Synthesis of Poly(Trimethylene Carbonate) from Amine Group Initiation: Role of Urethane Bonds in the Crystallinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Nuclear Magnetic Resonance Spectrometry (NMR)
2.2.2. Size Exclusion Chromatograms (SEC)
2.2.3. Fourier Transform Infrared Spectra (FT-IR)
2.2.4. Differential Scanning Calorimetry (DSC)
2.2.5. Thermogravimetric Analysis (ATG)
2.2.6. Mechanical Measurements
2.2.7. X-Ray Diffraction (XRD)
2.3. Synthesis
2.3.1. Typical Procedure for Polymerization of TMC Initiated by ROP-amine with Sn(Oct)2 (Same Protocol Has Been Performed with All the Amine Initiators)
2.3.2. Typical Procedure for Polymerization of TMC Initiated by ROP-Amine with TBD
3. Results and Discussion
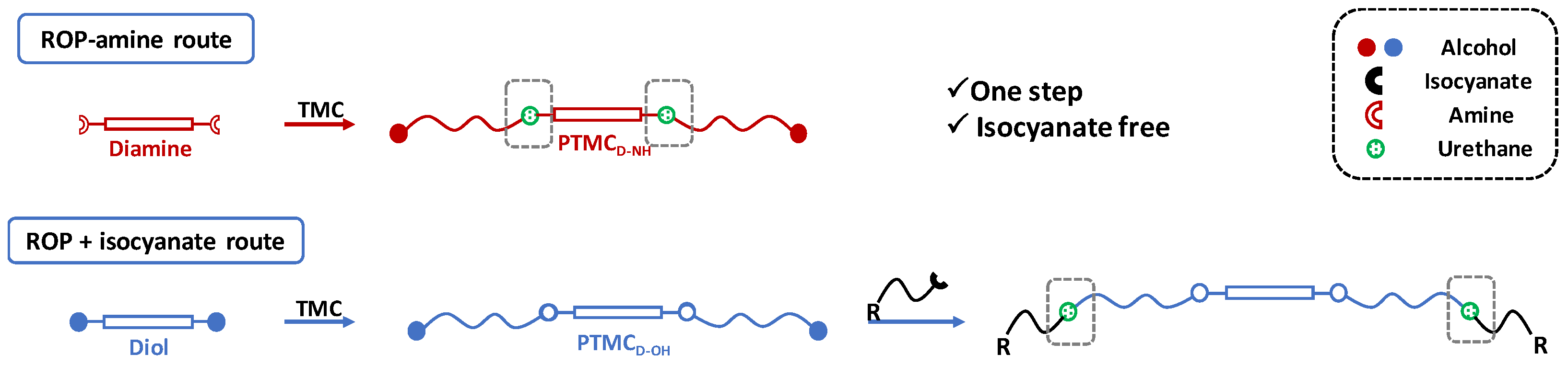
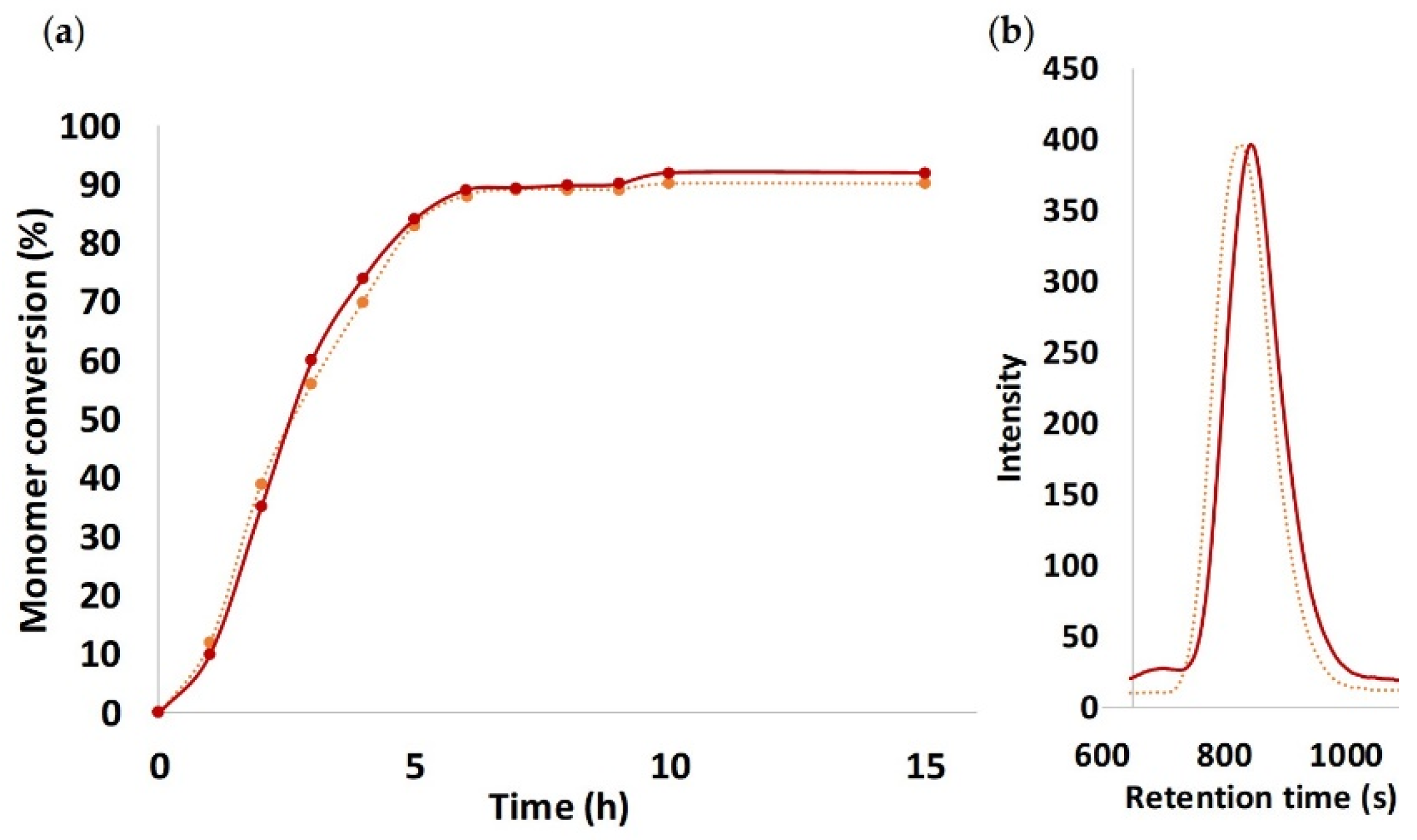
3.1. Synthesis of PTMC Homopolymer via ROP-Amine Route
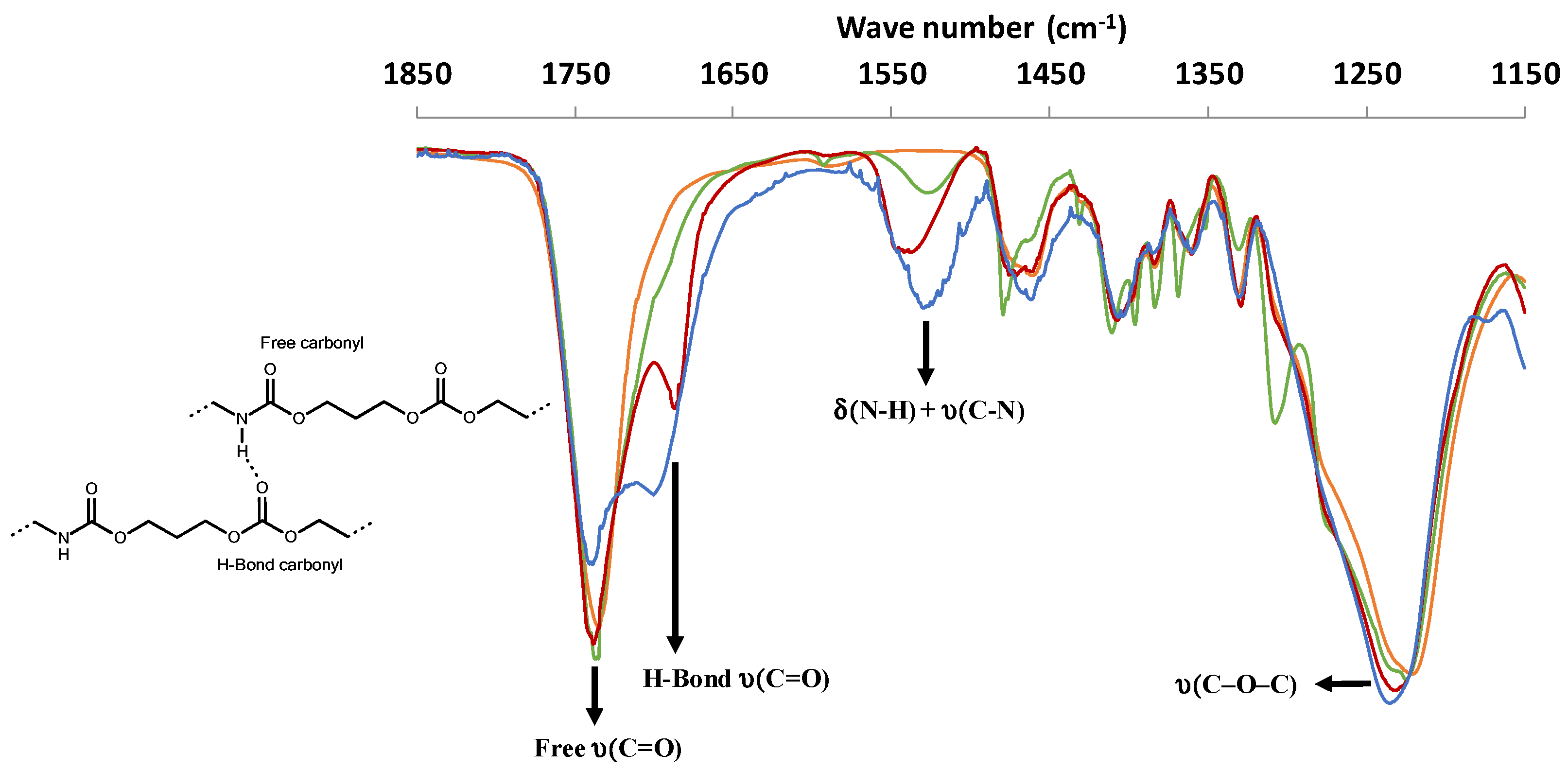
3.2. Urethane Bond Formation Determined by Infra-Red Analyses
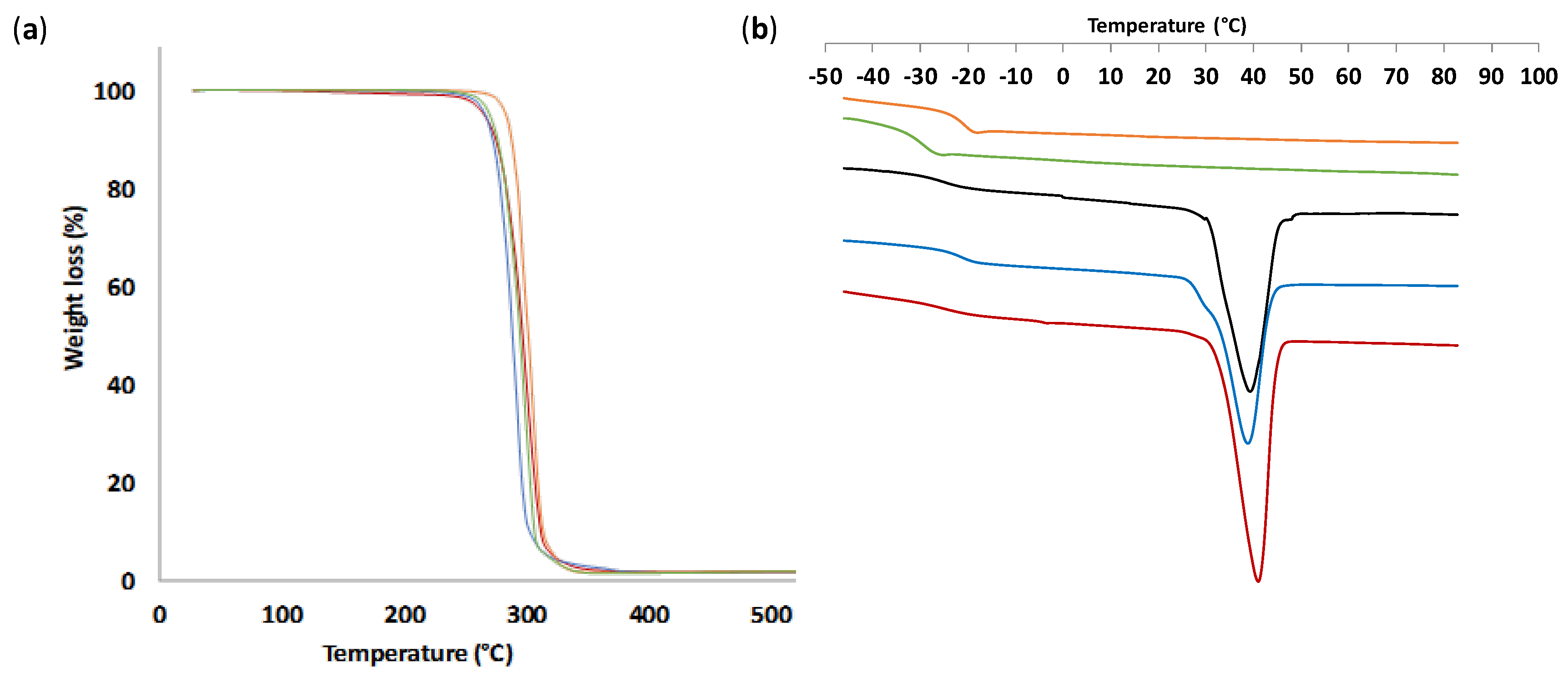
3.3. Thermal and Mechanical Properties of PTMC from ROP-Amine
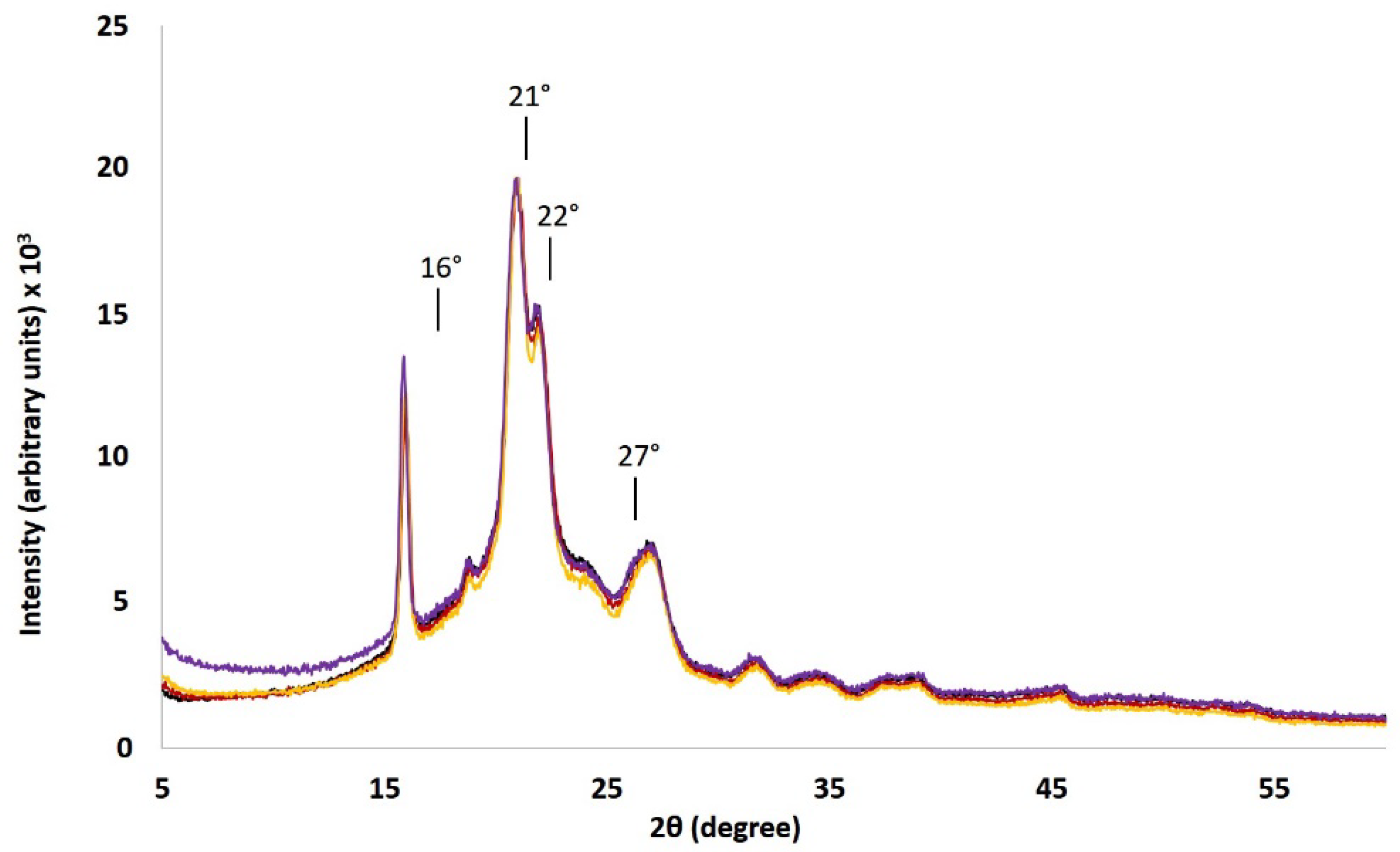
3.4. X-Ray Diffraction Analyses
3.5. Interest of Semi-Crystalline PTMC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vyner, M.C.; Li, A.; Amsden, B.G. The effect of poly(trimethylene carbonate) molecular weight on macrophage behavior and enzyme adsorption and conformation. Biomaterials 2014, 35, 9041–9048. