Conductive PEDOT:PSS-Based Organic/Inorganic Flexible Thermoelectric Films and Power Generators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
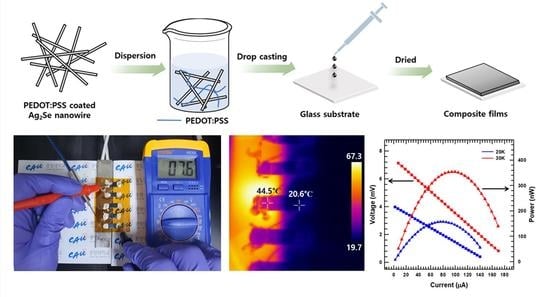
2.2. Synthesis of PEDOT:PSS-Coated Ag2Se NWs
2.3. Fabrication of the PEDOT:PSS-Coated Ag2Se NW/PEDOT:PSS Composite Film
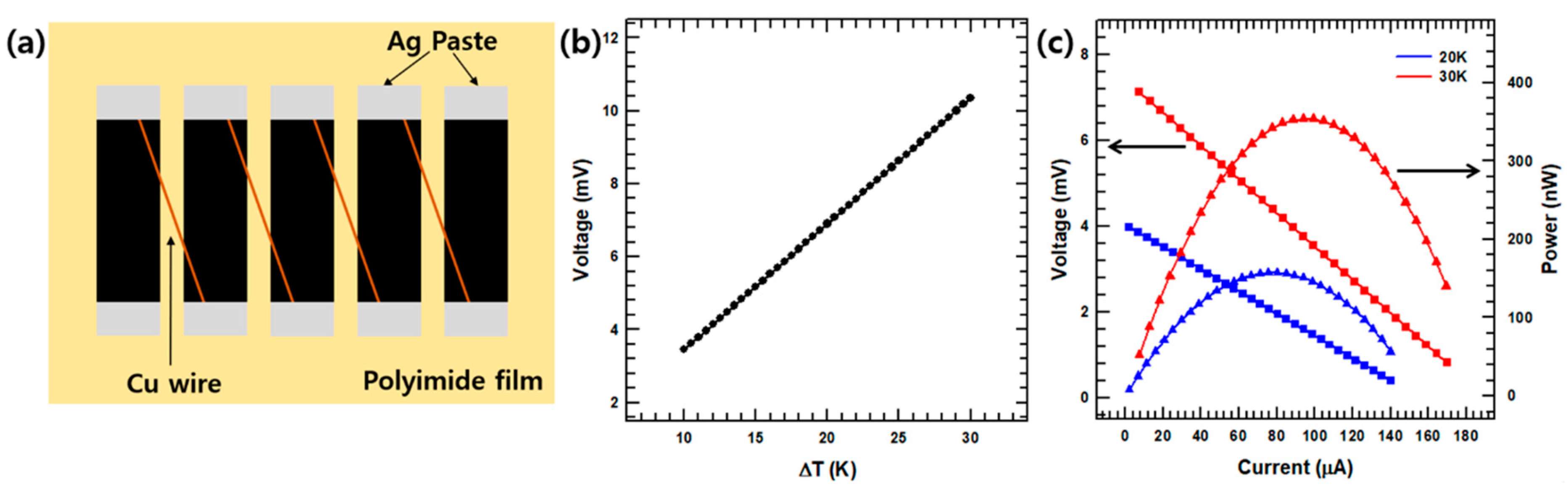
2.4. Device Preparation
2.5. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Zebarjadi, M.; Esfarjani, K.; Dresselhaus, M.; Ren, Z.; Chen, G. Perspectives on thermoelectrics: From fundamentals to device applications. Energy Environ. Sci. 2012, 5, 5147–5162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Organic thermoelectric materials: Emerging green energy materials converting heat to electricity directly and efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef]
- Kim, K.; Wie, J.; Kim, J. Syneristic interaction of P and N co-doping EDTA with controllable active EDTA-cobalt sites as efficient electrocatalyst for oxygen reduction reaction. J. Ind. Eng. Chem. 2020, 83, 252–259. [Google Scholar] [CrossRef]
- Zhou, W.; Fan, Q.; Zhang, Q.; Cai, L.; Li, K.; Gu, X.; Yang, F.; Zhang, N.; Wang, Y.; Liu, H. High-performance and compact-designed flexible thermoelectric modules enabled by a reticulate carbon nanotube architecture. Nat. Commun. 2017, 8, 1–9. [Google Scholar]
- Sotelo, A.; Depriester, M.; Torres, M.; Sahraoui, A.; Madre, M.; Diez, J. Effect of simultaneous K, and Yb substitution for Ca on the microstructural and thermoelectric characteristics of CaMnO3 ceramics. Ceram. Int. 2018, 44, 12697–12701. [Google Scholar] [CrossRef] [Green Version]
- Tiu, T.; Wang, C.; Hou, J.; Zhang, C.; Chen, H.; He, H.; Wang, N.; Wu, H.; Cao, G. Enhanced electron collection in perovskite solar cells employing thermoelectric NaCo2O4/TiO2 coaxial nanofibers. Small 2016, 12, 5146–5152. [Google Scholar]
- Park, D.; Ju, H.; Kim, J. One-pot fabrication of Ag–SrTiO3 nanocomposite and its enhanced thermoelectric properties. Ceram. Int. 2019, 45, 16969–16975. [Google Scholar] [CrossRef]
- Park, D.; Ju, H.; Kim, J. Effect of SrTiO3 Nanoparticles in Conductive Polymer on the Thermoelectric Performance for Efficient Thermoelectrics. Polymers 2020, 12, 777. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.E.; Ren, F.; Case, E.D.; Timm, E.J. Porosity dependence of elastic moduli in LAST (lead–antimony–silver–tellurium) thermoelectric materials. Mater. Chem. Phys. 2009, 118, 459–466. [Google Scholar] [CrossRef]
- Ren, F.; Case, E.; Timm, E.; Schock, H. Young’s modulus as a function of composition for an n-type lead–antimony–silver–telluride (LAST) thermoelectric material. Philos. Mag. 2007, 87, 4907–4934. [Google Scholar] [CrossRef]
