H+-Conducting Aromatic Multiblock Copolymer and Blend Membranes and Their Application in PEM Electrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer Synthesis
2.1.1. Multiblock-co-Ionomers (MBI)
2.1.2. Polymers for Three-Component Blend Membranes (3CBM)
2.2. Preparation of Multiblock-co-Ionomer Membranes and Ionically Cross-Linked Blend Membranes
2.3. Polymer and Membrane Characterization
2.3.1. Water Uptake (WU) and Hydration Number (λ) (Water Molecules Per SO3H Group)
2.3.2. Ion-Exchange Capacity (IEC)
2.3.3. Electrochemical Impedance Spectroscopy (EIS)
2.3.4. Thermal Stability via Thermogravimetry Coupled with a Fourier–Transform Infrared Spectrometer (TGA–FTIR)
2.3.5. Size Exclusion Chromatography (SEC)
2.3.6. Stress–Strain Measurements of Selected Membranes
2.3.7. Determination of H2 Permeability of the Membranes
2.4. PEM Water Electrolysis Experiments
2.4.1. Fabrication of Membrane Electrode Assemblies (MEAs)
2.4.2. Electrolysis Tests
3. Results
3.1. Preparation and Characterization of Ionomers and Ionomer Blend Membranes
3.1.1. Preparation and Characterization of the Aromatic Polymer Blocks
3.1.2. Preparation and Characterization of the Multiblock-co-Ionomers
3.1.3. Preparation and Characterization of the Polymers Used for the Preparation of Ternary Blend Membranes
3.1.4. Characterization Data of the Ternary Blend Membranes
3.2. Selected Characterization Results of Those Membranes Which Were Applied to Electrolysis
3.2.1. Stress–Strain Measurement Results
3.2.2. TGA-FTIR Coupling Results
3.2.3. Electrochemical Impedance in Dependence of Temperature
3.3. Hydrogen Permeability Measurements
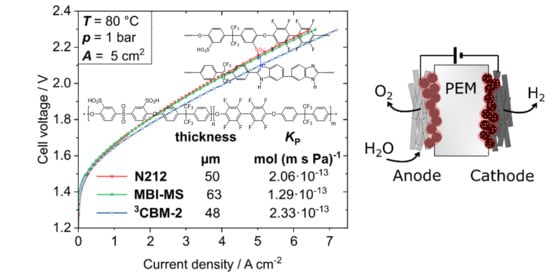
3.4. Water Electrolysis Polarization Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babic, U.; Suermann, M.; Büchi, F.N.; Gubler, L.; Schmidt, T.J. Critical Review—Identifying Critical Gaps for Polymer Electrolyte Water Electrolysis Development. J. Electrochem. Soc. 2017, 164, F387–F399. [Google Scholar] [CrossRef] [Green Version]
- Bessarabov, D.; Wang, H.; Li, H.; Zhao, N. PEM Electrolysis for Hydrogen Production: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1138775495. [Google Scholar]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Grot, W. Perfluorierte Ionenaustauscher-Membrane von hoher chemischer und thermischer Stabilität. Chem. Ing. Tech. 1972, 44, 167–169. [Google Scholar] [CrossRef]
- Grot, W. Use of Nafion Perfluorosulfonic Acid Products as Separators in Electrolytic Cells. Chem. Ing. Tech. 1978, 50, 299–301. [Google Scholar] [CrossRef]
- Grot, W.G. Perfluorinated ion exchange polymers and their use in research and industry. Macromol. Symp. 1994, 82, 161–172. [Google Scholar] [CrossRef]
- Ito, H.; Maeda, T.; Nakano, A.; Takenaka, H. Properties of Nafion membranes under PEM water electrolysis conditions. Int. J. Hydrogen Energy 2011, 36, 10527–10540. [Google Scholar] [CrossRef]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite Polymers Development and Application for Polymer Electrolyte Membrane Technologies—A Review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef]
- Bartmann, M.; Kowalczik, U. Zum Mechanismus der oxidativen Kupplung von Phenolen. Makromol. Chem. Phys. 1988, 189, 2285–2292. [Google Scholar] [CrossRef]
