2017, 152, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Matxain, J.M.; Asua, J.M.; Ruipérez, F. Design of new disulfide-based organic compounds for the improvement of self-healing materials. Phys. Chem. Chem. Phys. 2016, 18, 1758–1770. [Google Scholar] [CrossRef]
- Tesoro, G.C.; Sastri, V. Reversible crosslinking in epoxy resins. I. Feasibility studies. J. Appl. Polym. Sci. 1990, 39, 1425–1437. [Google Scholar] [CrossRef]
- Sastri, V.R.; Tesoro, G.C. Reversible crosslinking in epoxy resins. II. New approaches. J. Appl. Polym. Sci. 1990, 39, 1439–1457. [Google Scholar] [CrossRef]
- Engelberg, P.I.; Tesoro, G.C. Mechanical and thermal properties of epoxy resins with reversible crosslinks. Polym. Eng. Sci. 1990, 30, 303–307. [Google Scholar] [CrossRef]
- Johnson, L.M.; Ledet, E.; Huffman, N.D.; Swarner, S.L.; Shepherd, S.D.; Durham, P.G.; Rothrock, G.D. Controlled degradation of disulfide-based epoxy thermosets for extreme environments. Polymer 2015, 64, 84–92. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohishi, T.; Goseki, R.; Otsuka, H. Degradable epoxy resins prepared from diepoxide monomer with dynamic covalent disulfide linkage. Polymer 2016, 82, 319–326. [Google Scholar] [CrossRef]
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M.L.; Qi, H.J. Reprocessing and recycling of thermosetting polymers based on bond exchange reactions. RSC Adv. 2014, 4, 10108–10117. [Google Scholar] [CrossRef]
- Ding, Z.; Yuan, L.; Guan, Q.; Gu, A.; Liang, G. A reconfiguring and self-healing thermoset epoxy/chain-extended bismaleimide resin system with thermally dynamic covalent bonds. Polymer 2018, 147, 170–182. [Google Scholar] [CrossRef]
- Lu, L.; Fan, J.; Li, G. Intrinsic healable and recyclable thermoset epoxy based on shape memory effect and transesterification reaction. Polymer 2016, 105, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Pan, J.; Li, G. Recyclable high-performance epoxy based on transesterification reaction. J. Mater. Chem. A 2017, 5, 21505–21513. [Google Scholar] [CrossRef]
- Yue, L.; Bonab, V.S.; Yuan, D.; Patel, A.; Karimkhani, V.; Manas-Zloczower, I. Vitrimerization: A Novel Concept to Reprocess and Recycle Thermoset Waste via Dynamic Chemistry. Glob. Chall. 2019, 3, 1800076. [Google Scholar] [CrossRef] [Green Version]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Dyre, J.C. Colloquium: The glass transition and elastic models of glass-forming liquids. Rev. Mod. Phys. 2006, 78, 953–972. [Google Scholar] [CrossRef] [Green Version]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J. Am. Chem. Soc. 2012, 134, 7764–7767. [Google Scholar] [CrossRef]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Pei, Z.; Zhang, X.; Tao, L.; Wei, Y.; Ji, Y. Carbon nanotube-vitrimer composite for facile and efficient photo-welding of epoxy. Chem. Sci. 2014, 5, 3486–3492. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, Y.; Chen, Q.; Wei, Y.; Ji, Y. Regional Shape Control of Strategically Assembled Multishape Memory Vitrimers. Adv. Mater. 2016, 28, 156–160. [Google Scholar] [CrossRef]
- Liu, W.; Schmidt, D.F.; Reynaud, E. Catalyst Selection, Creep, and Stress Relaxation in High-Performance Epoxy Vitrimers. Ind. Eng. Chem. Res. 2017, 56, 2667–2672. [Google Scholar] [CrossRef]
- Ruiz De Luzuriaga, A.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horizons 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Ruiz De Luzuriaga, A.; Matxain, J.M.; Ruipérez, F.; Martin, R.; Asua, J.M.; Cabañero, G.; Odriozola, I. Transient mechanochromism in epoxy vitrimer composites containing aromatic disulfide crosslinks. J. Mater. Chem. C 2016, 4, 6220–6223. [Google Scholar] [CrossRef]