- Ju, H.; Kim, J. Preparation and structure dependent thermoelectric properties of nanostructured bulk bismuth telluride with graphen. J. Alloys Compd. 2016, 664, 639–647. [Google Scholar] [CrossRef]
- Ju, H.; Kim, M.; Kim, J. A facile fabrication of n-type Bi2Te3 nanowire/graphene layer-by-layer hybrid structures and their improved thermoelectric performance. Chem. Eng. J. 2015, 275, 102–112. [Google Scholar] [CrossRef]
- Ju, H.; Kim, J. The effect of temperature on thermoelectric properties of n-type Bi2Te3 nanowire/graphene layer-by-layer hybrid composites. Dalton Trans. 2015, 44, 11755–11762. [Google Scholar] [CrossRef]
- Li, Z.; Miao, N.; Zhou, J.; Sun, Z.; Liu, Z.; Xu, H. High thermoelectric performance of few-quintuple Sb2Te3 nanofilms. Nano Energy 2018, 43, 285–290. [Google Scholar] [CrossRef]
- Vieira, E.M.; Figueira, J.; Pires, A.L.; Grilo, J.; Silva, M.F.; Pereira, A.M.; Goncalves, L.M. Enhanced thermoelectric properties of Sb2Te3 and Bi2Te3 films for flexible thermal sensors. J. Alloys Compd. 2019, 774, 1102–1116. [Google Scholar] [CrossRef]
- Li, W.; Zheng, L.; Ge, B.; Lin, S.; Zhang, X.; Chen, Z.; Chang, Y.; Pei, Y. Promoting SnTe as an eco-friendly solution for p-PbTe thermoelectric via band convergence and interstitial defects. Adv. Mater. 2017, 29, 1605887. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, L.D. Charge and phonon transport in PbTe-based thermoelectric materials. NPJ Quantum Mater. 2018, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Sun, Y.; Pei, W.B.; Huang, L.; Xu, W.; Zhang, Q. Polypyrrole nanotube film for flexible thermoelectric application. Synth. Met. 2014, 196, 173–177. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.Y. Polypyrrole nanostructures and their thermoelectric performance. Mater. Chem. Front. 2017, 1, 380–386. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Wang, D.; Zhu, G.; Li, J. Preparation and thermoelectric properties of polythiophene/multiwalled carbon nanotube composites. Synth. Met. 2013, 181, 79–85. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Xu, J.; Song, H.; Lu, B.; Jiang, F.; Zhou, W.; Zhang, G.; Jiang, Q. Facile fabrication of PEDOT:PSS/polythiophenes bilayered nanofilms on pure organic electrodes and their thermoelectric performance. ACS Appl. Mater. Interfaces 2013, 5, 12811–12819. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Park, D.; Kim, J. Conductive polymer based high-performance hybrid thermoelectrics: Polyaniline/tin (II) sulfide nanosheet composites. Polymer 2019, 160, 24–29. [Google Scholar] [CrossRef]