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, S.; Vancso, G.J.; Grijpma, A.D.W.; Feijen, J. Enzymatic Surface Erosion of Poly(trimethylene carbonate) Films Studied by Atomic Force Microscopy. Biomacromolecules 2005, 6, 3404–3409. [Google Scholar] [CrossRef]
- Fukushima, K. Poly(trimethylene carbonate)-based polymers engineered for biodegradable functional biomaterials. Biomater. Sci. 2016, 4, 9–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Grijpma, D.W.; Feijen, J. Poly(trimethylene carbonate) and monomethoxy poly(ethylene glycol)-block-poly(trimethylene carbonate) nanoparticles for the controlled release of dexamethasone. J. Control. Release 2006, 111, 263–270. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.L.; Peng, J.R.; Zhang, L.; Qu, Y.; Chu, B.Y.; Dong, M.L.; Tan, L.W.; Qiana, Z.Y. Biodegradable Self-Assembled Micelles Based on MPEG-PTMC Copolymers: An Ideal Drug Delivery System for Vincristine. J. Biomed. Nanotechnol. 2017, 13, 427–436. [Google Scholar] [CrossRef]
- Blanquer, S.B.G.; Gebraad, A.W.H.; Miettinen, S.; Poot, A.A.; Grijpma, D.W.; Haimi, S.P. Differentiation of adipose stem cells seeded towards annulus fibrosus cells on a designed poly(trimethylene carbonate) scaffold prepared by stereolithography. J. Tissue Eng. Regen. Med. 2016, 11, 2752–2762. [Google Scholar] [CrossRef]
- Van Bochove, B.; Hannink, G.; Buma, P.; Grijpma, D.W. Preparation of Designed Poly(trimethylene carbonate) Meniscus Implants by Stereolithography: Challenges in Stereolithography. Macromol. Biosci. 2016, 16, 1853–1863. [Google Scholar] [CrossRef]