- Paolillo, S.; Grande, A.M. Towards multifunctional FRPCs for aerospace applications. In Proceedings of the 25th Conference of the Italian Association of Aeronautics and Astronautics (AIDAA), Rome, Italy, 9–12 September 2019; pp. 202–209. [Google Scholar]
- Zhou, F.; Guo, Z.; Wang, W.; Lei, X.; Zhang, B.; Zhang, H.; Zhang, Q. Preparation of self-healing, recyclable epoxy resins and low-electrical resistance composites based on double-disulfide bond exchange. Compos. Sci. Technol. 2018, 167, 79–85. [Google Scholar] [CrossRef]
- Biron, M. Material Selection for Thermoplastic Parts; William Andrew Publishing: Oxford, UK, 2016. [Google Scholar]
- Job, S.; Leeke, G.; Mativenga, P.T.; Oliveux, G.; Pickering, S.; Shuaib, N.A. Composites Recycling: Where Are We Now? Composites UK Ltd.: Berkhamsted, UK, 2016. [Google Scholar]
- Liu, T.; Hao, C.; Zhang, S.; Yang, X.; Wang, L.; Han, J.; Li, Y.; Xin, J.; Zhang, J. A Self-Healable High Glass Transition Temperature Bioepoxy Material Based on Vitrimer Chemistry. Macromolecules 2018, 51, 5577–5585. [Google Scholar] [CrossRef]
- Yang, X.; Guo, L.; Xu, X.; Shang, S.; Liu, H. A fully bio-based epoxy vitrimer: Self-healing, triple-shape memory and reprocessing triggered by dynamic covalent bond exchange. Mater. Des. 2020, 186, 108248. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Jin, K.; Torkelson, J.M. Vitrimers Designed Both to Strongly Suppress Creep and to Recover Original Cross-Link Density after Reprocessing: Quantitative Theory and Experiments. Macromolecules 2018, 51, 5537–5546. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. The New Plastics Economy: Rethinking the Future of Plastics & Catalysing Action; Ellen MacArthur Foundation: Cowes, UK, 2017. [Google Scholar]
- Post, W.; Susa, A.; Blaauw, R.; Molenveld, K.; Knoop, R.J.I. A Review on the Potential and Limitations of Recyclable Thermosets for Structural Applications. Polym. Rev. 2019, 60, 359–388. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.; Picchioni, F. Thermally Self-Healing Polymeric Materials: The Next Step to Recycling Thermoset Polymers? Macromolecules 2009, 42, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Dahlke, J.; Zechel, S.; Hager, M.D.; Schubert, U.S. How to Design a Self-Healing Polymer: General Concepts of Dynamic Covalent Bonds and Their Application for Intrinsic Healable Materials. Adv. Mater. Interfaces 2018, 5, 1800051. [Google Scholar] [CrossRef]
- Li, G. Self-Healing Composites: Shape Memory Polymer Based Structures; John Wiley & Sons: Chichester, UK, 2014; ISBN 9781118452462. [Google Scholar]
- Pernigoni, L.; Grande, A.M. Development of a supramolecular polymer based self-healing multilayer system for inflatable structures. Acta Astronaut. 2020, 177, 697–706. [Google Scholar] [CrossRef]








| Material Property | η Definition | Notes |
|---|---|---|
| Fracture toughness | KIC = fracture toughness (mode I) | |
| Strength | σ = stress X = tensile, compressive, impact, flexural | |
| Stiffness | E = Young’s modulus | |
| Strain Energy | U = strain energy |
| Healing Mechanism | Formulation | Healing Conditions | Tg | η+ | Test (Specimen) | Notes | Ref. |
|---|---|---|---|---|---|---|---|
| (°C) | (%) | ||||||
| Micro-Brownian motion | Bisphenol A-based epoxy-NMA-BDMA | 150 °C, 12 h | 120 | ~100 * | GIC (rectangular cast slab) | no clamping pressure required | [46] |
| Mech. interlocking | DGEBA-PACM | 185 °C, 1 h, 8–13 MPa | 162 | 71 ± 12 | PC (CT) | at stoichiometry | [47] |