- Ju, H.; Park, D.; Kim, J. Solution-processable flexible thermoelectric composite films based on conductive polymer/SnSe0.8S0.2 nanosheets/carbon nanotubes for wearable electronic applications. J. Mater. Chem. A 2018, 6, 5627–5634. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Wang, L.; Du, K.; Yin, Q.; Yin, Q. Polypyrrole/graphene/polyaniline ternary nanocomposite with high thermoelectric power factor. ACS Appl. Mater. Interfaces 2017, 9, 20124–20131. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Q.; Wang, L.; Chen, L. Abnormally enhanced thermoelectric transport properties of SWNT/PANI hybrid films by the strengthened PANI molecular ordering. Energ. Environ. Sci. 2014, 7, 3801–3807. [Google Scholar] [CrossRef]
- Luo, J.; Billep, D.; Waechtler, T.; Otto, T.; Toader, M.; Gordan, O.; Sheremet, E.; Martin, J.; Hietschold, M.; Zahn, D.R. Enhancement of the thermoelectric properties of PEDOT:PSS thin films by post-treatment. J. Mater. Chem. A 2013, 1, 7576–7583. [Google Scholar] [CrossRef]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef]
- Du, Y.; Cai, K.; Chen, S.; Cizek, P.; Lin, T. Facile preparation and thermoelectric properties of Bi2Te3 based alloy nanosheet/PEDOT:PSS composite films. ACS Appl. Mater. Interfaces 2014, 6, 5735–5743. [Google Scholar] [CrossRef]
- Gainza, J.; Serrano, S.F.; Rodrigues, J.E.; Huttel, Y.; Dura, O.J.; Koza, M.M.; Fernández, D.M.T.; Meléndez, J.J.; Márkus, B.G.; Simon, F. High-Performance n-type SnSe Thermoelectric Polycrystal Prepared by Arc-Melting. Cell Rep. Phys. Sci. 2020, 1, 100263. [Google Scholar] [CrossRef]
- Ge, Z.H.; Chang, Y.; Li, F.; Luo, J.; Fan, P. Improved thermoelectric properties of PEDOT:PSS polymer bulk prepared using spark plasma sintering. Chem. Commun. 2018, 54, 2429–2431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, J.; Katz, H.; Fang, F.; Opila, R. Promising thermoelectric properties of commercial PEDOT:PSS materials and their Bi2Te3 powder composites. ACS Appl. Mater. Interfaces 2010, 2, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Qiu, Y.; Cai, K.; Yao, Q.; Chen, S.; Chen, L.; He, J. High performance n-type Ag2Se film on nylon membrane for flexible thermoelectric power generator. Nat. Commun. 2019, 10, 1–7. [Google Scholar]
- Perez, T.J.A.; Caballero, C.O.; Vera, L.L.; Briones, F.; Martin, G.M. High Thermoelectric zT in n-Type Silver Selenide films at Room Temperature. Adv. Energy Mater. 2018, 8, 1702024. [Google Scholar] [CrossRef]