- Pêgo, A.P.; Grijpma, D.W.; Feijen, J. Enhanced mechanical properties of 1,3-trimethylene carbonate polymers and networks. Polymer 2003, 44, 6495–6504. [Google Scholar] [CrossRef]
- Castillo, R.V.; Fleury, G.; Navarro, C.; Couffin, A.; Bourissou, D.; Martin-Vaca, B. Impact of the architecture on the crystallization kinetics of poly(ε-caprolactone)/poly(trimethylene carbonate) block copolymers. Eur. Polym. J. 2017, 95, 711–727. [Google Scholar] [CrossRef]
- Schüller-Ravoo, S.; Feijen, J.; Grijpma, D.W. Flexible, elastic and tear-resistant networks prepared by photo-crosslinking poly(trimethylene carbonate) macromers. Acta Biomater. 2012, 8, 3576–3585. [Google Scholar] [CrossRef] [PubMed]
- Bat, E.; Kothman, B.H.M.; Higuera, G.A.; van Blitterswijk, C.A.; Feijen, J.; Grijpma, D.W. Ultraviolet light crosslinking of poly(trimethylene carbonate) for elastomeric tissue engineering scaffolds. Biomaterials 2010, 31, 8696–8705. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Li, M.; Gu, Z. The in Vitro and in Vivo Degradation of Cross-Linked Poly(trimethylene carbonate)-Based Networks. Polymers 2016, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.J.; Hendren, R.W.; Jensen, K.; Pitt, C.G. Synthesis, properties, and biodegradation of poly(1,3-trimethylene carbonate). Macromolecules 1991, 24, 1736–1740. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kojima, R. Crystal structure of poly(trimethylene carbonate). Macromolecules 2003, 36, 5139–5143. [Google Scholar] [CrossRef]