| Mech. interlocking + polyetherification-homopolymerization | 118 | 178 ± 56 | excess of epoxy groups | ||||
| Diffusion of thermoplastic healing agent | DGEBA-based epoxy-NMA + poly(bisphenol-A-co-epichlorohydrin) | 140 °C, 1 h | <74 * | 77 | GIC (CT) | 20 wt% thermopl. agent | [48] |
| 64 | KIC (CT) | ||||||
| E-glass + DGEBA-based epoxy-NMA + poly(bisphenol-A-co-epichlorohydrin) | 130 °C, 1 h | 74 | >30 | Visible damage area | 10 wt% thermopl. agent | ||
| 130 °C, 2 h | ~30 | optimized 7.5 wt thermopl. agent | [49] | ||||
| Differential expansive bleeding | DGEBA-DDS + PCL | 190 °C, 8 min, 18.7 kPa | 203 ^ | >100 | P and U at failure (SENB) | 15.5 wt% PCL, non-brittle behavior | [50] |
| CNFs + DGEBA/DGEBF epoxy + PCL | 175 °C, 10 min | 72 | 78 | peak bending P | 10 wt% PCL, 0.2 wt% CNFs | [54] | |
| 54–68 | 60 | WOF | [55] | ||||
| DGEBA-DDS + PCL | 150 °C, 30 min | 197 | 70–80 | PC (TDCB) | 25–26 vol% PCL | [51] | |
| E-glass + DGEBA-DDS + PCL | 150 °C, 30 min | 197^ | 82 | slope of P-Δl (DCB) | 25–26 vol% PCL, η increasing for subsequent healing cycles | [52] | |
| ~40 * | GIC (DCB) | ||||||
| 100 | CAI, C-scan (damaged area) | impact damage of 8 J | [53] | ||||
| Epoxy particles | Glass fiber + coldsetting epoxy + thermosetting epoxy particles | 120 °C, 10 min | n.a. | ~100 * | stiffness (TPB) | nearly full recovery in rigidity | [56] |
| >100 * | fatigue (SENT) | fatigue life extension | |||||
| Pressure delivery of healing agent | CFs + DGEBA-TETA + EMAA | 150 °C, 30 min | n.a. | 221 ± 17 | failure energy (DCB) | 15 vol% EMAA particles | [57] |
| 185 ± 26 | GIC (DCB) | ||||||
| 137 ± 10 | failure energy (DCB) | EMAA 2D fiber mesh, 4 interleaves | |||||
| 45 ± 9 | GIC (DCB) | ||||||
| CFs + DGEBA-TETA + EMAA | 150 °C, 30 min | n.a. | 223 | GIC (DCB) | 2-layers EMAA mesh | [59] | |
| 76 | flexural modulus (ENF) | ||||||
| CFs + TGDDM-DETDA + EMAA | 200 °C, 30 min, 20 kPa | 217 | 55 | GIC (DCB) | 2-step curing (5 h at 80 °C/8 h at 177 °C), 10 wt% EMAA pellets | [60] | |
| 230 °C, 30 min, 20 kPa | 105 | ||||||
| CFs + DGEBA-DETDA + EMAA | 150 °C, 30 min, 20 kPa | 138 | 82 | GIC (DCB) | 2-step curing (5 h at 80 °C/8 h at 177 °C), 10 wt% EMAA pellets | [61] | |
| 200 °C, 30 min, 20 kPa | 114 | ||||||
| CFs + DGEBA-TETA + EMAA | US: 20 kHz, 1.1 kW (~150 °C) | 142 | 135 | GIC (DCB) | 4 EMAA meshes per ply interface | [62] | |
| CFs + DGEBA-TETA + PEGMA | 150 °C, 30 min, 25 kPa | 83 | 57 | GIC (DCB) | 10 wt% thermopl. agent | [64] | |
| CFs + DGEBA-TETA + EMAA | 97 | 156 | |||||
| Thermoplastic melting and viscous flow into cracks upon heating | CFs + DGEBA-TETA + EVA | 150 °C, 30 min, 25 kPa | 97 | 103 | GIC (DCB) | 10 wt% thermopl. agent |
| Healing Mechanism | Formulation | Healing Conditions | Tg | η+ | Test (Specimen) | Notes | Ref. |
|---|---|---|---|---|---|---|---|
| (°C) | (%) | ||||||
| Interdiffusion + DA/rDA | DGEBA-PACM + DGEBA-FA-BMI healing gel | 10 µL gel on cracked surfaces, RT, 12 h, ~4.7 kPa | 160 ^ | 37 ± 8 | PC (CT) | healing gel at 90 °C for 1 h prior to insertion | [79] |
| DGEBA-FGE-PACM + BMI in DMF | 56 | 70 ± 22 | PC (CT) | 0.58 M of BMI in DMF | [80] | ||
| DA/rDA | 2MF-DGEBA + BMI | 150 °C, 5 min | 42 ^ | 122 | PC (TDCB) | 2-step synthesis, 20 wt% BMI | [83] |
| E-glass + DGEBA-FGE-PACM + BMI in DMF | 90 °C, 1 h and 22 °C, 12 h | ~72 * | ~55 * | Microdroplet single fiber pull-out test | η of fiber-resin interface, 0.25 w% FGE | [84] | |
| CFs-BMI + DGEBA-FGE-IPD | 90 °C, 1 h and RT, 24 h | 63 | 82 | Microdroplet single fiber pull-out test | CFs oxidized w/HNO3, reacted w/TEPA, immerged into BMI (in DMF) | [86] | |
