Kinetic and Thermal Study of Ethylene and Propylene Homo Polymerization Catalyzed by ansa-Zirconocene Activated with Alkylaluminum/Borate: Effects of Alkylaluminum on Polymerization Kinetics and Polymer Structure
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Polymerization
2.3. Characterization of Polymer
2.3.1. Nuclear Magnetic Resonance Spectroscopy (1H-NMR and 13C-NMR)
2.3.2. Gel Permeation Chromatography (GPC)
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Sulfur Content of the Quench-Labeled Samples
3. Results and Discussion
3.1. Ethylene Polymerization: Activity and Polymer Structure
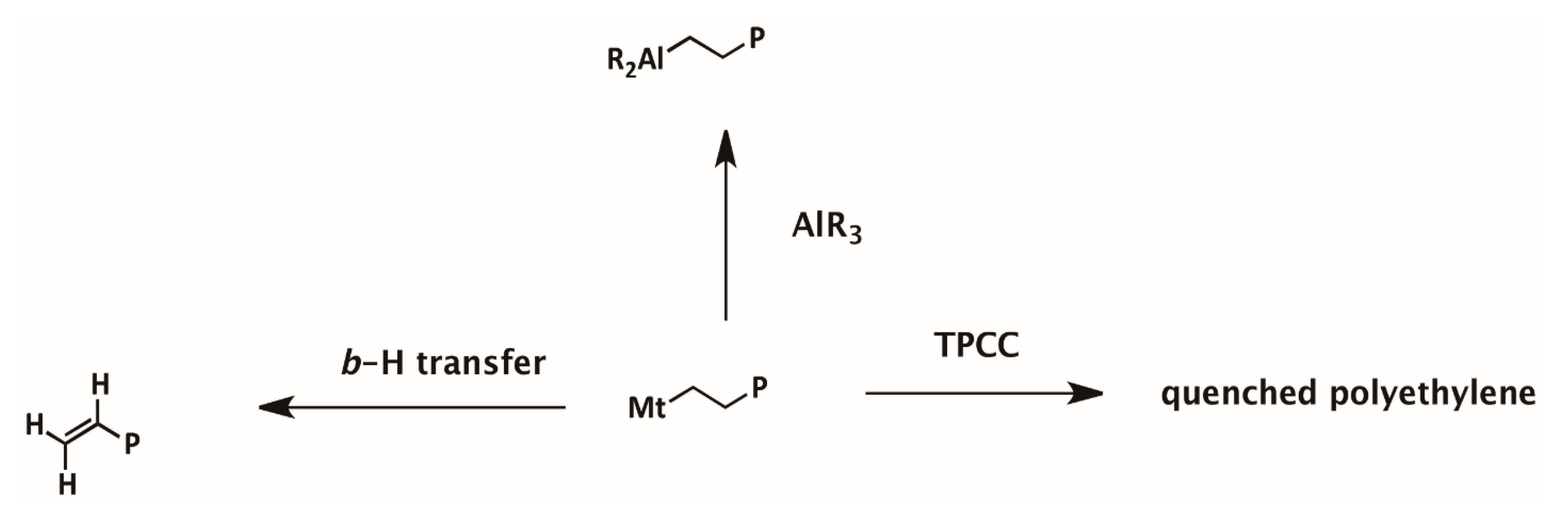
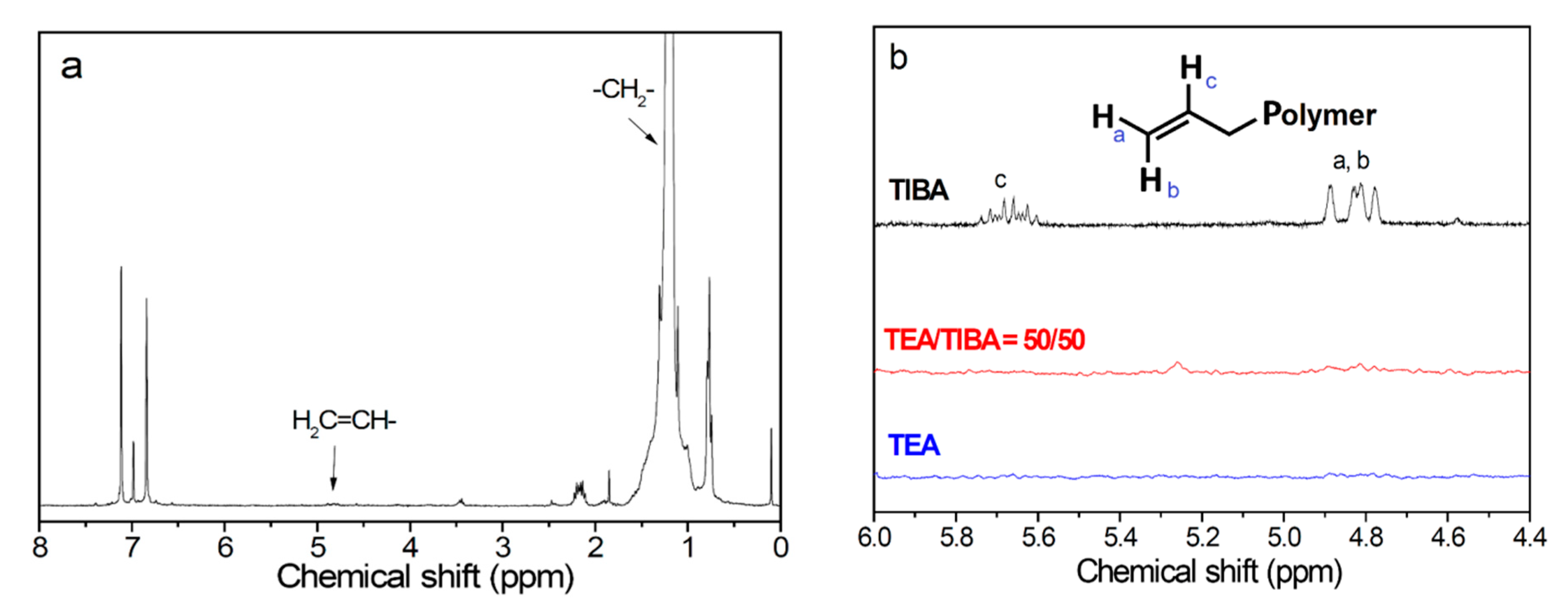
3.2. Ethylene Polymerization: Chain Transfer Reactions
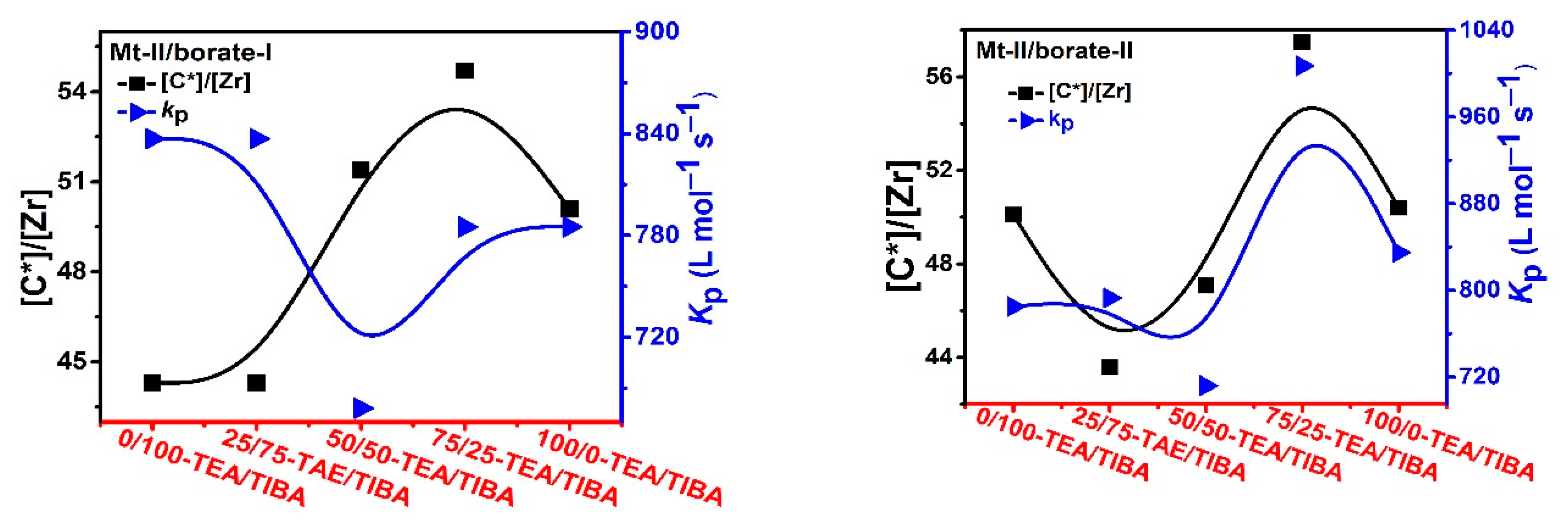
3.3. Ethylene Polymerization: Active Center Fraction and Mechanism
3.4. Propylene Polymerization: Active Center Fraction and Mechanism
4. Further Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for metal-catalyzed olefin polymerization: Activators, activation processes, and structure—Activity relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef] [PubMed]
- Wondimagegn, T.; Wang, D.; Razavi, A.; Ziegler, T. Computational design of C2-symmetric metallocene-based catalysts for the synthesis of high molecular weight polymers from ethylene/propylene copolymerization. Organometallics 2008, 27, 6434–6439. [Google Scholar] [CrossRef]
- Sarzotti, D.M.; Marshman, D.J.; Ripmeester, W.E.; Soares, J.B. A kinetic study of metallocene-catalyzed ethylene polymerization using different aluminoxane cocatalysts. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1677–1690. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene. Polymers 2020, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.E.; Ivchenko, P.V.; Bagrov, V.V.; Okumura, Y.; Elder, M.; Churakov, A.V. Asymmetric ansa-zirconocenes containing a 2-methyl-4-aryltetrahydroindacene fragment: Synthesis, structure, and catalytic activity in propylene polymerization and copolymerization. Organometallics 2011, 30, 5744–5752. [Google Scholar] [CrossRef]
- Yano, A.; Sone, M.; Yamada, S.; Hasegawa, S.; Sato, M.; Akimoto, A. Effect of ligand structures on high temperature homo-and copolymerization of ethylene by cationic hafnocene catalysts based on tetrakis (pentafluorophenyl) borate. J. Mol. Catal. A Chem. 2000, 156, 133–141. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef]
- Motolko, K.S.; Price, J.S.; Emslie, D.J.; Jenkins, H.A.; Britten, J.F. Zirconium complexes of a rigid, dianionic pincer ligand: Alkyl cations, arene coordination, and ethylene polymerization. Organometallics 2017, 36, 3084–3093. [Google Scholar] [CrossRef]
