Polylactide, Processed by a Foaming Method Using Compressed Freon R134a, for Tissue Engineering
Abstract
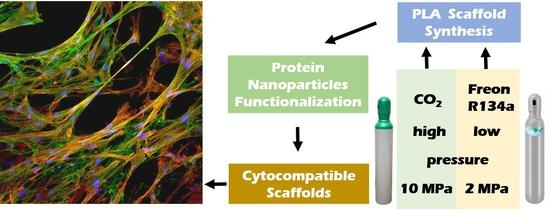
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polymer Disks
2.2.2. 3D Porous Scaffold Fabrication Using Compressed Fluids
2.2.3. Porous Scaffold Characterization
Solid Density and Porosity
Morphology by Scanning Electron Microscopy (SEM)
Micro X-ray Computed Tomography
Rheological Properties
2.2.4. pNPs Production and Purification
2.2.5. Porous Scaffold Functionalization with pNPs
2.2.6. Cell Culture and Viability Assay
2.2.7. Immunofluorescence Assays
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation of 3D Porous Scaffolds
3.2. 3D Porous Scaffold Characterization
3.3. Surface Functionalization of 3D Porous Scaffolds with GPF-Based pNPs
3.4. Cytocompatibility of 3D Porous Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 31. [Google Scholar] [CrossRef]
- Kanczler, J.; Oreffo, R. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cells Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Kanczler, J.; Ginty, P.J.; Barry, J.J.; Clarke, N.M.; Howdle, S.M.; Shakesheff, K.; Oreffo, R.O. The effect of mesenchymal populations and vascular endothelial growth factor delivered from biodegradable polymer scaffolds on bone formation. Biomaterials 2008, 29, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 922–939. [Google Scholar] [CrossRef]
- Guo, J.L.; Kim, Y.S.; Mikos, A.G. Biomacromolecules for Tissue Engineering: Emerging Biomimetic Strategies. Biomacromolecules 2019, 20, 2904–2912. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, E.; Kiran, E. Foaming of polymers with supercritical fluids and perspectives on the current knowledge gaps and challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Iglesias-Mejuto, A.; García-González, C. Solvent-Free Approaches for the Processing of Scaffolds in Regenerative Medicine. Polymers 2020, 12, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, L.J.; Hutter, V.; Tai, H.; Howdle, S.M.; Shakesheff, K.M. The effect of processing variables on morphological and mechanical properties of supercritical CO2 foamed scaffolds for tissue engineering. Acta Biomater. 2012, 8, 61–71. [Google Scholar] [CrossRef] [Green Version]
- García-González, C.A.; Concheiro, A.; Alvarez-Lorenzo, C. Processing of Materials for Regenerative Medicine Using Supercritical Fluid Technology. Bioconjugate Chem. 2015, 26, 1159–1171. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. Supercritical fluids in 3-D tissue engineering. J. Supercrit. Fluids 2012, 69, 97–107. [Google Scholar] [CrossRef]
- Duarte, A.; Mano, J.; Reis, R.L. Supercritical fluids in biomedical and tissue engineering applications: A review. Int. Mater. Rev. 2009, 54, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Bhamidipati, M.; Scurto, A.M.; Detamore, M.S. The Future of Carbon Dioxide for Polymer Processing in Tissue Engineering. Tissue Eng. Part B Rev. 2013, 19, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, O.R.; Lewis, A.L.; Whitaker, M.J.; Tai, H.; Shakesheff, K.; Howdle, S.M. Applications of supercritical CO2 in the fabrication of polymer systems for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.-B.; Lee, C.-H.; Chen, A.-Z. Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications. Adv. Health Mater. 2017, 6, 1700433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, L.; Danen, K.; Kemmere, M.; Keurentjes, J. A parametric study into the morphology of polystyrene-co-methyl methacrylate foams using supercritical carbon dioxide as a blowing agent. Polymer 2007, 48, 3771–3780. [Google Scholar] [CrossRef]