- Pêgo, A.P.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Copolymers of trimethylene carbonate and ε-caprolactone for porous nerve guides: Synthesis and properties. J. Biomater. Sci. Polym. Ed. 2001, 12, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Grijpma, D.W.; Feijen, J. Triblock Copolymers Based on 1,3-Trimethylene Carbonate and Lactide as Biodegradable Thermoplastic Elastomers. Macromol. Chem. Phys. 2004, 205, 867–875. [Google Scholar] [CrossRef]
- Li, X.; Mignard, N.; Taha, M.; Prochazka, F.; Chen, J.; Zhang, S.; Becquart, F. Thermoreversible Supramolecular Networks from Poly(trimethylene Carbonate) Synthesized by Condensation with Triuret and Tetrauret. Macromolecules 2019, 52, 6585–6599. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M.; Pikus, S.; Skrzypiec, K. The synthesis and characterization of new thermoplastic poly(carbonate-urethane) elastomers derived from HDI and aliphatic–aromatic chain extenders. Eur. Polym. J. 2009, 45, 2629–2643. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Lannutti, J.L.; Wagner, W.R.; Guan, J. Synthesis, characterization and surface modification of low moduli poly(ether carbonate urethane)ureas for soft tissue engineering. Acta Biomater. 2009, 5, 2901–2912. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, X.; Yang, J.; Wang, Y.; Zhang, Z.; Hou, Y.; Lin, F.; Li, Y. Influence of Hard Segments on the Thermal, Phase-Separated Morphology, Mechanical, and Biological Properties of Polycarbonate Urethanes. Appl. Sci. 2017, 7, 306. [Google Scholar] [CrossRef]
- Ma, Z.; Hong, Y.; Nelson, D.M.; Pichamuthu, J.E.; Leeson, C.E.; Wagner, W.R. Biodegradable Polyurethane Ureas with Variable Polyester or Polycarbonate Soft Segments: Effects of Crystallinity, Molecular Weight, and Composition on Mechanical Properties. Biomacromolecules 2011, 12, 3265–3274. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, M.; Basko, M.; Biedroń, T.; Wojtczak, E.; Michalski, A. Polymerization of lactide initiated by primary amines and catalyzed by a protic acid. Eur. Polym. J. 2015, 71, 380–388. [Google Scholar] [CrossRef]
- Kowalski, A.; Libiszowski, J.; Biela, T.; Cypryk, M.; Duda, A.; Penczek, S. Kinetics and Mechanism of Cyclic Esters Polymerization Initiated with Tin(II) Octoate. Polymerization of ε-Caprolactone and l,l-Lactide Co-initiated with Primary Amines. Macromolecules 2005, 38, 8170–8176. [Google Scholar] [CrossRef]
- Clark, L.; Cushion, M.G.; Dyer, H.E.; Schwarz, A.D.; Duchateau, R.; Mountford, P. Dicationic and zwitterionic catalysts for the amine-initiated, immortal ring-opening polymerisation of rac-lactide: Facile synthesis of amine-terminated, highly heterotactic PLA. Chem. Commun. 2010, 46, 273–275. [Google Scholar] [CrossRef]