- Lamb, J.V.; Buffet, J.C.; Turner, Z.R.; Khamnaen, T.; O’Hare, D. Metallocene Polyethylene Wax Synthesis. Macromolecules 2020, 53, 5847–5856. [Google Scholar] [CrossRef]
- Kaminsky, W. New polymers by metallocene catalysis. Macromol. Chem. Phys. 1996, 197, 3907–3945. [Google Scholar] [CrossRef]
- Kaminsky, W.; Bark, A.; Arndt, M. New polymers by homogenous zirconocene/aluminoxane catalysts. In Makromolekulare Chemie. Macromol. Symposia; Wiley: New York, NY, USA, 1991; pp. 83–93. [Google Scholar]
- Kaminsky, W. Highly active metallocene catalysts for olefin polymerization. J. Chem. Soc. Dalton Trans. 1998, 1413–1418. [Google Scholar] [CrossRef]
- Ehm, C.; Vittoria, A.; Goryunov, G.P.; Izmer, V.V.; Kononovich, D.S.; Samsonov, O.V.; Budzelaar, P.H.M.; Voskoboynikov, A.Z.; Busico, V.; Uborsky, D.V. On the limits of tuning comonomer affinity of ‘Spaleck-type’ ansa-zirconocenes in ethene/1-hexene copolymerization: A high-throughput experimentation/QSAR approach. Dalton Trans. 2020, 49, 10162–10172. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, M. Cationic group 4 metallocene complexes and their role in polymerisation catalysis: The chemistry of well defined Ziegler catalysts. J. Chem. Soc. Dalton Trans. 1996, 255–270. [Google Scholar] [CrossRef]
- Bochmann, M. The chemistry of catalyst activation: The case of group 4 polymerization catalysts. Organometallics 2010, 29, 4711–4740. [Google Scholar] [CrossRef]
- Bochmann, M.; Lancaster, S.J. Base-free cationic zirconium benzyl complexes as highly active polymerization catalysts. Organometallics 1993, 12, 633–640. [Google Scholar] [CrossRef]
- González-Pelayo, S.; Bernardo, O.; Borge, J.; López, L. Synthesis of Metallocene Analogues of the Phenethylamine and Tetrahydroisoquinoline Scaffolds via Regioselective Ring Opening of 2-Aryl-N-Sulfonyl Aziridines. Adv. Synth. Catal. 2020. [Google Scholar] [CrossRef]
- Bochmann, M. Kinetic and mechanistic aspects of metallocene polymerisation catalysts. J. Organomet. Chem. 2004, 689, 3982–3998. [Google Scholar] [CrossRef]
- Lin, S.; Tagge, C.D.; Waymouth, R.M.; Nele, M.; Collins, S.; Pinto, J. Kinetics of propylene polymerization using bis (2-phenylindenyl) zirconium dichloride/methylaluminoxane. J. Am. Chem. Soc. 2000, 122, 11275–11285. [Google Scholar] [CrossRef]
- Ghiotto, F.; Pateraki, C.; Severn, J.R.; Friederichs, N.; Bochmann, M. Rapid evaluation of catalysts and MAO activators by kinetics: What controls polymer molecular weight and activity in metallocene/MAO catalysts? Dalton Trans. 2013, 42, 9040–9048. [Google Scholar] [CrossRef] [PubMed]