- Gimeno, M.; Ventosa, N.; Sala, S.; Veciana, J. Use of 1,1,1,2-Tetrafluoroethane (R-134a)-Expanded Liquids as Solvent Media for Ecoefficient Particle Design with the DELOS Crystallization Process. Cryst. Growth Des. 2006, 6, 23–25. [Google Scholar] [CrossRef]
- Huchon, G.; Hofbauer, P.; Cannizzaro, G.; Iacono, P.; Wald, F. Comparison of the safety of drug delivery via HFA- and CFC-metered dose inhalers in CAO. Eur. Respir. J. 2000, 15, 663–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.-M.; Zhang, M.; Wu, Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef]
- del Río, E.P.; Martínez-Miguel, M.; Veciana, J.; Ratera, I.; Guasch, J. Artificial 3D Culture Systems for T Cell Expansion. ACS Omega 2018, 3, 5273–5280. [Google Scholar] [CrossRef] [PubMed]
- del Río, E.P.; Santos, F.; Rodriguez, X.R.; Martínez-Miguel, M.; Roca-Pinilla, R.; Arís, A.; Garcia-Fruitos, E.; Veciana, J.; Spatz, J.P.; Ratera, I.; et al. CCL21-loaded 3D hydrogels for T cell expansion and differentiation. Biomaterials 2020, 259, 120313. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Ferrer-Miralles, N.; Garcia-Fruitos, E.; Mitraki, A.; Peternel, S.; Rinas, U.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Vazquez, E.; Villaverde, A. Bacterial inclusion bodies are industrially exploitable amyloids. FEMS Microbiol. Rev. 2019, 43, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Rinas, U.; Garcia-Fruitos, E.; Corchero, J.L.; Vazquez, E.; Seras-Franzoso, J.; Villaverde, A. Bacterial Inclusion Bodies: Discovering Their Better Half. Trends Biochem. Sci. 2017, 42, 726–737. [Google Scholar] [CrossRef]
- Seras-Franzoso, J.; Steurer, C.; Roldán, M.; Vendrell, M.; Vidaurre-Agut, C.; Tarruella, A.; Saldaña, L.; Vilaboa, N.; Parera, M.; Elizondo, E.; et al. Functionalization of 3D scaffolds with protein-releasing biomaterials for intracellular delivery. J. Control. Release 2013, 171, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tatkiewicz, W.I.; Seras-Franzoso, J.; Fruitos, E.G.; Vazquez, E.; Kyvik, A.R.; Guasch, J.; Villaverde, A.; Veciana, J.; Ratera, I. Surface-Bound Gradient Deposition of Protein Nanoparticles for Cell Motility Studies. ACS Appl. Mater. Interfaces 2018, 10, 25779–25786. [Google Scholar] [CrossRef]
- Tatkiewicz, W.I.; Seras-Franzoso, J.; Garcia-Fruitos, E.; Vazquez, E.; Ventosa, N.; Peebo, K.; Ratera, I.; Villaverde, A.; Veciana, J. Two-Dimensional Microscale Engineering of Protein-Based Nanoparticles for Cell Guidance. ACS Nano 2013, 7, 4774–4784. [Google Scholar] [CrossRef]
- Seras-Franzoso, J.; Díez-Gil, C.; Vazquez, E.; García-Fruitós, E.; Cubarsi, R.; Ratera, I.; Veciana, J.; Villaverde, A. Bioadhesiveness and efficient mechanotransduction stimuli synergistically provided by bacterial inclusion bodies as scaffolds for tissue engineering. Nanomedicine 2012, 7, 79–93. [Google Scholar] [CrossRef]
