3D-Printed Poly(ε-Caprolactone)/Hydroxyapatite Scaffolds Modified with Alkaline Hydrolysis Enhance Osteogenesis In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of 3D-Printed Scaffolds
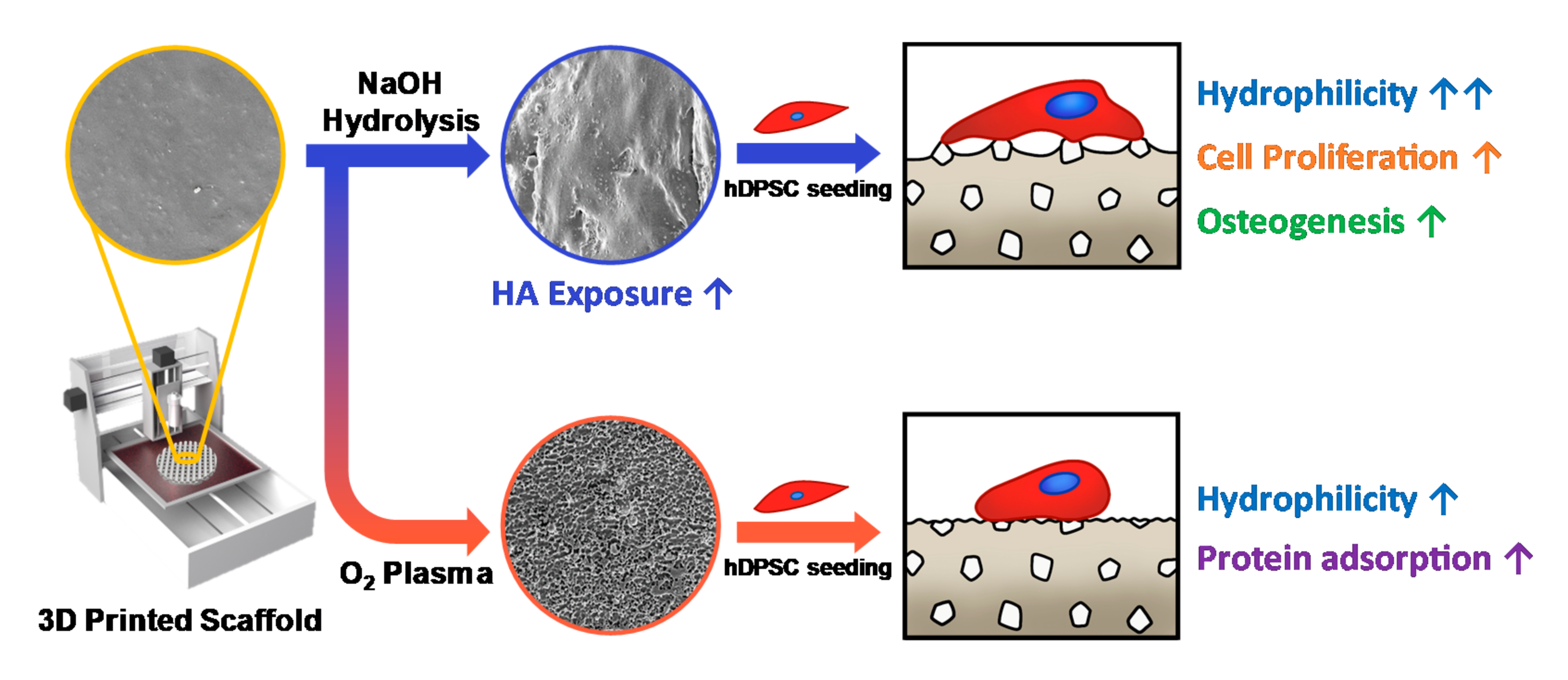
2.2. Surface Modification of 3D-Printed Scaffolds
2.3. Characteristics of 3D-Printed Scaffolds
2.4. Cell Attachment and Proliferation on the 3D-Printed Scaffolds
2.5. Osteogenic Differentiation of hDPSCs on 3D Scaffolds
3. Results and Discussion
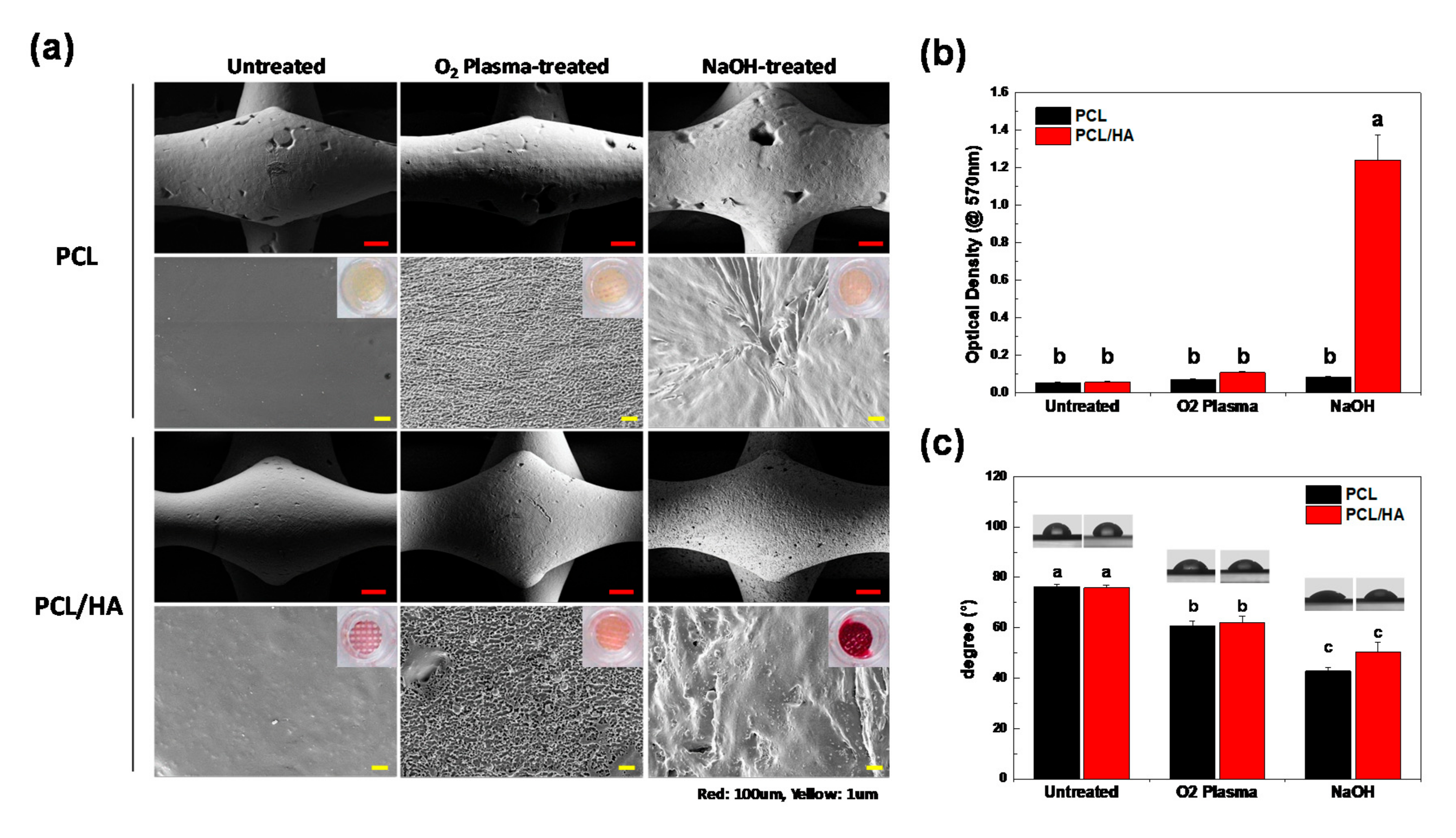
3.1. Characteristics of 3D-Printed Scaffolds
3.2. Cell Attachment and Proliferation on the 3D-Printed Scaffolds
3.3. Osteogenic Differentiation of hDPSCs on 3D Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mistry, A.S.; Mikos, A.G. Tissue engineering strategies for bone regeneration. In Regenerative Medicine II; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–22. [Google Scholar]
- Janicki, P.; Schmidmaier, G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury 2011, 42, S77–S81. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.; Arts, J.C.; Schmidmaier, G.; Larsson, S. What should be the characteristics of the ideal bone graft substitute? Injury 2011, 42, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef]
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A review of three-dimensional printing in tissue engineering. Tissue Eng. Part B Rev. 2016, 22, 298–310. [Google Scholar] [CrossRef]
- Song, X.; Tetik, H.; Jirakittsonthon, T.; Parandoush, P.; Yang, G.; Lee, D.; Ryu, S.; Lei, S.; Weiss, M.L.; Lin, D. Biomimetic 3D printing of hierarchical and interconnected porous hydroxyapatite structures with high mechanical strength for bone cell culture. Adv. Eng. Mater. 2019, 21, 1800678. [Google Scholar] [CrossRef]
- Daly, A.C.; Freeman, F.E.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv. Healthc. Mater. 2017, 6, 1700298. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A review on the recent research of polycaprolactone (PCL). In Proceedings of the Advanced Materials Research; Trans Tech Publications Ltd.: Baech, Switzerland, 2016; pp. 249–255. [Google Scholar]
- Vyas, C.; Zhang, J.; Øvrebø, Ø.; Huang, B.; Roberts, I.; Setty, M.; Allardyce, B.; Haugen, H.; Rajkhowa, R.; Bartolo, P. 3D printing of silk microparticle reinforced polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2020, 118, 111433. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef]