- Tritto, I.; Donetti, R.; Sacchi, M.C.; Locatelli, P.; Zannoni, G. Dimethylzirconocene− Methylaluminoxane Catalyst for Olefin Polymerization: NMR Study of Reaction Equilibria. Macromolecules 1997, 30, 1247–1252. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Semikolenova, N.V.; Zakharov, V.A.; Talsi, E.P. Mechanism of dimethylzirconocene activation with methylaluminoxane: NMR monitoring of intermediates at high Al/Zr ratios. Macromol. Chem. Phys. 2000, 201, 558–567. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Semikolenova, N.V.; Yudaev, D.V.; Ystenes, M.; Rytter, E.; Zakharov, V.A.; Talsi, E.P. 1H and 13C NMR Study of the Intermediates Formed by (Cp-R)2ZrCl2 Activation with MAO and AlMe3/[CPh3][B(C6F5)4]. Correlation of Spectroscopic and Ethene Polymerization Data. Macromol. Chem. Phys. 2003, 204, 1110–1117. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Babushkin, D.E.; Talsi, E.P.; Voskoboynikov, A.Z.; Gritzo, H.; Schröder, L.; Damrau, H.-R.H.; Wieser, U.; Schaper, F.; Brintzinger, H.H. ansa-Titanocene Catalysts for α-Olefin Polymerization. Syntheses, Structures, and Reactions with Methylaluminoxane and Boron-Based Activators. Organometallics 2005, 24, 894–904. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Brintzinger, H.-H. Activation of dimethyl zirconocene by methylaluminoxane (MAO) size estimate for Me-MAO-anions by pulsed field-gradient NMR. J. Am. Chem. Soc. 2002, 124, 12869–12873. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Z.; Guo, W.; Khan, A.; Fu, Z.; Xu, J.; Fan, Z. Kinetics and mechanism of metallocene-catalyzed olefin polymerization: Comparison of ethylene, propylene homopolymerizations, and their copolymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 867–875. [Google Scholar] [CrossRef]
- Desert, X.; Carpentier, J.-F.; Kirillov, E. Quantification of active sites in single-site group 4 metal olefin polymerization catalysis. Coord. Chem. Rev. 2019, 386, 50–68. [Google Scholar] [CrossRef]
- Altaf, A.A.; Badshah, A.; Khan, N.; Marwat, S.; Ali, S. Zirconium complexes in homogeneous ethylene polymerization. J. Coord. Chem. 2011, 64, 1815–1836. [Google Scholar] [CrossRef]
- Nelsen, D.L.; Anding, B.J.; Sawicki, J.L.; Christianson, M.D.; Arriola, D.J.; Landis, C.R. Chromophore quench-labeling: An approach to quantifying catalyst speciation as demonstrated for (EBI) ZrMe2/B(C6F5)3-catalyzed polymerization of 1-hexene. ACS Catal. 2016, 6, 7398–7408. [Google Scholar] [CrossRef]
- Ali, A.; Liu, X.; Guo, Y.; Akram, M.A.; Wu, H.; Liu, W.; Khan, A.; Jiang, B.; Fu, Z.; Fan, Z. Kinetics and mechanism of ethylene and propylene polymerizations catalyzed with ansa-zirconocene activated by borate/TIBA. J. Organomet. Chem. 2020, 922, 121366. [Google Scholar] [CrossRef]