- Pesarrodona, M.; Ferrer-Miralles, N.; Unzueta, U.; Gener, P.; Tatkiewicz, W.; Abasolo, I.; Ratera, I.; Veciana, S.J.; Schwartz, A.; Villaverde, A. Intracellular targeting of CD44+ cells with self-assembling, protein only nanoparticles. Int. J. Pharm. 2014, 473, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Seras-Franzoso, J.; Tsimbouri, P.M.; Burgess, K.V.; Unzueta, U.; Garcia-Fruitos, E.; Vazquez, E.; Villaverde, A.; Dalby, M.J. Topographically targeted osteogenesis of mesenchymal stem cells stimulated by inclusion bodies attached to polycaprolactone surfaces. Nanomedicine 2014, 9, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Tatkiewicz, W.I.; Seras-Franzoso, J.; García-Fruitós, E.; Vazquez, E.; Kyvik, A.R.; Ventosa, N.; Guasch, J.; Villaverde, A.; Veciana, J.; Ratera, I. High-Throughput Cell Motility Studies on Surface-Bound Protein Nanoparticles with Diverse Structural and Compositional Characteristics. ACS Biomater. Sci. Eng. 2019, 5, 5470–5480. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Tang, Y.X.; Thankappan, S.K.; Lee, P.; Fard, S.E.; Harmon, M.D.; Tran, K.; Yu, X. Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine. Nat. Synth. Biomed. Polym. Elsevier Sci. 2014, 351–371. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- James, R.; Manoukian, O.S.; Kumbar, S.G. Poly(lactic acid) for delivery of bioactive macromolecules. Adv. Drug Deliv. Rev. 2016, 107, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Barry, J.; Gidda, H.; Scotchford, C.; Howdle, S. Porous methacrylate scaffolds: Supercritical fluid fabrication and in vitro chondrocyte responses. Biomaterials 2004, 25, 3559–3568. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef]
- Zhong, T.; Deng, C.; Gao, Y.; Chen, M.; Zuo, B. Studies of in situ-forming hydrogels by blending PLA-PEG-PLA copolymer with silk fibroin solution. J. Biomed. Mater. Res. Part A 2012, 100, 1983–1989. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.-F.; Turng, L.-S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [Green Version]
- Unzueta, U.; Cespedes, M.V.; Sala, R.; Alamo, P.; Sánchez-Chardi, A.; Pesarrodona, M.; Sánchez-García, L.; Cano-Garrido, O.; Villaverde, A.; Vázquez, E.; et al. Release of targeted protein nanoparticles from functional bacterial amyloids: A death star-like approach. J. Control. Release 2018, 279, 29–39. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Fages, J. Extrusion assisted by supercritical CO2: A review on its application to biopolymers. J. Supercrit. Fluids 2017, 120, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Ventosa, N.; Sala, A.S.; Veciana, J.; Torres, J.; Llibre, J. Depressurization of an Expanded Liquid Organic Solution (DELOS): A New Procedure for Obtaining Submicron- or Micron-Sized Crystalline Particles. Cryst. Growth Des. 2001, 1, 299–303. [Google Scholar] [CrossRef]
- Mooney, D.; Baldwin, D.F.; Suh, N.P.; Vacanti, J.P.; Langer, R. Novel approach to fabricate porous sponges of poly(d,l-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 1996, 17, 1417–1422. [Google Scholar] [CrossRef]
- Quirk, R.A.; France, R.M.; Shakesheff, K.M.; Howdle, S.M. Supercritical fluid technologies and tissue engineering scaffolds. Curr. Opin. Solid State Mater. Sci. 2004, 8, 313–321. [Google Scholar] [CrossRef]
- Hatami, T.; Johner, J.C.F.; De Castro, K.C.; Mei, L.H.I.; Vieira, M.G.A.; Meireles, M.A.A. New Insight into a Step-by-Step Modeling of Supercritical CO2 Foaming to Fabricate Poly(ε-caprolactone) Scaffold. Ind. Eng. Chem. Res. 2020, 59, 20033–20044. [Google Scholar] [CrossRef]