- Xu, T.; Wu, D.; Seo, S.-J.; Woo, J.-J.; Wu, L.; Moon, S.-H. Proton exchange composite membranes from blends of brominated and sulfonated poly(2,6-dimethyl-1,4-phenylene oxide). J. Appl. Polym. Sci. 2012, 124, 3511–3519. [Google Scholar] [CrossRef]
- Noshay, A.; Robeson, L.M. Sulfonated polysulfone. J. Appl. Polym. Sci. 1976, 20, 1885–1903. [Google Scholar] [CrossRef]
- Xing, P.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Wang, K.; Kaliaguine, S. Synthesis and characterization of sulfonated poly(ether ether ketone) for proton exchange membranes. J. Membr. Sci. 2004, 229, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Cichon, P.J.; Krüger, A.J.; Krieg, H.M.; Bessarabov, D.; Aniol, K.; Kerres, J. Sulfonated poly(arylene thioether phosphine oxide)s and poly(arylene ether phosphine oxide)s PBI-blend membranes and their performance in SO2 electrolysis. Int. J. Hydrogen Energy 2016, 41, 4521–4537. [Google Scholar] [CrossRef]
- Adamski, M.; Skalski, T.J.G.; Britton, B.; Peckham, T.J.; Metzler, L.; Holdcroft, S. Highly Stable, Low Gas Cross-over, Proton-Conducting Phenylated Polyphenylenes. Angew. Chem. 2017, 56, 9058–9061. [Google Scholar] [CrossRef]
- Schuster, M.; Kreuer, K.-D.; Andersen, H.T.; Maier, J. Sulfonated Poly(phenylene sulfone) Polymers as Hydrolytically and Thermooxidatively Stable Proton Conducting Ionomers. Macromolecules 2007, 40, 598–607. [Google Scholar] [CrossRef]
- Büchi, F.N.; Gupta, B.; Haas, O.; Scherer, G.G. Study of radiation-grafted FEP-G-polystyrene membranes as polymer electrolytes in fuel cells. Electrochim. Acta 1995, 40, 345–353. [Google Scholar] [CrossRef]
- Holmes, T.; Skalski, T.J.G.; Adamski, M.; Holdcroft, S. Stability of Hydrocarbon Fuel Cell Membranes: Reaction of Hydroxyl Radicals with Sulfonated Phenylated Polyphenylenes. Chem. Mater. 2019, 31, 1441–1449. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Lufrano, F.; Staiti, P.; Aricò, A.S. Electrochemical characterization of a PEM water electrolyzer based on a sulfonated polysulfone membrane. J. Membr. Sci. 2013, 448, 209–214. [Google Scholar] [CrossRef]
- Chae, K.-J.; Kim, K.-Y.; Choi, M.-J.; Yang, E.; Kim, I.S.; Ren, X.; Lee, M. Sulfonated polyether ether ketone (SPEEK)-based composite proton exchange membrane reinforced with nanofibers for microbial electrolysis cells. Chem. Eng. J. 2014, 254, 393–398. [Google Scholar] [CrossRef]
- Jang, I.-Y.; Kweon, O.-H.; Kim, K.-E.; Hwang, G.-J.; Moon, S.-B.; Kang, A.-S. Application of polysulfone (PSf)– and polyether ether ketone (PEEK)–tungstophosphoric acid (TPA) composite membranes for water electrolysis. J. Membr. Sci. 2008, 322, 154–161. [Google Scholar] [CrossRef]
- Smith, D.W.; Oladoyinbo, F.O.; Mortimore, W.A.; Colquhoun, H.M.; Thomassen, M.S.; Ødegård, A.; Guillet, N.; Mayousse, E.; Klicpera, T.; Hayes, W. A Microblock Ionomer in Proton Exchange Membrane Electrolysis for the Production of High Purity Hydrogen. Macromolecules 2013, 46, 1504–1511. [Google Scholar] [CrossRef]
- Schönberger, F.; Kerres, J. Novel multiblock-co-ionomers as potential polymer electrolyte membrane materials. J. Polym. Sci. A Polym. Chem. 2007, 45, 5237–5255. [Google Scholar] [CrossRef]
- Takamuku, S.; Jannasch, P. Multiblock Copolymers Containing Highly Sulfonated Poly(arylene sulfone) Blocks for Proton Conducting Electrolyte Membranes. Macromolecules 2012, 45, 6538–6546. [Google Scholar] [CrossRef]