- Söhling, N.; Neijhoft, J.; Nienhaus, V.; Acker, V.; Harbig, J.; Menz, F.; Ochs, J.; Verboket, R.D.; Ritz, U.; Blaeser, A. 3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds. Materials 2020, 13, 1836. [Google Scholar] [CrossRef] [PubMed]
- Declercq, H.A.; Desmet, T.; Berneel, E.E.; Dubruel, P.; Cornelissen, M.J. Synergistic effect of surface modification and scaffold design of bioplotted 3-D poly-ε-caprolactone scaffolds in osteogenic tissue engineering. Acta Biomater. 2013, 9, 7699–7708. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, J.; Chen, Y.; Song, D.; Gao, Z.; Zhang, X.; Bai, Y.; Saris, P.E.; Feng, H.; Xu, H. Design of antibacterial biointerfaces by surface modification of poly (ε-caprolactone) with fusion protein containing hydrophobin and PA-1. Colloids Surf. B Biointerfaces 2017, 151, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Can-Herrera, L.; Ávila-Ortega, A.; de la Rosa-García, S.; Oliva, A.; Cauich-Rodríguez, J.; Cervantes-Uc, J. Surface modification of electrospun polycaprolactone microfibers by air plasma treatment: Effect of plasma power and treatment time. Eur. Polym. J. 2016, 84, 502–513. [Google Scholar] [CrossRef]
- Pulyala, P.; Singh, A.; Dias-Netipanyj, M.F.; Cogo, S.C.; Santos, L.S.; Soares, P.; Gopal, V.; Suganthan, V.; Manivasagam, G.; Popat, K.C. In-Vitro cell adhesion and proliferation of adipose derived stem cell on hydroxyapatite composite surfaces. Mater. Sci. Eng. C 2017, 75, 1305–1316. [Google Scholar] [CrossRef]
- Fang, J.; Li, P.; Lu, X.; Fang, L.; Lü, X.; Ren, F. A strong, tough, and osteoconductive hydroxyapatite mineralized polyacrylamide/dextran hydrogel for bone tissue regeneration. Acta Biomater. 2019, 88, 503–513. [Google Scholar] [CrossRef]
- Dai, C.; Li, Y.; Pan, W.; Wang, G.; Huang, R.; Bu, Y.; Liao, X.; Guo, K.; Gao, F. Three-dimensional high-porosity chitosan/honeycomb porous carbon/hydroxyapatite scaffold with enhanced osteoinductivity for bone regeneration. ACS Biomater. Sci. Eng. 2019, 6, 575–586. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Turng, L.-S. Comparison between PCL/hydroxyapatite (HA) and PCL/halloysite nanotube (HNT) composite scaffolds prepared by co-extrusion and gas foaming. Mater. Sci. Eng. C 2017, 72, 53–61. [Google Scholar] [CrossRef]