- Liu, Z.; Somsook, E.; Landis, C.R. A 2H-labeling scheme for active-site counts in metallocene-catalyzed alkene polymerization. J. Am. Chem. Soc. 2001, 123, 2915–2916. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.; Dahlmann, M.; Erker, G.; Bergander, K. Arriving at an experimental estimate of the intrinsic activation barrier of olefin insertion into the Zr− C bond of an active metallocene Ziegler catalyst. J. Am. Chem. Soc. 1998, 120, 5643–5652. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Semikolenova, N.V.; Panchenko, V.N.; Zakharov, V.A.; Brintzinger, H.H.; Talsi, E.P. Activation of rac-Me2Si (ind)2ZrCl2 by Methylalumoxane Modified by Aluminum Alkyls: An EPR Spin-Probe, 1H NMR, and Polymerization Study. Macromol. Chem. Phys. 2006, 207, 327–335. [Google Scholar] [CrossRef]
- Panin, A.; Sukhova, T.; Bravaya, N. Triisobutylaluminum as cocatalyst for zirconocenes. I. Sterically opened zirconocene/triisobutylaluminum/perfluorophenylborate as highly effective ternary catalytic system for synthesis of low molecular weight polyethylenes. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1901–1914. [Google Scholar] [CrossRef]
- Franceschini, F.C.; Tavares, T.T.d.R.; Greco, P.P.; Galland, G.B.; dos Santos, J.H.; Soares, J.B. Effects of the type and concentration of alkylaluminum cocatalysts on the molar mass of polypropylene made with in situ supported metallocene catalysts. J. Appl. Polym. Sci. 2005, 95, 1050–1055. [Google Scholar] [CrossRef]
- Dong, C.; Niu, H.; Dong, J.-Y. “Two-in-One” catalysis of broad/bimodal molecular-weight-distribution polypropylene by a combination of Ziegler–Natta and metallocene catalysts. Appl. Catal. A Gen. 2014, 484, 142–147. [Google Scholar] [CrossRef]
- Huang, R.; Liu, D.; Wang, S.; Mao, B. Preparation of spherical MgCl2 supported bis (imino) pyridyl iron (II) precatalyst for ethylene polymerization. J. Mol. Catal. A Chem. 2005, 233, 91–97. [Google Scholar] [CrossRef]
- Nejabat, G.R.; Nekoomanesh, M.; Arabi, H.; Salehi-Mobarakeh, H.; Zohuri, G.-H.; Mortazavi, M.-M.; Ahmadjo, S.; Miller, S.A. Study of Ziegler-Natta/(2-PhInd)2ZrCl2 hybrid catalysts performance in slurry propylene polymerization. Polyolefins J. 2015. [Google Scholar] [CrossRef]
- Guo, Y.; He, F.; Zhang, Z.; Khan, A.; Fu, Z.; Xu, J.; Fan, Z. Influence of trimethylaluminum on kinetics of rac-Et (Ind) 2ZrCl2/aluminoxane catalyzed ethylene polymerization. J. Organomet. Chem. 2016, 808, 109–116. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Fu, Z.; Fan, Z. Effect of internal electron donor on the active center distribution in MgCl2-supported Ziegler–Natta catalyst. Catal. Commun. 2015, 69, 147–149. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, P.; Zhang, S.; Jiang, B.; Khan, A.; Zhu, L.; Fu, Z.; Fan, Z. Study on 2-thiophenecarbonyl chloride-quenched olefin polymerization with α-diimine nickel catalysts. Iran. Polym. J. 2018, 27, 153–159. [Google Scholar] [CrossRef]