- Ghassemi, H.; McGrath, J.E.; Zawodzinski, T.A. Multiblock sulfonated–fluorinated poly(arylene ether)s for a proton exchange membrane fuel cell. Polymer 2006, 47, 4132–4139. [Google Scholar] [CrossRef]
- Einsla, M.L.; Kim, Y.S.; Hawley, M.; Lee, H.-S.; McGrath, J.E.; Liu, B.; Guiver, M.D.; Pivovar, B.S. Toward Im-proved Conductivity of Sulfonated Aromatic Proton Exchange Membranes at Low Relative Humidity. Chem. Mater. 2008, 20, 5636–5642. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Hickner, M.A.; Yu, X.; Li, Y.; Glass, T.E.; McGrath, J.E. Influence of chemical composition and sequence length on the transport properties of proton exchange membranes. J. Polym. Sci. B Polym. Phys. 2006, 44, 2226–2239. [Google Scholar] [CrossRef]
- Cui, W.; Kerres, J.; Eigenberger, G. Development and characterization of ion-exchange polymer blend membranes. Sep. Purif. Technol. 1998, 14, 145–154. [Google Scholar] [CrossRef]
- Kerres, J.A. Development of ionomer membranes for fuel cells. J. Membr. Sci. 2001, 185, 3–27. [Google Scholar] [CrossRef]
- Kerres, J.A. Blended and Cross-Linked Ionomer Membranes for Application in Membrane Fuel Cells. Fuel Cells 2005, 5, 230–247. [Google Scholar] [CrossRef]
- Kerres, J.A. Design Concepts for Aromatic Ionomers and Ionomer Membranes to be Applied to Fuel Cells and Electrolysis. Polym. Rev. 2015, 55, 273–306. [Google Scholar] [CrossRef]
- Kerres, J.; Ullrich, A.; Meier, F.; Häring, T. Synthesis and characterization of novel acid–base polymer blends for application in membrane fuel cells. Solid State Ion. 1999, 125, 243–249. [Google Scholar] [CrossRef]
- Li, Q.F.; Rudbeck, H.C.; Chromik, A.; Jensen, J.O.; Pan, C.; Steenberg, T.; Calverley, M.; Bjerrum, N.J.; Kerres, J. Properties, degradation and high temperature fuel cell test of different types of PBI and PBI blend membranes. J. Membr. Sci. 2010, 347, 260–270. [Google Scholar] [CrossRef]
- Van Zyl, A.J.; Kerres, J.A.; Cui, W.; Junginger, M. Application of new sulfonated ionomer membranes in the separation of pentene and pentane by facilitated transport. J. Membr. Sci. 1997, 137, 173–185. [Google Scholar] [CrossRef]
- Gogel, V.; Jörissen, L.; Chromik, A.; Schönberger, F.; Lee, J.; Schäfer, M.; Krajinovic, K.; Kerres, J. Ionomer Membrane and MEA Development for DMFC. Sep. Sci. Technol. 2008, 43, 3955–3980. [Google Scholar] [CrossRef]
- Peach, R.; Krieg, H.M.; Krüger, A.J.; van der Westhuizen, D.; Bessarabov, D.; Kerres, J. Comparison of ionically and ionical-covalently cross-linked polyaromatic membranes for SO2 electrolysis. Int. J. Hydrogen Energy 2014, 39, 28–40. [Google Scholar] [CrossRef]
- Chromik, A.; dos Santos, A.R.; Turek, T.; Kunz, U.; Häring, T.; Kerres, J. Stability of acid-excess acid–base blend membranes in all-vanadium redox-flow batteries. J. Membr. Sci. 2015, 476, 148–155. [Google Scholar] [CrossRef]
- Krajinovic, K.; Kaz, T.; Haering, T.; Gogel, V.; Kerres, J. Highly Sulphonated Multiblock-co-polymers for Direct Methanol Fuel Cells. Fuel Cells 2011, 11, 787–800. [Google Scholar] [CrossRef]
