Studies of the Sulfonated Hydrogenated Styrene–Isoprene–Styrene Block Copolymer and Its Surface Properties, Cytotoxicity, and Platelet-Contacting Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
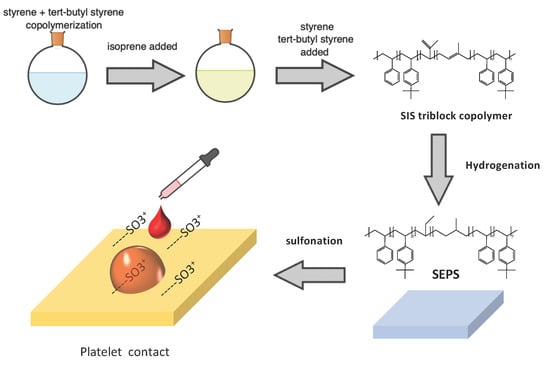
2.3. Synthesis of the SIS Triblock Copolymer
2.4. Hydrogenation of the SIS Block Copolymer
2.5. Sulfonation of the SEPS Block Copolymer
2.6. Streaming Potential
2.7. Cytotoxicity Assay
2.8. In Vitro Platelet Adhesion Test
3. Results
3.1. Material Characterization
3.2. Cytotoxicity Test
3.3. In Vitro Platelet Adhesion Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tokhadze, N.; Chennell, P.; Bernard, L.; Lambert, C.; Pereira, B.; Mailhot-Jensen, B.; Sautou, V. Impact of alternative materials to plasticized PVC infusion tubings on drug sorption and plasticizer release. Sci Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.X.; Liu, R.; Tang, X.Z.; Han, W. A Drug-in-Adhesive Matrix Based on Thermoplastic Elastomer: Evaluation of Percutaneous Absorption, Adhesion, and Skin Irritation. Aaps Pharmscitech 2012, 13, 1179–1189. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.J.; Xin, Z.R.; Xu, S.A.; Shi, H.C.; Yang, H.W.; Song, L.J.; Yan, S.J.; Luan, S.F.; Yin, J.H.; Khan, A.F.; et al. Enzyme-mimicking polymer brush-functionalized surface for combating biomaterial-associated infections. Appl. Surf. Sci. 2017, 423, 869–880. [Google Scholar] [CrossRef]
- Luan, S.F.; Zhao, J.; Yang, H.W.; Shi, H.C.; Jin, J.; Li, X.M.; Liu, J.C.; Wang, J.W.; Yin, J.H.; Stagnaro, P. Surface modification of poly(styrene-b-(ethylene-co-butylene)-b-styrene) elastomer via UV-induced graft polymerization of N-vinyl pyrrolidone. Colloids Surf. B Biointerfaces 2012, 93, 127–134. [Google Scholar] [CrossRef]
- Li, X.M.; Luan, S.F.; Shi, H.C.; Yang, H.W.; Song, L.J.; Jin, J.; Yin, J.H.; Stagnaro, P. Improved biocompatibility of poly (styrene-b-(ethylene-co-butylene)-b-styrene) elastomer by a surface graft polymerization of hyaluronic acid. Colloids Surf. B Biointerfaces 2013, 102, 210–217. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Crespo-Amoros, J.E.; Parres, F.; Samper, M.D. Influence of Ultraviolet Radiation Exposure Time on Styrene-Ethylene-Butadiene-Styrene (SEBS) Copolymer. Polymers 2020, 12, 862. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Z.; Xu, X.D.; Chen, R.H.; Zhao, J.R.; Cui, L.L.; Sheng, G.K.; Shi, Q.; Wong, S.C.; Yin, J.H. Bioinspired Antioxidant Defense System Constructed by Antioxidants-Eluting Electrospun F127-Based Fibers. ACS Appl. Mater. Interfaces 2017, 9, 38313–38322. [Google Scholar] [CrossRef]
- Hou, J.W.; Shi, Q.; Ye, W.; Fan, Q.F.; Shi, H.C.; Wong, S.C.; Xu, X.D.; Yin, J.H. A novel hydrophilic polymer-brush pattern for site-specific capture of blood cells from whole blood. Chem. Commun. 2015, 51, 4200–4203. [Google Scholar] [CrossRef]
- Orujalipoor, I.; Polat, K.; Huang, Y.C.; Ide, S.; Sen, M.; Jeng, U.S.; Agceli, G.K.; Cihangir, N. Partially sulfonated styrene-(ethylene-butylene)-styrene copolymers: Nanostructures, bio and electro-active properties. Mater. Chem. Phys. 2019, 225, 399–405. [Google Scholar] [CrossRef]
- Ghanooni, S.; Nikfarjam, N.; Makvandi, P. Surface Reactive and Active Polymers. In Reactive and Functional Polymers; Gutiérrez, T.J., Ed.; Springer: Cham, Switzerland, 2020; Volume 4. [Google Scholar]
- Makvandi, P.; Iftekhar, S.; Pizzetti, F.; Zarepour, A.; Zare, E.N.; Ashrafizadeh, M.; Agarwal, T.; Padil, V.V.T.; Mohammadinejad, R.; Sillanpaa, M.; et al. Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: A review. Environ. Chem. Lett. 2020, 1–29. [Google Scholar] [CrossRef]