- Dubdub, I.; Al-Yaari, M. Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction. Polymers 2020, 12, 891. [Google Scholar] [CrossRef]
- He, F.; Wang, D.; Jiang, B.; Zhang, Z.; Cheng, Z.; Fu, Z.; Zhang, Q.; Fan, Z. Introducing electron-donating substituents on ligand backbone of α-diimine nickel complex and the effects on catalyst thermal stability in ethylene polymerization. Inorg. Chim. Acta 2019, 486, 704–710. [Google Scholar] [CrossRef]
- Yang, P.; Zhong, W.; Jiang, B.; Zhang, B.; Fu, Z.; Fan, Z. Determination and tracing of active and dormant propagation chains in 1-hexene polymerization with supported Ziegler-Natta catalyst. Appl. Catal. A Gen. 2020, 595, 117469. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, B.; He, F.; Fu, Z.; Xu, J.; Fan, Z. Comparative Study on Kinetics of Ethylene and Propylene Polymerizations with Supported Ziegler–Natta Catalyst: Catalyst Fragmentation Promoted by Polymer Crystalline Lamellae. Polymers 2019, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The influence of Ziegler-Natta and metallocene catalysts on polyolefin structure, properties, and processing ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Akram, M.A.; Guo, Y.; Wu, H.; Liu, W.; Khan, A.; Liu, X.; Fu, Z.; Fan, Z. Ethylene–propylene copolymerization and their terpolymerization with dienes using ansa-Zirconocene catalysts activated by borate/alkylaluminum. J. Macromol. Sci. Part A 2020, 57, 156–164. [Google Scholar] [CrossRef]
- Khan, A.; Guo, Y.; Fu, Z.; Fan, Z. Kinetics of short-duration ethylene polymerization with MgCl2-supported Ziegler-Natta catalyst: Two-stage initiation evidenced by changes in active center concentration. J. Appl. Polym. Sci. 2017, 134, 45187. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, B.; Guo, Y.; Ali, A.; Guo, W.; Fu, Z.; Fan, Z. Effects of titanium dispersion state on distribution and reactivity of active centers in propylene polymerization with MgCl2-supported Ziegler-Natta catalysts: A kinetic study based on active center counting. ChemCatChem 2020, 12, 5140–5148. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Kostjuk, S.V.; Kaputsky, F.N.; Nedorezova, P.M.; Aladyshev, A.M. Effect of Different Aluminum Alkyls on the Metallocene/Methylaluminoxane Catalyzed Polymerization of Higher α-Olefins and Styrene. Macromol. Chem. Phys. 2008, 209, 1255–1265. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, X.; Weng, Y.; Fu, Z.; He, A.; Fan, Z. Mechanistic study on comonomer effect in ethylene/1-hexene copolymerization with TiCl4/MgCl2 model Ziegler-Natta catalysts. J. Catal. 2019, 369, 324–334. [Google Scholar] [CrossRef]