- Park, D.; Kim, M.; Kim, J. Fabrication of PEDOT:PSS/Ag2Se Nanowires for Polymer-Based Thermoelectric Applications. Polymers 2020, 12, 2932. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, Y.; Cai, K.; Ding, Y.; Wang, M.; Jiang, C.; Yao, Q.; Huang, C.; Chen, L.; He, J. Ultrahigh power factor and flexible silver selenide-based composite film for thermoelectric devices. Energ. Environ. Sci. 2020, 13, 1240–1249. [Google Scholar] [CrossRef]
- Qu, J.; Goubet, N.; Livache, C.; Martinez, B.; Amelot, D.; Gréboval, C.; Chu, A.; Ramade, J.; Cruguel, H.; Ithurria, S. Intraband mid-infrared transitions in Ag2Se nanocrystals: Potential and limitations for Hg-free low-cost photodetection. J. Phys. Chem. C 2018, 122, 18161–18167. [Google Scholar] [CrossRef]
- Pei, J.; Chen, G.; Jia, D.; Jin, R.; Xu, H.; Chen, D. Rapid synthesis of Ag2Se dendrites with enhanced electrical performance by microwave-assisted solution method. New J. Chem. 2013, 37, 323–328. [Google Scholar] [CrossRef]
- Tan, L.; Fu, J.; Liu, S. Growth of photoluminescent Ag2Se nanowires from a simple precursor solution. Cryst. Eng. Comm. 2014, 16, 10534–10538. [Google Scholar] [CrossRef]
- Jiang, C.; Ding, Y.; Cai, K.; Tong, L.; Lu, Y.; Zhao, W.; Wei, P. Ultrahigh Performance of n-Type Ag2Se Films for Flexible Thermoelectric Power Generators. ACS Appl. Mater. Interfaces 2020, 12, 9646–9655. [Google Scholar] [CrossRef]
- Ju, H.; Kim, J. Chemically exfoliated SnSe nanosheets and their SnSe/poly(3, 4-ethylenedioxythiophene): Poly(styrenesulfonate) composite films for polymer based thermoelectric applications. ACS Nano 2016, 10, 5730–5739. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, Y.; Cai, K.; Li, X.; Gao, M.; He, J. Ultrahigh Performance PEDOT/Ag2Se/CuAgSe Composite Film for Wearable Thermoelectric Power Generators. Mater. Today Phys. 2020, 14, 100223. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.; Kim, M.; Kim, J. Conductive PEDOT:PSS-Based Organic/Inorganic Flexible Thermoelectric Films and Power Generators. Polymers 2021, 13, 210. https://doi.org/10.3390/polym13020210
Park D, Kim M, Kim J. Conductive PEDOT:PSS-Based Organic/Inorganic Flexible Thermoelectric Films and Power Generators. Polymers. 2021; 13(2):210. https://doi.org/10.3390/polym13020210
Chicago/Turabian StylePark, Dabin, Minsu Kim, and Jooheon Kim. 2021. "Conductive PEDOT:PSS-Based Organic/Inorganic Flexible Thermoelectric Films and Power Generators" Polymers 13, no. 2: 210. https://doi.org/10.3390/polym13020210
APA StylePark, D., Kim, M., & Kim, J. (2021). Conductive PEDOT:PSS-Based Organic/Inorganic Flexible Thermoelectric Films and Power Generators. Polymers, 13(2), 210. https://doi.org/10.3390/polym13020210