- Milovac, D.; Gamboa-Martínez, T.C.; Ivankovic, M.; Ferrer, G.G.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: In Vitro cell culture studies. Mater. Sci. Eng. C 2014, 42, 264–272. [Google Scholar] [CrossRef]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef]
- Liu, W.; Zhan, J.; Su, Y.; Wu, T.; Wu, C.; Ramakrishna, S.; Mo, X.; Al-Deyab, S.S.; El-Newehy, M. Effects of plasma treatment to nanofibers on initial cell adhesion and cell morphology. Colloids Surf. B Biointerfaces 2014, 113, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Arolkar, G.; Salgo, M.; Kelkar-Mane, V.; Deshmukh, R. The study of air-plasma treatment on corn starch/poly (ε-caprolactone) films. Polym. Degrad. Stab. 2015, 120, 262–272. [Google Scholar] [CrossRef]
- Cheng, Q.; Lee, B.L.-P.; Komvopoulos, K.; Yan, Z.; Li, S. Plasma surface chemical treatment of electrospun poly (L-lactide) microfibrous scaffolds for enhanced cell adhesion, growth, and infiltration. Tissue Eng. Part A 2013, 19, 1188–1198. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jung, S.-C.; Kook, M.-S.; Kim, B.-H. In Vitro study of 3D PLGA/n-HAp/β-TCP composite scaffolds with etched oxygen plasma surface modification in bone tissue engineering. Appl. Surf. Sci. 2016, 388, 321–330. [Google Scholar] [CrossRef]
- Yeo, A.; Wong, W.J.; Teoh, S.H. Surface modification of PCL-TCP scaffolds in rabbit calvaria defects: Evaluation of scaffold degradation profile, biomechanical properties and bone healing patterns. J. Biomed. Mater. Res. Part A 2010, 93, 1358–1367. [Google Scholar] [CrossRef]
- Khanna-Jain, R.; Mannerström, B.; Vuorinen, A.; Sándor, G.K.; Suuronen, R.; Miettinen, S. Osteogenic differentiation of human dental pulp stem cells on β-tricalcium phosphate/poly (l-lactic acid/caprolactone) three-dimensional scaffolds. J. Tissue Eng. 2012, 3, 2041731412467998. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhang, F.; Wang, L.; Chen, B.; Reynolds, M.A.; Ma, J.; Schneider, A.; Gu, N.; Xu, H.H. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 423–433. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kwon, J.S.; Park, S.H.; Park, J.H.; Jang, S.H.; Yin, X.Y.; Yun, J.-H.; Kim, J.H.; Min, B.H.; Lee, J.H. A computer-designed scaffold for bone regeneration within cranial defect using human dental pulp stem cells. Sci. Rep. 2015, 5, 12721. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Singh, A.K.; Kar, N.; Dravid, A.; Bellare, J. Modelling and optimization of NaOH-etched 3-D printed PCL for enhanced cellular attachment and growth with minimal loss of mechanical strength. Mater. Sci. Eng. C 2019, 98, 602–611. [Google Scholar] [CrossRef]