- Liu, Z.; Somsook, E.; White, C.B.; Rosaaen, K.A.; Landis, C.R. Kinetics of initiation, propagation, and termination for the [rac-(C2H4(1-indenyl)2)ZrMe][MeB(C6F5)3]-catalyzed polymerization of 1-hexene. J. Am. Chem. Soc. 2001, 123, 11193–11207. [Google Scholar] [CrossRef] [PubMed]
- Sillars, D.R.; Landis, C.R. Catalytic propene polymerization: Determination of propagation, termination, and epimerization kinetics by direct NMR observation of the (EBI)Zr(MeB(C6F5)3) Propenyl catalyst species. J. Am. Chem. Soc. 2003, 125, 9894–9895. [Google Scholar] [CrossRef] [PubMed]
- Valencia López, L.A.; Enríquez-Medrano, F.J.; Maldonado Textle, H.; Soriano Corral, F.; López González, H.R.; St Thomas, C.; Hernández Gámez, F.; Olivares Romero, J.L.; Díaz de León Gómez, R.E. The Influence of co-catalyst in the Polymerization of 1, 3-butadiene Catalyzed by Neodymium Chloride Tripentanolate. J. Mex. Chem. Soc. 2016, 60, 141–147. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Q.; Fu, Z.; Fan, Z. Improving microisotacticity of Ziegler–Natta catalyzed polypropylene by using triethylaluminum/triisobutylaluminum mixtures as cocatalyst. Polymer 2014, 55, 4865–4872. [Google Scholar] [CrossRef]
- Spaleck, W.; Kueber, F.; Winter, A.; Rohrmann, J.; Bachmann, B.; Antberg, M.; Dolle, V.; Paulus, E.F. The influence of aromatic substituents on the polymerization behavior of bridged zirconocene catalysts. Organometallics 1994, 13, 954–963. [Google Scholar] [CrossRef]
- Carvill, A.; Tritto, I.; Locatelli, P.; Sacchi, M.C. Polymer microstructure as a probe into hydrogen activation effect in ansa-zirconocene/methylaluminoxane catalyzed propene polymerizations. Macromolecules 1997, 30, 7056–7062. [Google Scholar] [CrossRef]
- Huang, J.; Rempel, G.L. Kinetic study of propylene polymerization using Et (H4Ind) 2ZrCl2/methylalumoxane catalysts. Ind. Eng. Chem. Res. 1997, 36, 1151–1157. [Google Scholar] [CrossRef]
- Estrada, J.V.; Hamielec, A. Modelling of ethylene polymerization with Cp2ZrCl2MAO catalyst. Polymer 1994, 35, 808–818. [Google Scholar] [CrossRef]


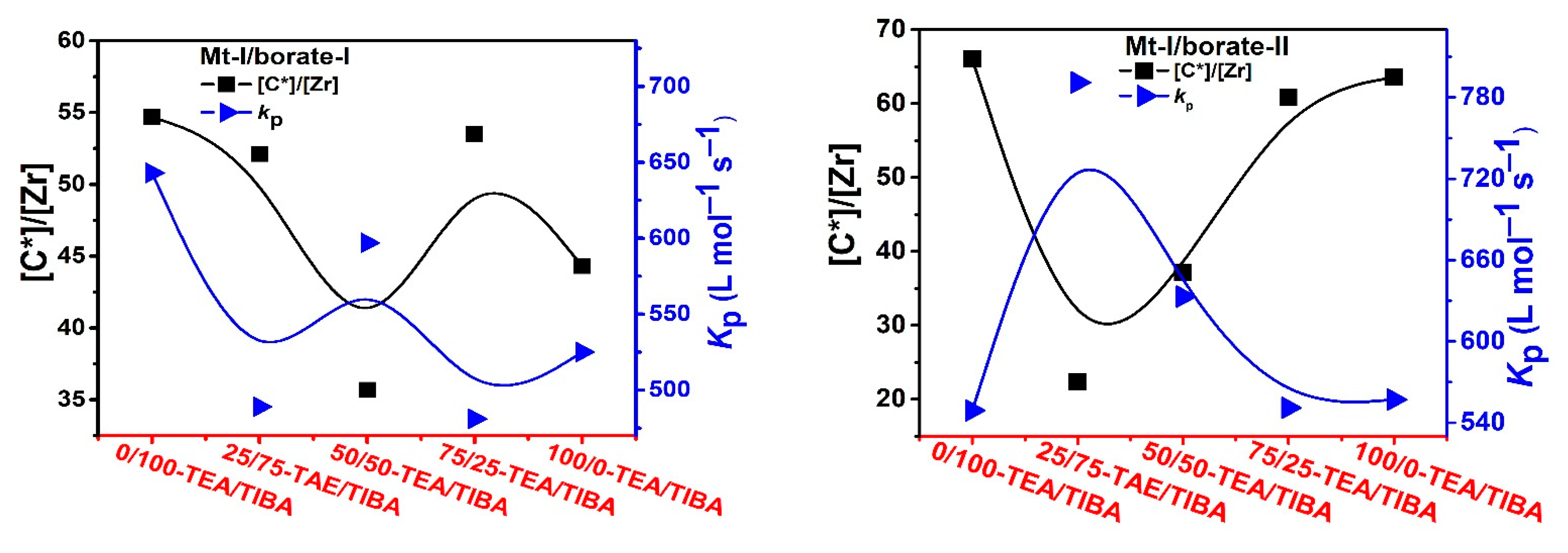
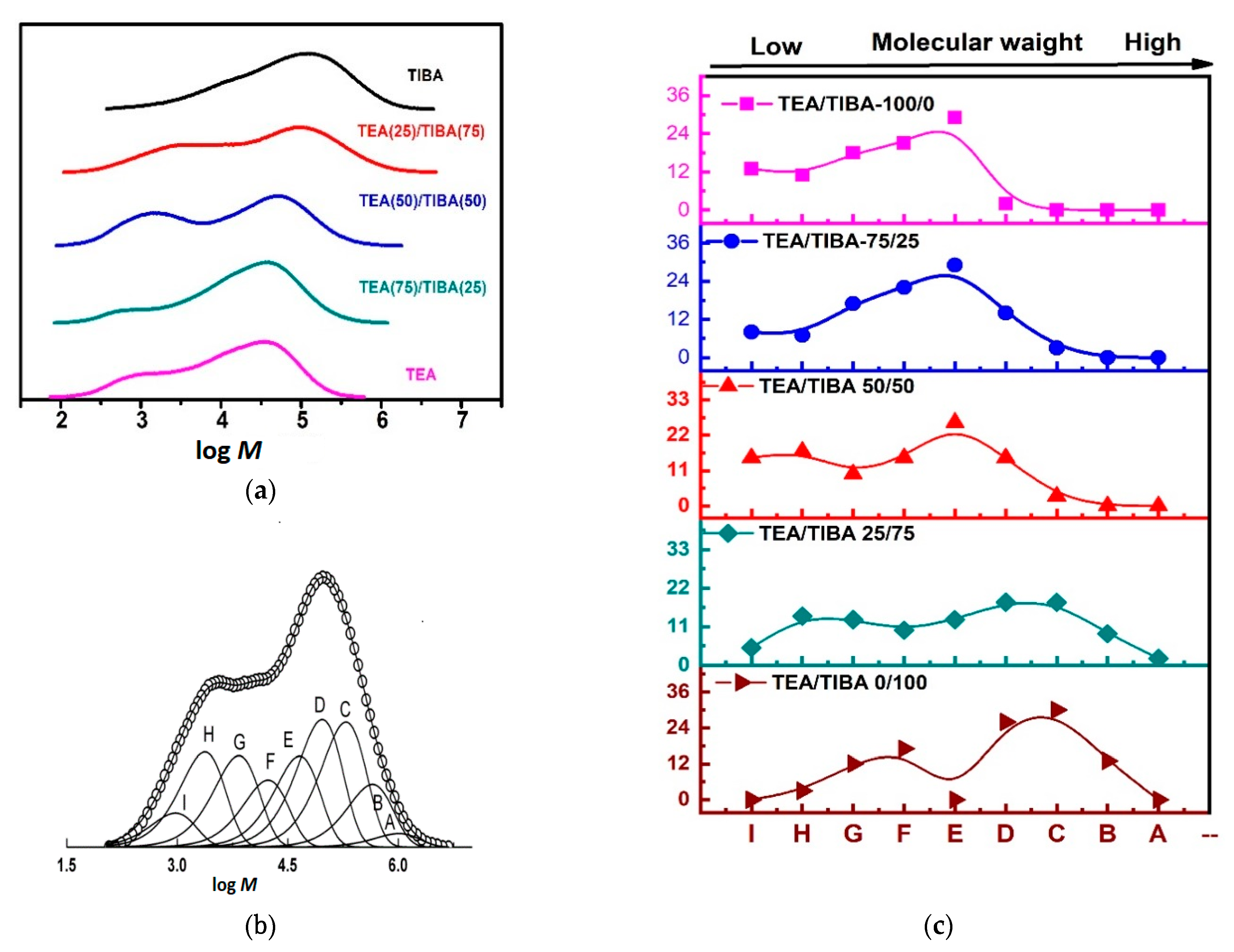
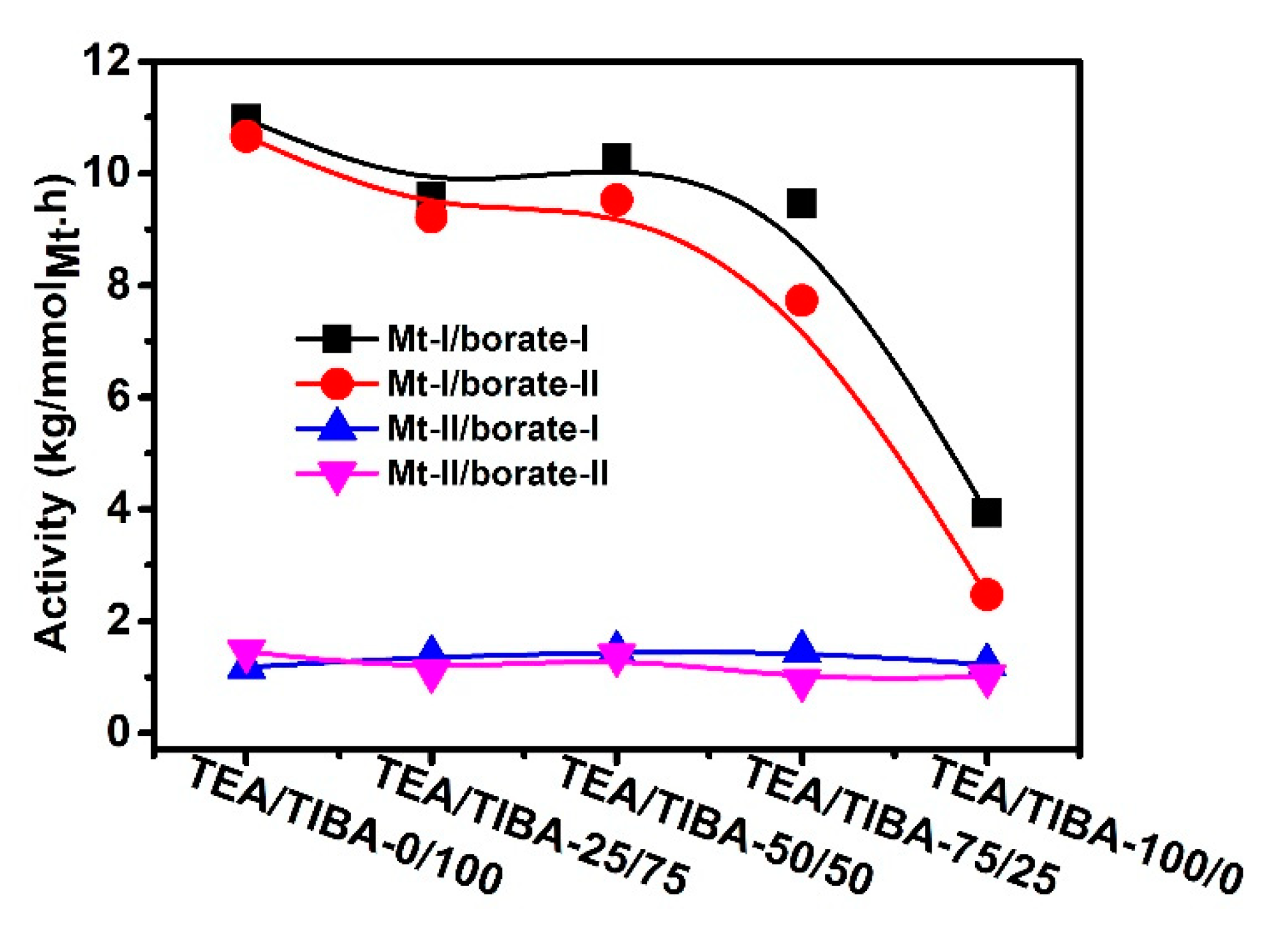

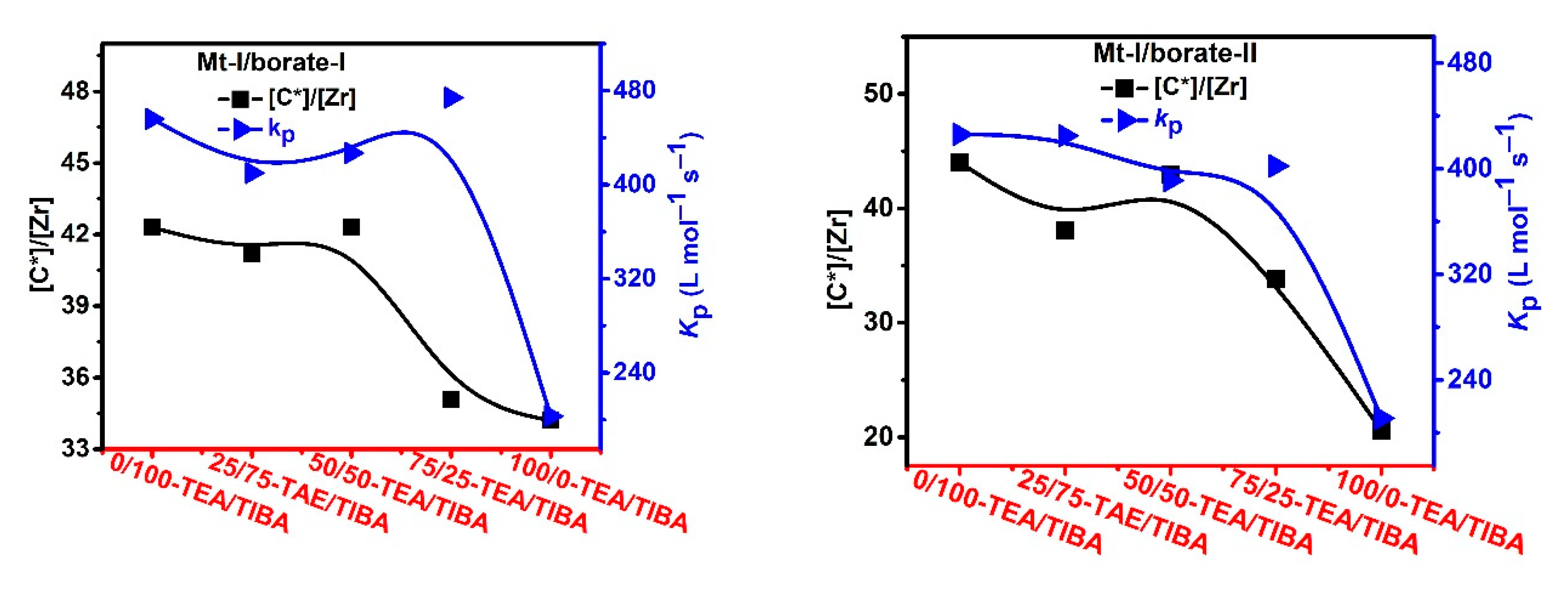

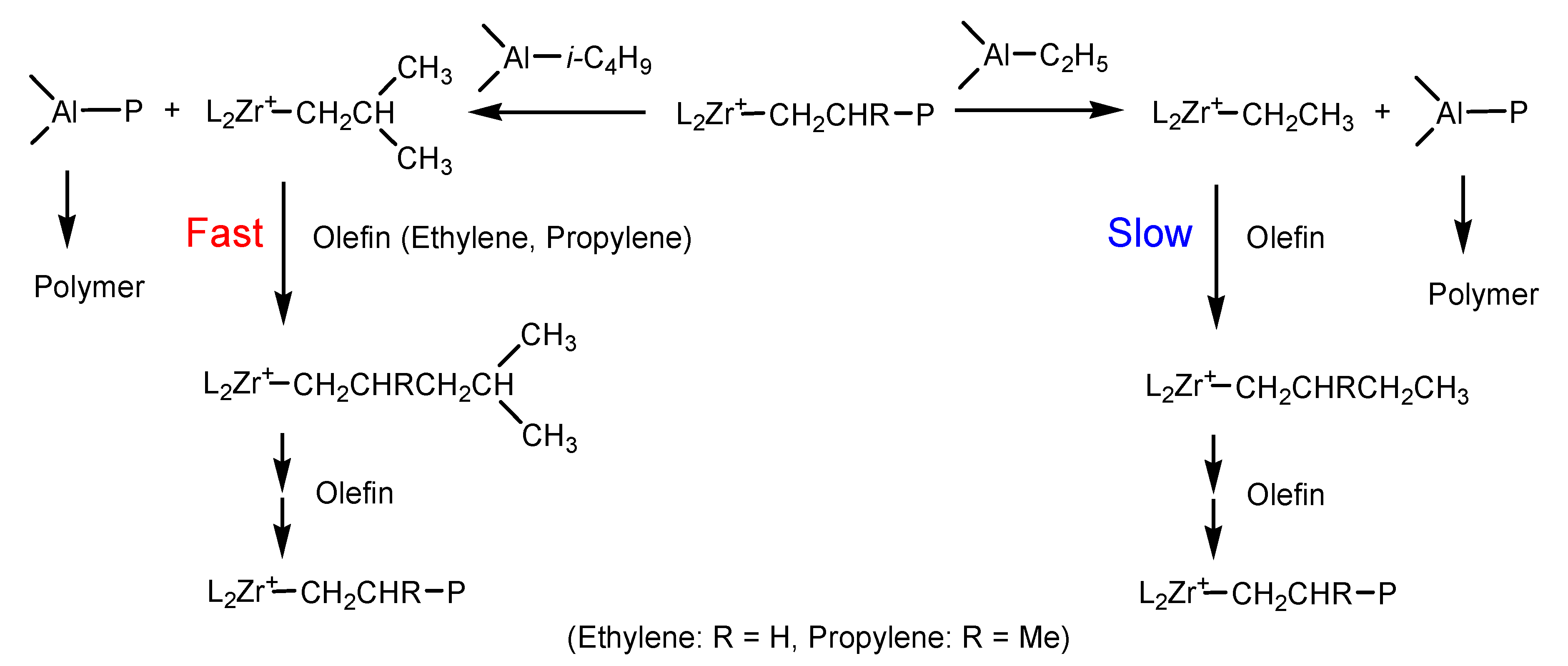
| Run | Borate | Triethylaluminium (TEA)/Triisobutylaluminium (TIBA) (mol/mol) | Activity (kg/mmolMt·h) | [C*]/[Zr] (%) | kp (L/mol·s) | Mw b (kg/mol) | Ɖ b | Tmc (°C) | ΔHm c (J/g) |
|---|---|---|---|---|---|---|---|---|---|
| 1.1 | I | 0/100 | 3.05 | 54.7 | 643 | 163 | 10.3 | 126 | 179 |
| 1.2 | I | 25/75 | 2.21 | 52.1 | 489 | 113 | 24.2 | 130 | 196 |
| 1.3 | I | 50/50 | 1.85 | 35.7 | 597 | 48 | 18.0 | 130 | 203 |
| 1.4 | I | 75/25 | 2.23 | 53.5 | 481 | 44 | 10.9 | 131 | 206 |
| 1.5 | I | 100/0 | 2.02 | 44.3 | 525 | 31 | 10.2 | 120 | 106 |