- Kerres, J.; Schönberger, F.; Chromik, A.; Häring, T.; Li, Q.; Jensen, J.O.; Pan, C.; Noyé, P.; Bjerrum, N.J. Partially Fluorinated Arylene Polyethers and Their Ternary Blend Membranes with PBI and H3PO4. Part I. Synthesis and Characterisation of Polymers and Binary Blend Membranes. Fuel Cells 2008, 8, 175–187. [Google Scholar] [CrossRef]
- Kerres, J.A.; Xing, D.; Schönberger, F. Comparative investigation of novel PBI blend ionomer membranes from nonfluorinated and partially fluorinated poly arylene ethers. J. Polym. Sci. B Polym. Phys. 2006, 44, 2311–2326. [Google Scholar] [CrossRef]
- Kerres, J.; Ullrich, A.; Hein, M. Preparation and characterization of novel basic polysulfone polymers. J. Polym. Sci. A Polym. Chem. 2001, 39, 2874–2888. [Google Scholar] [CrossRef]
- Titvinidze, G.; Wohlfarth, A.; Kreuer, K.-D.; Schuster, M.; Meyer, W.H. Reinforcement of Highly Proton Conducting Multi-Block Copolymers by Online Crosslinking. Fuel Cells 2014, 14, 325–331. [Google Scholar] [CrossRef]
- Cho, H.; Krieg, H.M.; Kerres, J.A. Performances of Anion-Exchange Blend Membranes on Vanadium Redox Flow Batteries. Membranes 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkov, V.I.; Chernyak, A.V.; Golubenko, D.V.; Tverskoy, V.A.; Lochin, G.A.; Odjigaeva, E.S.; Yaroslavtsev, A.B. Hydration and Diffusion of H+, Li+, Na+, Cs+ Ions in Cation-Exchange Membranes Based on Polyethylene- and Sulfonated-Grafted Polystyrene Studied by NMR Technique and Ionic Conductivity Measurements. Membranes 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Bülbül, E.; Atanasov, V.; Mehlhorn, M.; Bürger, M.; Chromik, A.; Häring, T.; Kerres, J. Highly phosphonated polypentafluorostyrene blended with polybenzimidazole: Application in vanadium redox flow battery. J. Membr. Sci. 2019, 570, 194–203. [Google Scholar] [CrossRef]
- Cooper, K.R. Progress Toward Accurate Through-Plane Ion Transport Resistance Measurement of Thin Solid Electrolytes. J. Electrochem. Soc. 2010, 157, B1731. [Google Scholar] [CrossRef] [Green Version]
- Kerres, J.; Ullrich, A.; Hein, M.; Gogel, V.; Friedrich, K.A.; Jörissen, L. Cross-Linked Polyaryl Blend Membranes for Polymer Electrolyte Fuel Cells. Fuel Cells 2004, 4, 105–112. [Google Scholar] [CrossRef]
- Pei, P.; Wu, Z.; Li, Y.; Jia, X.; Chen, D.; Huang, S. Improved methods to measure hydrogen crossover current in proton exchange membrane fuel cell. Appl. Energy 2018, 215, 338–347. [Google Scholar] [CrossRef]
- Trinke, P.; Haug, P.; Brauns, J.; Bensmann, B.; Hanke-Rauschenbach, R.; Turek, T. Hydrogen Crossover in PEM and Alkaline Water Electrolysis: Mechanisms, Direct Comparison and Mitigation Strategies. J. Electrochem. Soc. 2018, 165, F502–F513. [Google Scholar] [CrossRef]
- Trinke, P.; Bensmann, B.; Reichstein, S.; Hanke-Rauschenbach, R.; Sundmacher, K. Hydrogen Permeation in PEM Electrolyzer Cells Operated at Asymmetric Pressure Conditions. J. Electrochem. Soc. 2016, 163, F3164–F3170. [Google Scholar] [CrossRef]
- Mayerhöfer, B.; McLaughlin, D.; Böhm, T.; Hegelheimer, M.; Seeberger, D.; Thiele, S. Bipolar Membrane Elec-trode Assemblies for Water Electrolysis. ACS Appl. Energy Mater. 2020, 3, 9635–9644. [Google Scholar] [CrossRef]
- Bühler, M.; Hegge, F.; Holzapfel, P.; Bierling, M.; Suermann, M.; Vierrath, S.; Thiele, S. Optimization of anodic porous transport electrodes for proton exchange membrane water electrolyzers. J. Mater. Chem. A 2019, 7, 26984–26995. [Google Scholar] [CrossRef]