- Tapia-Lopez, L.V.; Esparza-Ponce, H.E.; Luna-Velasco, A.; Garcia-Casillas, P.E.; Castro-Carmona, H.; Castro, J.S. Bioactivation of zirconia surface with laminin protein coating via plasma etching and chemical modification. Surf. Coat. Technol. 2020, 402, 126307. [Google Scholar] [CrossRef]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. Part A 2003, 67, 531–537. [Google Scholar] [CrossRef]
- Jaidev, L.; Chatterjee, K. Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mater. Des. 2019, 161, 44–54. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Roh, H.-S.; Lee, C.-M.; Hwang, Y.-H.; Kook, M.-S.; Yang, S.-W.; Lee, D.; Kim, B.-H. Addition of MgO nanoparticles and plasma surface treatment of three-dimensional printed polycaprolactone/hydroxyapatite scaffolds for improving bone regeneration. Mater. Sci. Eng. C 2017, 74, 525–535. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, J.E.; Han, J.; Jeong, S.; Lim, J.W.; Lee, M.C.; Son, H.; Kim, H.B.; Choung, Y.-H.; Seonwoo, H.; et al. 3D-Printed Poly(ε-Caprolactone)/Hydroxyapatite Scaffolds Modified with Alkaline Hydrolysis Enhance Osteogenesis In Vitro. Polymers 2021, 13, 257. https://doi.org/10.3390/polym13020257
Park S, Kim JE, Han J, Jeong S, Lim JW, Lee MC, Son H, Kim HB, Choung Y-H, Seonwoo H, et al. 3D-Printed Poly(ε-Caprolactone)/Hydroxyapatite Scaffolds Modified with Alkaline Hydrolysis Enhance Osteogenesis In Vitro. Polymers. 2021; 13(2):257. https://doi.org/10.3390/polym13020257
Chicago/Turabian StylePark, Sangbae, Jae Eun Kim, Jinsub Han, Seung Jeong, Jae Woon Lim, Myung Chul Lee, Hyunmok Son, Hong Bae Kim, Yun-Hoon Choung, Hoon Seonwoo, and et al. 2021. "3D-Printed Poly(ε-Caprolactone)/Hydroxyapatite Scaffolds Modified with Alkaline Hydrolysis Enhance Osteogenesis In Vitro" Polymers 13, no. 2: 257. https://doi.org/10.3390/polym13020257
APA StylePark, S., Kim, J. E., Han, J., Jeong, S., Lim, J. W., Lee, M. C., Son, H., Kim, H. B., Choung, Y.-H., Seonwoo, H., Chung, J. H., & Jang, K.-J. (2021). 3D-Printed Poly(ε-Caprolactone)/Hydroxyapatite Scaffolds Modified with Alkaline Hydrolysis Enhance Osteogenesis In Vitro. Polymers, 13(2), 257. https://doi.org/10.3390/polym13020257