| 1.6 | II | 0/100 | 3.14 | 66.0 | 549 | n.d | n.d | 130 | 209 |
| 1.7 | II | 25/75 | 1.54 | 22.4 | 791 | n.d | n.d | 128 | 223 |
| 1.8 | II | 50/50 | 2.04 | 37.2 | 633 | n.d | n.d | 128 | 201 |
| 1.9 | II | 75/20 | 2.90 | 60.8 | 551 | n.d | n.d | 129 | 225 |
| 1.10 | II | 100/0 | 3.07 | 63.6 | 557 | n.d | n.d | 120 | 116 |
| Run | Borate | TEA/TIBA (mol/mol) | Activity (kg/mmolMt·h) | [C*]/[Zr] (%) | kp (L/mol·s) | Mwb (kg/mol) | Ɖ b | Tmc (°C) | ΔHm c (J/g) |
|---|---|---|---|---|---|---|---|---|---|
| 2.1 | I | 25/75 | 3.22 | 44.3 | 837 | 2.4 | 2.2 | 122 | 214 |
| 2.2 | I | 25/75 | 3.22 | 44.3 | 837 | 2.4 | 2.2 | 122 | 214 |
| 2.3 | I | 50/50 | 3.02 | 51.4 | 678 | 8.2 | 3.3 | 106 | 54 |
| 2.4 | I | 75/25 | 3.72 | 54.7 | 785 | 2.6 | 2.2 | 123 | 212 |
| 2.5 | I | 100/0 | 3.41 | 50.1 | 785 | 2.9 | 2.5 | 101 | 156 |
| 2.6 | II | 0/100 | 3.00 | 43.6 | 793 | n.d | n.d | 122 | 226 |
| 2.7 | II | 25/75 | 2.90 | 47.1 | 712 | n.d | n.d | 122 | 170 |
| 2.8 | II | 50/50 | 5.02 | 57.5 | 1007 | n.d | n.d | 117 | 157 |
| 2.9 | II | 75/20 | 3.65 | 50.4 | 835 | n.d | n.d | 123 | 207 |
| 2.10 | II | 100/0 | 3.10 | 49.4 | 722 | n.d | n.d | 101 | 158 |
| Run | Borate | TEA/TIBA (mol/mol) | Activity (kg/mmolMt·h) | [C*]/[Zr] (%) | kp (L/mol·s) | Mwb (kg/mol) | Ɖ b | Tmc (°C) | ΔHm c (J/g) |
|---|---|---|---|---|---|---|---|---|---|
| 3.1 | I | 0/100 | 10.97 | 42.3 | 456 | 59.6 | 4.5 | 158 | 75 |
| 3.2 | I | 25/75 | 9.60 | 41.2 | 410 | 33.6 | 3.5 | 158 | 83 |
| 3.3 | I | 50/50 | 10.27 | 42.3 | 427 | n.d | n.d | 158 | 97 |
| 3.4 | I | 75/25 | 9.46 | 35.1 | 474 | n.d | n.d | 157 | 106 |
| 3.5 | I | 100/0 | 3.94 | 34.2 | 203 | 12.6 | 2.4 | 158 | 104 |
| 3.6 | II | 0/100 | 10.66 | 44.0 | 426 | n.d | n.d | 158 | 62 |
| 3.7 | II | 25/75 | 9.22 | 38.1 | 425 | n.d | n.d | 158 | 89 |
| 3.8 | II | 50/50 | 9.53 | 42.9 | 391 | n.d | n.d | 158 | 109 |
| 3.9 | II | 75/20 | 7.73 | 33.8 | 402 | n.d | n.d | 158 | 112 |
| 3.10 | II | 100/0 | 2.47 | 20.6 | 211 | n.d | n.d | 159 | 122 |
| Run | Borate | TEA/TIBA (mol/mol) | Activity (kg/mmolMt·h) | [C*]/[Zr] (%) | kp (L/mol·s) | Mwb (kg/mol) | Ɖ b | Tmc (°C) | ΔHmc (J/g) |
|---|---|---|---|---|---|---|---|---|---|
| 4.1 | I | 0/100 | 1.18 | 17.5 | 118 | 59.6 | 4.5 | 122 | 51 |
| 4.2 | I | 25/75 | 1.37 | 21.5 | 112 | 34.4 | 3.4 | 115 | 51 |
| 4.3 | I | 50/50 | 1.44 | 22.5 | 113 | 16.1 | 2.8 | 91 | 51 |
| 4.4 | I | 75/25 | 1.46 | 21.2 | 122 | n.d | n.d | 115 | 56 |
| 4.5 | I | 100/0 | 1.22 | 19.8 | 109 | 12.7 | 2.2 | 106 | 49 |
| 4.6 | II | 0/100 | 1.46 | 18.8 | 137 | n.d | n.d | 122 | 57 |
| 4.7 | II | 25/75 | 1.10 | 17.5 | 111 | n.d | n.d | 116 | 48 |
| 4.8 | II | 50/50 | 1.39 | 19.9 | 123 | n.d | n.d | 92 | 52 |
| 4.9 | II | 75/20 | 0.94 | 12.7 | 130 | n.d | n.d | 115 | 54 |