- Krajinovic, K. Synthese und Charakterisierung von Multiblock-co-Poly(aryl)-Ionomer(blend)membranen für den Einsatz in Direktmethanolbrennstoffzellen. Master’s Thesis, Universität Stuttgart, Stuttgart, Germany, 2011. [Google Scholar]
- Liu, B.; Robertson, G.P.; Guiver, M.D.; Sun, Y.-M.; Liu, Y.-L.; Lai, J.-Y.; Mikhailenko, S.; Kaliaguine, S. Sulfonated poly(aryl ether ether ketone ketone)s containing fluorinated moieties as proton exchange membrane materials. J. Polym. Sci. B Polym. Phys. 2006, 44, 2299–2310. [Google Scholar] [CrossRef] [Green Version]
- Kerres, J. Covalent-Ionically Cross-linked Poly(Etheretherketone)-Basic Polysulfone Blend Ionomer Mem-branes. Fuel Cells 2006, 6, 251–260. [Google Scholar] [CrossRef]
- Yu, T.H.; Sha, Y.; Liu, W.-G.; Merinov, B.V.; Shirvanian, P.; Goddard, W.A. Mechanism for degradation of Nafion in PEM fuel cells from quantum mechanics calculations. J. Am. Chem. Soc. 2011, 133, 19857–19863. [Google Scholar] [CrossRef]
- Freger, V. Hydration of ionomers and Schroeder’s paradox in Nafion. J. Phys. Chem. B 2009, 113, 24–36. [Google Scholar] [CrossRef]
- Kreuer, K.-D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [Green Version]
- Albert, A.; Barnett, A.O.; Thomassen, M.S.; Schmidt, T.J.; Gubler, L. Radiation-Grafted Polymer Electrolyte Membranes for Water Electrolysis Cells: Evaluation of Key Membrane Properties. ACS Appl. Mater. Interfaces 2015, 7, 22203–22212. [Google Scholar] [CrossRef]
- Klose, C.; Saatkamp, T.; Münchinger, A.; Bohn, L.; Titvinidze, G.; Breitwieser, M.; Kreuer, K.-D.; Vierrath, S. All-Hydrocarbon MEA for PEM Water Electrolysis Combining Low Hydrogen Crossover and High Efficiency. Adv. Energy Mater. 2020, 10, 1903995. [Google Scholar] [CrossRef]
- Chae, J.E.; Lee, S.Y.; Baek, S.Y.; Song, K.H.; Park, C.H.; Kim, H.-J.; Lee, K.-S. High-performance multiblock PEMs containing a highly acidic fluorinated-hydrophilic domain for water electrolysis. J. Membr. Sci. 2021, 638, 119694. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Tang, H.; Lin, Y.; Pan, M. Hydrogen crossover through perfluorosulfonic acid membranes with variable side chains and its influence in fuel cell lifetime. Int. J. Hydrogen Energy 2014, 39, 15989–15995. [Google Scholar] [CrossRef]
- Wei, G.; Xu, L.; Huang, C.; Wang, Y. SPE water electrolysis with SPEEK/PES blend membrane. Int. J. Hydrogen Energy 2010, 35, 7778–7783. [Google Scholar] [CrossRef]











| Name | Ratio r (a) | ncalc (b) | Mw kDa | Mn kDa | PDI (c) | nGPC (d) | IECdir meq/g | IECtot meq/g | Yield % | TSO3H-onset (e) °C |
|---|---|---|---|---|---|---|---|---|---|---|
| PKK long | 0.993 | 143 | 18.8 | 10.2 | 1.8 | 14 | 0.42 | 0.85 | 70 | 290 |
| PKK middle | 0.972 | 36 | 12.3 | 8.3 | 1.5 | 12 | 1.41 | 1.77 | 42 | 280 |
| PKK short | 0.955 | 22 | 6.6 | 5.1 | 1.3 | 7 | 1.08 | 1.47 | 39 | 270 |
| Name | Ratio r (a) | ncalc (b) | Mw kDa | Mn kDa | PDI (c) | nGPC (d) | Yield % | TSO3H-onset (e) °C |
|---|---|---|---|---|---|---|---|---|
| PFS long | 0.936 | 15 | 24.7 | 10.1 | 2.4 | 16 | 81 | 450 |
| PFS short | 0.818 | 5 | 9.0 | 4.7 | 2.0 | 8 | 85 | 420 |
| Name/Type | Composition PKK + PFS (a) | Mw kDa | Mn kDa | PDI (b) | nGPC (c) blocks | IECdir meq/g | IECtot meq/g | TSO3H-onset (d) °C | TCO onset °C (d) |