- Markočič, E.; Škerget, M.; Knez, Ž. Effect of Temperature and Pressure on the Behavior of Poly(ε-caprolactone) in the Presence of Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2013, 52, 15594–15601. [Google Scholar] [CrossRef]
- Hatami, T.; Mei, L.H.I.; Shabanian, S.R. Modeling of Two-Step Supercritical CO 2 Foaming to Fabricate Poly(ε-caprolactone) Scaffolds. Chem. Eng. Technol. 2021, 44, 1233–1240. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of Carriers Controlling Phenotypic Expression in BMP-Induced Osteogenesis and Chondrogenesis. J. Bone Jt. Surg.-Am. Vol. 2001, 83, S105–S115. [Google Scholar] [CrossRef]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore Size of Porous Hydroxyapatite as the Cell-Substratum Controls BMP-Induced Osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Götz, H.; Müller, M.; Emmel, A.; Holzwarth, U.; Erben, R.; Stangl, R. Effect of surface finish on the osseointegration of laser-treated titanium alloy implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J.; Little, D.G.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur. Cells Mater. 2013, 26, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Sarazin, P.; Roy, X.; Favis, B.D. Controlled preparation and properties of porous poly(l-lactide) obtained from a co-continuous blend of two biodegradable polymers. Biomaterials 2004, 25, 5965–5978. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kang, S.G.; Kim, E.S.; Cho, S.H.; Lee, J.H. Fabrication and characterization of hydrophilic poly(lactic-co-glycolic acid)/poly(vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials 2003, 24, 4011–4021. [Google Scholar] [CrossRef]
- Wirtz, D.C.; Schiffers, N.; Pandorf, T.; Radermacher, K.; Weichert, D.; Forst, R. Critical evaluation of known bone material properties to realize anisotropic FE-simulation of the proximal femur. J. Biomech. 2000, 33, 1325–1330. [Google Scholar] [CrossRef]
- Mathieu, L.M.; Mueller, T.L.; Bourban, P.-E.; Pioletti, D.; Müller, R.; Månson, J.-A.E. Architecture and properties of anisotropic polymer composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Chastain, S.R.; Kundu, A.K.; Dhar, S.; Calvert, J.W.; Putnam, A.J. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J. Biomed. Mater. Res. A 2006, 78, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Marei, N.H.; El-Sherbiny, I.M.; Lotfy, A.; El-Badawy, A.; El-Badri, N. Mesenchymal stem cells growth and proliferation enhancement using PLA vs PCL based nanofibrous scaffolds. Int. J. Biol. Macromol. 2016, 93, 9–19. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovic, J. Supercritical CO2-assisted production of PLA and PLGA foams for controlled thymol release. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 394–404. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen—Glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Adams, U.J.; Laurencin, C.T.; Nukavarapu, S.P. Optimally Porous and Biomechanically Compatible Scaffolds for Large-Area Bone Regeneration. Tissue Eng. Part A 2012, 18, 1376–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobbert, F.S.L.; Zadpoor, A.A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J. Mater. Chem. B 2017, 5, 6175–6192. [Google Scholar] [CrossRef] [Green Version]