- Girard, J.; Brunetto, P.S.; Braissant, O.; Rajacic, Z.; Khanna, N.; Landmann, R.; Daniels, A.U.; Fromm, K.M. Development of a polystyrene sulfonate/silver nanocomposite with self-healing properties for biomaterial applications. Comptes Rendus Chim. 2013, 16, 550–556. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.W.; Liu, H.X.; He, H.; Yadava, N.; Chambers, J.J.; Thayumanavan, S. Anionic Polymers Promote Mitochondrial Targeting of Delocalized Lipophilic Cations. Bioconjugate Chem. 2020, 31, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Paluck, S.J.; McGahran, A.J.; Maynard, H.D. Poly(vinyl sulfonate) Facilitates bFGF-Induced Cell Proliferation. Biomacromolecules 2015, 16, 2684–2692. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Zhu, Y.Z.; Li, X.; Liu, X.M.; Yeung, K.W.K.; Wu, S.L.; Wang, X.B.; Cui, Z.D.; Yang, X.J.; Chu, P.K. Surface functionalization of biomaterials by radical polymerization. Prog. Mater. Sci. 2016, 83, 191–235. [Google Scholar] [CrossRef]
- Lv, J.H.; Jin, J.; Han, Y.Y.; Jiang, W. Effect of end-grafted PEG conformation on the hemocompatibility of poly(styrene-b-(ethylene-co-butylene)-b-styrene). J Biomat. Sci. Polym. E 2019, 30, 1670–1685. [Google Scholar] [CrossRef]
- Laprade, E.J.; Liaw, C.Y.; Jiang, Z.; Shull, K.R. Mechanical and Microstructural Characterization of Sulfonated Pentablock Copolymer Membranes. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 39–47. [Google Scholar] [CrossRef]
- Geise, G.M.; Freeman, B.D.; Paul, D.R. Characterization of a sulfonated pentablock copolymer for desalination applications. Polymer 2010, 51, 5815–5822. [Google Scholar] [CrossRef]
- Chen, S.H.; Willis, C.; Shull, K.R. Water transport and mechanical response of block copolymer ion-exchange membranes for water purification. J. Membr. Sci. 2017, 544, 388–396. [Google Scholar] [CrossRef]
- Tsai, B.H.; Chuang, Y.H.; Cheng, C.H.; Lin, J.C. Sulfonation and Characterization of Tert-Butyl Styrene/Styrene/Isoprene Copolymer and Polypropylene Blends for Blood Compatibility Applications. Polymers 2020, 12, 1351. [Google Scholar] [CrossRef]
- Velichkova, R.; Toncheva, V.; Antonov, C.; Alexandrov, V.; Pavlova, S.; Dubrovina, L.; Gladkova, E. Styrene Isoprene Block Copolymers. 2. Hydrogenation and Solution Properties. J. Appl. Polym. Sci. 1991, 42, 3083–3090. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Hu, Y.M.; Wang, Y.R. Synthesis and characterisation of HSIBR used as viscosity index improver for lubricants. Lubr. Sci. 2012, 24, 188–197. [Google Scholar] [CrossRef]
- Li, W. Hydrogenated styrene-isoprene-butadiene rubber: Optimisation of hydrogenation conditions and performance evaluation as viscosity index improver. Lubr. Sci. 2015, 27, 279–296. [Google Scholar] [CrossRef]
- Mather, B.D.; Beyer, F.L.; Long, T.E. Synthesis and SAXS characterization of sulfonated styrene-ethylene/propylene-styrene triblock copolymers. Abstr. Pap. Am. Chem. S 2006, 231, 258. [Google Scholar]
- Ma, Z.F.; Liu, S.; Ke, Y.; Wang, H.Z.; Chen, R.H.; Xiang, Z.H.; Xie, Z.G.; Shi, Q.; Yin, J.H. Biomimetic nano-NOS mediated local NO release for inhibiting cancer-associated platelet activation and disrupting tumor vascular barriers. Biomaterials 2020, 255, 120141. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Shi, Q.; Hou, J.W.; Gao, J.; Li, C.M.; Jin, J.; Shi, H.C.; Yin, J.H. Fabricating bio-inspired micro/nano-particles by polydopamine coating and surface interactions with blood platelets. Appl. Surf. Sci. 2015, 351, 236–242. [Google Scholar] [CrossRef]