| 4.0 | II | 100/0 | 1.01 | 17.4 | 102 | n.d | n.d | 106 | 47 |
| M | Cocatalyst | a Activit | [C*]/[Zr] (%) | kp(L/mol·s) | Mwb (kg/mol) | Ɖ b | Tmc (°C) | ΔHm c (J/g) |
|---|---|---|---|---|---|---|---|---|
| E | Methylaluminoxane (MAO) | 3.37 | 40.4 | 592 | 272 | 3.1 | 134.9 | 76.9 |
| E | Methylaluminoxanes (MMAO) | 2.16 | 67.8 | 330 | 195 | 3.0 | n.d | n.d |
| E | dMAO | 5.2 | 45.5 | 804 | 290 | 3.6 | n.d | n.d |
| E | TIBA-borate-I | 3.24 | 63.3 | 591 | 2.8 | 2.2 | 122 | 214 |
| E | TEA borate-I | 3.41 | 50.1 | 785 | 2.9 | 2.5 | 101 | 156 |
| E | TIBA-borate-II | 3.00 | 43.6 | 793 | n.d | n.d | 122 | 226 |
| E | TEA borate-II | 3.10 | 49.4 | 722 | n.d | n.d | 101 | 158 |
| P | MMAO | 3.8 | 24.3 | 156 | 15.2 | 1.8 | n.d | n.d |
| P | TIBA-borate-I | 1.18 | 17.5 | 118 | 59.6 | 4.5 | 122 | 51 |
| p | TIBA borate-I | 1.22 | 19.8 | 109 | 12.7 | 2.2 | 106 | 49 |
| p | TEA-borate-II | 1.46 | 18.8 | 137 | n.d | n.d | 122 | 57 |
| p | TIBA borate-II | 1.01 | 17.4 | 102 | n.d | n.d | 106 | 47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Muhammad, N.; Hussain, S.; Jamil, M.I.; Uddin, A.; Aziz, T.; Tufail, M.K.; Guo, Y.; Wei, T.; Rasool, G.; et al. Kinetic and Thermal Study of Ethylene and Propylene Homo Polymerization Catalyzed by ansa-Zirconocene Activated with Alkylaluminum/Borate: Effects of Alkylaluminum on Polymerization Kinetics and Polymer Structure. Polymers 2021, 13, 268. https://doi.org/10.3390/polym13020268
Ali A, Muhammad N, Hussain S, Jamil MI, Uddin A, Aziz T, Tufail MK, Guo Y, Wei T, Rasool G, et al. Kinetic and Thermal Study of Ethylene and Propylene Homo Polymerization Catalyzed by ansa-Zirconocene Activated with Alkylaluminum/Borate: Effects of Alkylaluminum on Polymerization Kinetics and Polymer Structure. Polymers. 2021; 13(2):268. https://doi.org/10.3390/polym13020268
Chicago/Turabian StyleAli, Amjad, Nadeem Muhammad, Shahid Hussain, Muhammad Imran Jamil, Azim Uddin, Tariq Aziz, Muhammad Khurram Tufail, Yintian Guo, Tiantian Wei, Ghulam Rasool, and et al. 2021. "Kinetic and Thermal Study of Ethylene and Propylene Homo Polymerization Catalyzed by ansa-Zirconocene Activated with Alkylaluminum/Borate: Effects of Alkylaluminum on Polymerization Kinetics and Polymer Structure" Polymers 13, no. 2: 268. https://doi.org/10.3390/polym13020268
APA StyleAli, A., Muhammad, N., Hussain, S., Jamil, M. I., Uddin, A., Aziz, T., Tufail, M. K., Guo, Y., Wei, T., Rasool, G., Fan, Z., & Guo, L. (2021). Kinetic and Thermal Study of Ethylene and Propylene Homo Polymerization Catalyzed by ansa-Zirconocene Activated with Alkylaluminum/Borate: Effects of Alkylaluminum on Polymerization Kinetics and Polymer Structure. Polymers, 13(2), 268. https://doi.org/10.3390/polym13020268