- Seras-Franzoso‡, J.; Tatkiewicz‡, W.I.; Vazquez, E.; García-Fruitós, E.; Ratera, I.; Veciana, J.; Villaverde, A. Integrating mechanical and biological control of cell proliferation through bioinspired multieffector materials. Nanomedicine 2015, 10, 873–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seras-Franzoso, J.; Peebo, K.; García-Fruitós, E.; Vázquez, E.; Rinas, U.; Villaverde, A. Improving protein delivery of fibroblast growth factor-2 from bacterial inclusion bodies used as cell culture substrates. Acta Biomater. 2014, 10, 1354–1359. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, E.; Corchero, J.L.; Burgueño, J.F.; Seras-Franzoso, J.; Kosoy, A.; Bosser, R.; Mendoza, R.; Martínez-Láinez, J.M.; Rinas, U.; Fernández, E.; et al. Functional Inclusion Bodies Produced in Bacteria as Naturally Occurring Nanopills for Advanced Cell Therapies. Adv. Mater. 2012, 24, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.D.; Cooper, A.I. Synthesis of Polystyrene by Dispersion Polymerization in 1,1,1,2-Tetrafluoroethane (R134a) Using Inexpensive Hydrocarbon Macromonomer Stabilizers. Macromolecules 2003, 36, 7534–7542. [Google Scholar] [CrossRef]






| Stage | Processing Parameters | Material | ||||
|---|---|---|---|---|---|---|
| PLA | PLA | PLA | PLA | PLGA | ||
| Polymer disk preparation | Thermal annealing | Yes | Yes | Yes | Yes | No |
| m (g) | 0.4 | 0.4 | 0.8 | 0.8 | 0.3 | |
| T (°C) | 150 | 150 | 150 | 150 | - | |
| Applied pressure (Kg) | 3000 | 3000 | 3000 | 3000 | 500 | |
| Porous scaffold fabrication | Compressed fluid | Freon R134a | Freon R134a | scCO2 | scCO2 | Freon R134a |
| Tw (°C) | 40 | 40 | 35 | 35 | 35 | |
| Pw (MPa) | 2 | 2 | 10.3 | 10.3 | 2 | |
| Soaking time, t (h) | 3 | 3 | 2 | 2 | 2 | |
| pNP Scaffold decoration | - | No | Yes | No | Yes | No |
| Material | Total Porosity, PT (%) | Closed Porosity, Pclosed (%) | Open Porosity, PO (%) |
|---|---|---|---|
| PLA-Freon R134a | 92.8 ± 2.0 | 83.2 ± 3.3 | 9.6 ± 1.3 |
| PLA-scCO2 | 82.3 ± 0.9 | 56.9 ± 7.5 | 25.4 ± 6.6 |
| PLGA-Freon R134a | 96.7 ± 0.8 | 89.1 ± 3.3 | 7.6 ± 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguado, M.; Saldaña, L.; Pérez del Río, E.; Guasch, J.; Parera, M.; Córdoba, A.; Seras-Franzoso, J.; Cano-Garrido, O.; Vázquez, E.; Villaverde, A.; et al. Polylactide, Processed by a Foaming Method Using Compressed Freon R134a, for Tissue Engineering. Polymers 2021, 13, 3453. https://doi.org/10.3390/polym13203453
Aguado M, Saldaña L, Pérez del Río E, Guasch J, Parera M, Córdoba A, Seras-Franzoso J, Cano-Garrido O, Vázquez E, Villaverde A, et al. Polylactide, Processed by a Foaming Method Using Compressed Freon R134a, for Tissue Engineering. Polymers. 2021; 13(20):3453. https://doi.org/10.3390/polym13203453
Chicago/Turabian StyleAguado, María, Laura Saldaña, Eduardo Pérez del Río, Judith Guasch, Marc Parera, Alba Córdoba, Joaquín Seras-Franzoso, Olivia Cano-Garrido, Esther Vázquez, Antonio Villaverde, and et al. 2021. "Polylactide, Processed by a Foaming Method Using Compressed Freon R134a, for Tissue Engineering" Polymers 13, no. 20: 3453. https://doi.org/10.3390/polym13203453
APA StyleAguado, M., Saldaña, L., Pérez del Río, E., Guasch, J., Parera, M., Córdoba, A., Seras-Franzoso, J., Cano-Garrido, O., Vázquez, E., Villaverde, A., Veciana, J., Ratera, I., Vilaboa, N., & Ventosa, N. (2021). Polylactide, Processed by a Foaming Method Using Compressed Freon R134a, for Tissue Engineering. Polymers, 13(20), 3453. https://doi.org/10.3390/polym13203453