- Sato, H.; Tanaka, Y. H-1-Nmr Study of Polyisoprenes. J. Polym. Sci. Part A Polym. Chem. 1979, 17, 3551–3558. [Google Scholar] [CrossRef]
- Patiño, D.; Correa, E.; Acevedo-Morantes, M. Effect of sulfonation and diethanolamine addition on the mechanical and physicochemical properties of SEPS copolymer. J. Phys. Conf. Ser. 2016, 687, 012056. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Q.; Zeng, X.R.; Wu, W.Q. Epoxidation of styrene-isoprene-styrene block copolymer and its use for hot-melt pressure sensitive adhesives. Polym. Plast Technol. 2008, 47, 978–983. [Google Scholar] [CrossRef]
- Vargantwar, P.H.; Brannock, M.C.; Smith, S.D.; Spontak, R.J. Midblock sulfonation of a model long-chain poly(p-tert-butylstyrene-b-styrene-b-p-tert-butylstyrene) triblock copolymer. J. Mater. Chem. 2012, 22, 25262–25271. [Google Scholar] [CrossRef]
- Hylton, D.M.; Shalaby, S.W.; Latour, R.A. Direct correlation between adsorption-induced changes in protein structure and platelet adhesion. J. Biomed. Mater. Res. A 2005, 73a, 349–358. [Google Scholar] [CrossRef]
- Grasel, T.G.; Cooper, S.L. Properties and biological interactions of polyurethane anionomers: Effect of sulfonate incorporation. J. Biomed. Mater. Res. 1989, 23, 311–338. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Lin, J.C. Surface characterization and in vitro platelet compatibility study of surface sulfonated chitosan membrane with amino group protection-deprotection strategy. J. Biomat. Sci. Polym. E 2008, 19, 291–310. [Google Scholar] [CrossRef]










| S5T5 | S6T4 | S7T3 | S8T2 | S9T1 | |
|---|---|---|---|---|---|
| Styrene feeding weight (g) | 1.0 | 1.2 | 1.4 | 1.6 | 1.8 |
| tert-butyl styrene (tbs) feeding weight (g) | 1.0 | 0.8 | 0.6 | 0.4 | 0.2 |
| Isoprene feeding weight (g) | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Styrene/tbs (experimental weight ratio) a | 0.67 | 0.79 | 0.91 | 1.05 | 1.16 |
| Styrenic block (theoretical mole %) | 0.18 | 0.18 | 0.19 | 0.20 | 0.20 |
| Styrenic block (experimental mole %) a | 0.39 | 0.36 | 0.37 | 0.37 | 0.34 |
| 1,4 isoprene/3,4 isoprene (molar ratio) a | 15.45 | 14.02 | 13.37 | 14.24 | 15.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, B.-H.; Lin, T.-A.; Cheng, C.-H.; Lin, J.-C. Studies of the Sulfonated Hydrogenated Styrene–Isoprene–Styrene Block Copolymer and Its Surface Properties, Cytotoxicity, and Platelet-Contacting Characteristics. Polymers 2021, 13, 235. https://doi.org/10.3390/polym13020235
Tsai B-H, Lin T-A, Cheng C-H, Lin J-C. Studies of the Sulfonated Hydrogenated Styrene–Isoprene–Styrene Block Copolymer and Its Surface Properties, Cytotoxicity, and Platelet-Contacting Characteristics. Polymers. 2021; 13(2):235. https://doi.org/10.3390/polym13020235
Chicago/Turabian StyleTsai, Bin-Hong, Tse-An Lin, Chi-Hui Cheng, and Jui-Che Lin. 2021. "Studies of the Sulfonated Hydrogenated Styrene–Isoprene–Styrene Block Copolymer and Its Surface Properties, Cytotoxicity, and Platelet-Contacting Characteristics" Polymers 13, no. 2: 235. https://doi.org/10.3390/polym13020235
APA StyleTsai, B.-H., Lin, T.-A., Cheng, C.-H., & Lin, J.-C. (2021). Studies of the Sulfonated Hydrogenated Styrene–Isoprene–Styrene Block Copolymer and Its Surface Properties, Cytotoxicity, and Platelet-Contacting Characteristics. Polymers, 13(2), 235. https://doi.org/10.3390/polym13020235