- Alba, A.; du Boullay, O.T.; Martin-Vaca, B.; Bourissou, D. Direct ring-opening of lactide with amines: Application to the organo-catalyzed preparation of amide end-capped PLA and to the removal of residual lactide from PLA samples. Polym. Chem. 2015, 6, 989–997. [Google Scholar] [CrossRef]
- Toshikj, N.; Robin, J.-J.; Blanquer, S. A simple and general approach for the synthesis of biodegradable triblock copolymers by organocatalytic ROP from poly(lactide) macroinitiators. Eur. Polym. J. 2020, 127, 109599. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Li, Z.; Wang, X.; Dong, H.; Sun, H.; Yang, K.; Gebru, H.; Guo, K. H-bonding binary organocatalysis promoted amine-initiated ring-opening polymerizations of lactide from polysarcosine to diblock copolymers. Eur. Polym. J. 2017, 97, 389–396. [Google Scholar] [CrossRef]
- Liu, J.Y.; Liu, L.J. Ring Ppening Polymerization of ε-Caprolactone Initiated by Natural Amino Acids. Macromolecules 2004, 37, 2674–2676. [Google Scholar] [CrossRef]
- Mattia, J.; Painter, P. A Comparison of Hydrogen Bonding and Order in a Polyurethane and Poly(urethane−urea) and Their Blends with Poly(ethylene glycol). Macromolecules 2007, 40, 1546–1554. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Su, W.; Feng, J.; Wang, H.-F.; Zhang, X.-Z.; Zhuo, R.-X. Controllable preparation of poly(alkylene carbonate)s and observation on their structure-related odd-even effect. Polymer 2010, 51, 1010–1015. [Google Scholar] [CrossRef]