| GR-BMI + TF | 800 W, 2 min (microwave), 4 min (IR) | 100 | ~84 * | σT (dog-bone) | 0,5 wt% graphene dispersed in NMP and BMI, TF added | [87] | |
| GNS + FDB-OGE-D230 | ~0.2 W cm−2, ~20 min (IR) | 57 | 93 | Lap shear strength | 0.5 wt% GNS | [88] | |
| MWCNTs + EpF-BMI | 140 °C, 40 min | 71 | ~80 | KIC | multiple healing cycles | [89] | |
| B-GNPs + DGEBA-FGE-BMI | 130 °C, 2 h and 80 °C, 2 h | n.a. | ~87 | σF (dog-bone type V) | first study on bulk epoxy matrix healing | [90] | |
| Alkoxyamines | diEP-DGEBA-DETA | 90 °C, 1.5 h in Ar | ~45 * | 62 | impact test | not feasible in air (O2 deactivation) | [91] |
| diEP-DGEBA-PETMP | 25 °C | 0–10 | 60 | σT (dog-bone) | sub-ambient Tg | [92] | |
| Imines | EN-VAN-AP | 100 °C, 60 s, 10 N, then 120 °C, 4 h | 71 | ~100 * | σT (dog-bone) | simple reprocessability | [93] |
| Tetrasulfides | EPS-4SH | 65 °C, 20 min | −46 | 100 | cohesion test, optical microscopy | cross-lining catalyzed w/1 wt% DMAP | [100] |
| ER-OMAS | 70 °C, 10 min, air, 30 kPa | −11 | 100 | gap closure efficiency | ~600 μm-thick film | [101] | |
| 70 °C, 2 h | 70 | Interfacial strength recovery (SENT) | crack on healed SENT formed at same location as pristine SENT | ||||
| 60 | KIC (DENT) | J-integral for η evaluation | [102] | ||||
| E-glass + DGEBA-aliphatic amine-APTS-tetrathiol-TEA-BDS | 85 °C, 16 h, 2 bar | ~50 * | 80 * | low-velocity impact (damaged area reduction) | composite prep by VARIM, impact energy 8 J | [103] | |
| Transesterification | DGEBA-CBMI | 200 °C, 2 h | 125 | ~78 | KIC (SENT) | referred to highest CBMI wt% | [111] |
| DGEBA-tricarballylic acid | 150 °C, 18 h | 56–59 | 59 | σT | compression programming | [112] | |
| DGEBA-phthalic anhydride | 150 °C, 10 h, 12 MPa | 98 | 88 | σT | powder state, 32 h milling time | [113] | |
| Vitrimer (transesterification) | DGEBA-di/tricarboxylic acid | 240 °C, 3 min | ~15 * | ~100 * | σT (dog-bone) | 10 mol% Zn(acac)2 catalyst | [114] |
| DGEBA-di/tricarboxylic acid | 25% comp, 125 °C, 1 h | ~15 | ~77 * | Lap shear PC | Zn(OAc)2 catalyst, Mohr clamp | [117] | |
| MWCNTs + DGEBA-adipic acid | ~0.15 W cm−2, 30 s (IR) | ~40 | ~80 * | PC (film) | TBD catalyst, photo-welding | [119] | |
| Vitrimer (aromatic disulfide exchange) | DGEBA-4AFD | 200 °C, 5 min, 100 bar | 130 | ~100 | DMA measurements | mechanochromic effect | [122] |
| BDSER-PPy | 180 °C, 20 min, 20 MPa | 133 ^ | ~100 | σT (dog-bone) | reprocessable composites w/MWCNTs | [125] | |
| Bio-Vitrimer (transesterification) | TEP-MHHPA | 220 °C, 5 min | 187 | 70 | crack width reduction | Zn(acac)2 catalyst | [128] |
| ESO-FPA | 180 °C, 60 min | 65 | ~100 | optical microscopy | [129] | ||
| Bio-Vitrimer (disulfide exchange) | IS-ECH-4AFD | 100 °C, 60 min | 41 | 100 | optical microscopy | multiple reprocessing cycles | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolillo, S.; Bose, R.K.; Santana, M.H.; Grande, A.M. Intrinsic Self-Healing Epoxies in Polymer Matrix Composites (PMCs) for Aerospace Applications. Polymers 2021, 13, 201. https://doi.org/10.3390/polym13020201
Paolillo S, Bose RK, Santana MH, Grande AM. Intrinsic Self-Healing Epoxies in Polymer Matrix Composites (PMCs) for Aerospace Applications. Polymers. 2021; 13(2):201. https://doi.org/10.3390/polym13020201
Chicago/Turabian StylePaolillo, Stefano, Ranjita K. Bose, Marianella Hernández Santana, and Antonio M. Grande. 2021. "Intrinsic Self-Healing Epoxies in Polymer Matrix Composites (PMCs) for Aerospace Applications" Polymers 13, no. 2: 201. https://doi.org/10.3390/polym13020201