|---|---|---|---|---|---|---|---|---|---|
| MBI-LL | Long + Long | 104.3 | 41.3 | 2.5 | 4 | 0.40 | 0.62 | 291 | 409 |
| MBI-LS | Long + Short | 67.7 | 25.3 | 2.7 | 3 | 0.88 | 0.99 | 270 | 497 |
| MBI-ML | Middle + Long | 79.9 | 21.9 | 3.6 | 2 | 0.65 | 0.92 | - | - |
| MBI-MS | Middle + Short | 63.8 | 22.2 | 2.9 | 3 | 0.57 | 0.90 | 303 | 475 |
| MBI-SL | Short + Long | gel | gel | - | - | 0.64 | 1.01 | - | - |
| MBI-SS | Short + Short | 110.6 | 19.5 | 5.7 | 4 | 0.49 | 0.80 | 287 | 457 |
| Type | Thickness μm (a) | IECdir meq/g | IECtot meq/g | TSO3H onset °C (b) | TCO onset °C (b) | WU 25°/90 °C % (c) | λ 25°/90°(d) | Tg [°C] (e) | Specific Imped. Ω*cm (f) | Conductivity mS/cm (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| MBI-LL | 105 | 0.52 | 0.66 | 323 | 471 | 20.7/23.2 | 17.4/19.5 | 178.4 | 17.1 | 58.5 |
| MBI-LS | 126 | 0.98 | 1.1 | 342 | 477 | 19.3/25.3 | 9.7/12.8 | 191.8 | 13.3 | 75.2 |
| MBI-ML | Brittle | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. |
| MBI-MS | 82 | 1.1 | 1.34 | 309 | 446 | 48.1/60.5 | 19.9/25.1 | - | 9.8 | 102.0 |
| MBI-SL | Brittle | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. |
| MBI-SS | Brittle | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. |
| Name | Mw kDa | Mn kDa | PDI (b) | nGPC (c) | IECtot meq/g | DS (e) | TSO3H onset °C (f) | TCO onset °C (f) |
|---|---|---|---|---|---|---|---|---|
| SFS001 | 211.5 (a) | 67.5 (a) | 3.1 | 85 | 2.31 | - | 308 | 451 |
| PSU-py | 38.3 | 14.3 | 2.7 | 21 | - | 2.13 | - | 310 |
| F6-PBI | 847.3 | 66.1 | 12.8 | 123 | 3.74 (d) | - | - | 500 |
| Name | Ratio SFS/PSU-py/ F6PBI (a) | Thickness μm (b) | IECdir meq/g | IECtot meq/g | TSO3H onset °C (c) | TCO onset °C (c) | WU 25°/90 °C % (d) | λ 25°/90° (e) | Tg °C (f) | Spec. Imped. Ω*cm (g) | Cond. mS/cm (h) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ³C-BM-1 | 85:12:3 | 100 | 1.38 | 1.71 | 310 | 425 | 76/130 | 24.7/42.2 | 314 | 17.8 | 56.3 |
| ³C-BM-2 | 85:10:5 | 25 | 1.19 | 1.79 | 275 | 380 | 61/108 | 18.9/33.5 | - | 7.3 | 137.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, J.; Mayerhöfer, B.; Trinke, P.; Bensmann, B.; Hanke-Rauschenbach, R.; Krajinovic, K.; Thiele, S.; Kerres, J. H+-Conducting Aromatic Multiblock Copolymer and Blend Membranes and Their Application in PEM Electrolysis. Polymers 2021, 13, 3467. https://doi.org/10.3390/polym13203467
Bender J, Mayerhöfer B, Trinke P, Bensmann B, Hanke-Rauschenbach R, Krajinovic K, Thiele S, Kerres J. H+-Conducting Aromatic Multiblock Copolymer and Blend Membranes and Their Application in PEM Electrolysis. Polymers. 2021; 13(20):3467. https://doi.org/10.3390/polym13203467
Chicago/Turabian StyleBender, Johannes, Britta Mayerhöfer, Patrick Trinke, Boris Bensmann, Richard Hanke-Rauschenbach, Katica Krajinovic, Simon Thiele, and Jochen Kerres. 2021. "H+-Conducting Aromatic Multiblock Copolymer and Blend Membranes and Their Application in PEM Electrolysis" Polymers 13, no. 20: 3467. https://doi.org/10.3390/polym13203467
APA StyleBender, J., Mayerhöfer, B., Trinke, P., Bensmann, B., Hanke-Rauschenbach, R., Krajinovic, K., Thiele, S., & Kerres, J. (2021). H+-Conducting Aromatic Multiblock Copolymer and Blend Membranes and Their Application in PEM Electrolysis. Polymers, 13(20), 3467. https://doi.org/10.3390/polym13203467