- Ji, L.-J.; Lai, K.-L.; He, B.; Wang, G.; Song, L.-Q.; Wu, Y.; Gu, Z.-W. Study on poly(l-lactide-co-trimethylene carbonate): Synthesis and cell compatibility of electrospun film. Biomed. Mater. 2010, 5, 045009. [Google Scholar] [CrossRef] [PubMed]
- Geven, M.A.; Sprecher, C.; Guillaume, O.; Eglin, D.; Grijpma, D.W. Micro-porous composite scaffolds of photo-crosslinked poly(trimethylene carbonate) and nano-hydroxyapatite prepared by low-temperature extrusion-based additive manufacturing. Polym. Adv. Technol. 2016, 28, 1226–1232. [Google Scholar] [CrossRef]



| Initiator Formula | Initiator Name | Abbreviation of PTMC | F1 | DPnNMR | DPnSEC | Ð3 |
|---|---|---|---|---|---|---|
 | 1,6-hexanediol | PTMCD-OH | D 2 | 93 | 110 | 1.8 |
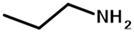 | propylamine | PTMCM-NH | M 2 | 88 | 86 | 1.9 |
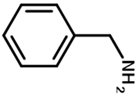 | benzylamine | PTMCM-NH-Φ | M | 88 | 86 | 1.9 |
 | 1,6-hexanediamine | PTMCD-NH | D | 102 | 86 | 1.9 |
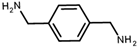 | p-xylenediamine | PTMCD-NH-Φ | D | 108 | 98 | 1.7 |
 | tris (2-aminoethyl)amine | PTMCT-NH | T 2 | 98 | 83 | 3.4 |
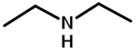 | bis(3-aminopropyl)amine | PTMCM-S-NH | Ms 2 | 94 | 108 | 1.6 |
 | L-phenylalanine methyl ester | PTMCM-AAP-NH | M | 143 | 72 | 1.6 |
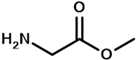 | glycine methyl ester | PTMCM-AAG-NH | M | 147 | 70 | 1.7 |
| PTMC | Tg 1 (°C) | Tm 1 (°C) | ∆Hm1 (J·g−1) | T10% 2 (°C) |
|---|---|---|---|---|
| PTMCD-OH | −20.0 | - | - | 288 |
| PTMCM-NH | −30.0 | - | - | 276 |
| PTMCD-NH | −25.0 | 40.8 | 52.5 | 275 |
| PTMCD-NH-Φ | −24.6 | 39.2 | 49.4 | 276 |
| PTMCT-NH | −22.3 | 38.7 | 37.3 | 273 |
| PTMC | DPnth | DPnNMR1 | Tg 2 (°C) | Tm 2 (°C) | ∆Hm2 (J·g−1) |
|---|---|---|---|---|---|
| PTMCD-NH | 100 | 102 | −25.0 | 40.8 | 52.5 |
| PTMCD-NH | 200 | 196 | −27.8 | 41.9 | 51.7 |
| PTMCD-NH | 500 | 460 | −26.4 | 40.7 | 48.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brossier, T.; Volpi, G.; Lapinte, V.; Blanquer, S. Synthesis of Poly(Trimethylene Carbonate) from Amine Group Initiation: Role of Urethane Bonds in the Crystallinity. Polymers 2021, 13, 280. https://doi.org/10.3390/polym13020280
Brossier T, Volpi G, Lapinte V, Blanquer S. Synthesis of Poly(Trimethylene Carbonate) from Amine Group Initiation: Role of Urethane Bonds in the Crystallinity. Polymers. 2021; 13(2):280. https://doi.org/10.3390/polym13020280
Chicago/Turabian StyleBrossier, Thomas, Gael Volpi, Vincent Lapinte, and Sebastien Blanquer. 2021. "Synthesis of Poly(Trimethylene Carbonate) from Amine Group Initiation: Role of Urethane Bonds in the Crystallinity" Polymers 13, no. 2: 280. https://doi.org/10.3390/polym13020280
APA StyleBrossier, T., Volpi, G., Lapinte, V., & Blanquer, S. (2021). Synthesis of Poly(Trimethylene Carbonate) from Amine Group Initiation: Role of Urethane Bonds in the Crystallinity. Polymers, 13(2), 280. https://doi.org/10.3390/polym13020280







