Facile Fabrication of Eucommia Rubber Composites with High Shape Memory Performance
Abstract
:1. Introduction
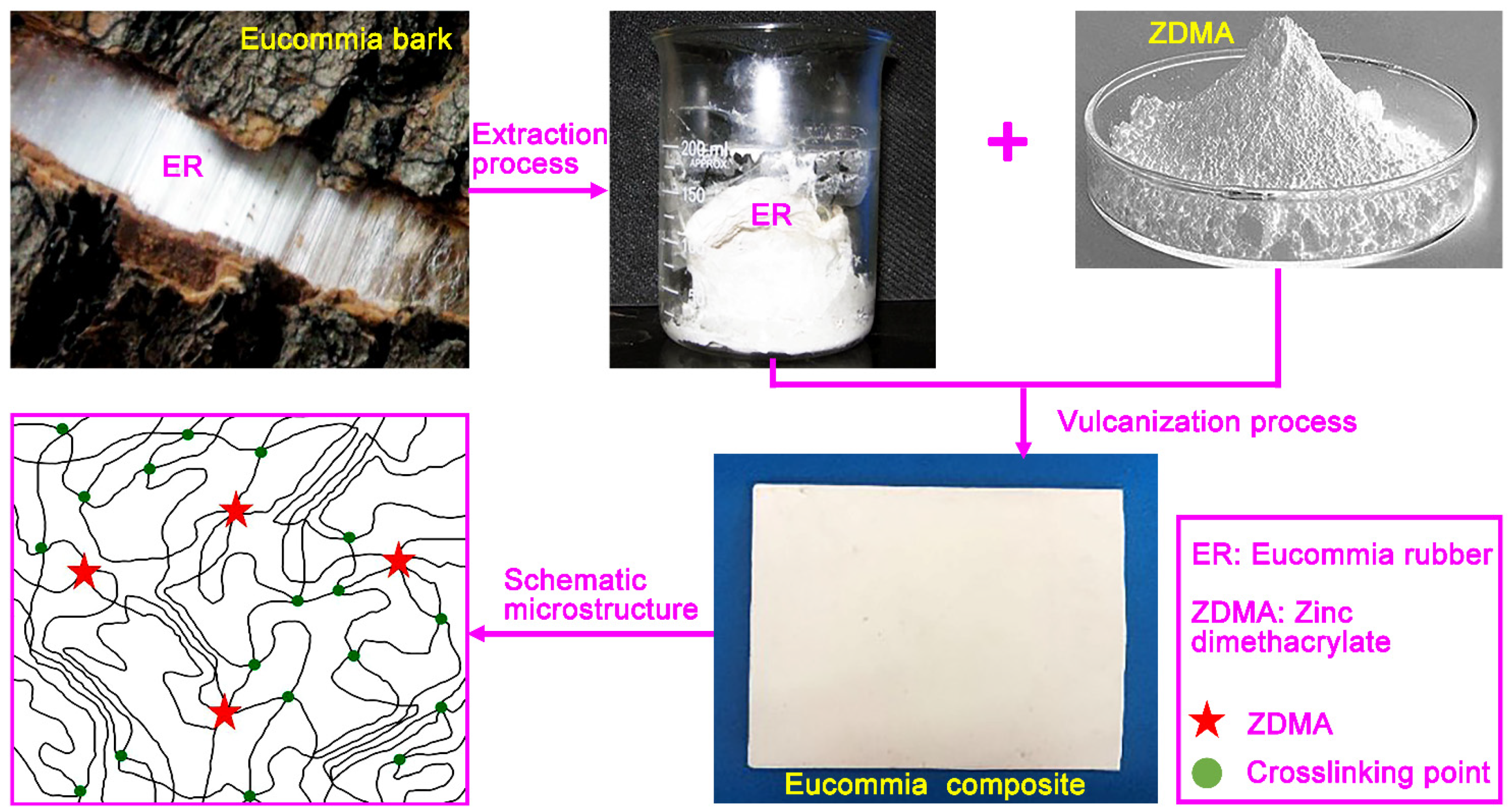
2. Materials and Methods
3. Results and Discussion
3.1. Properties of ER Composites
3.1.1. Cure Characteristics of ER Composites
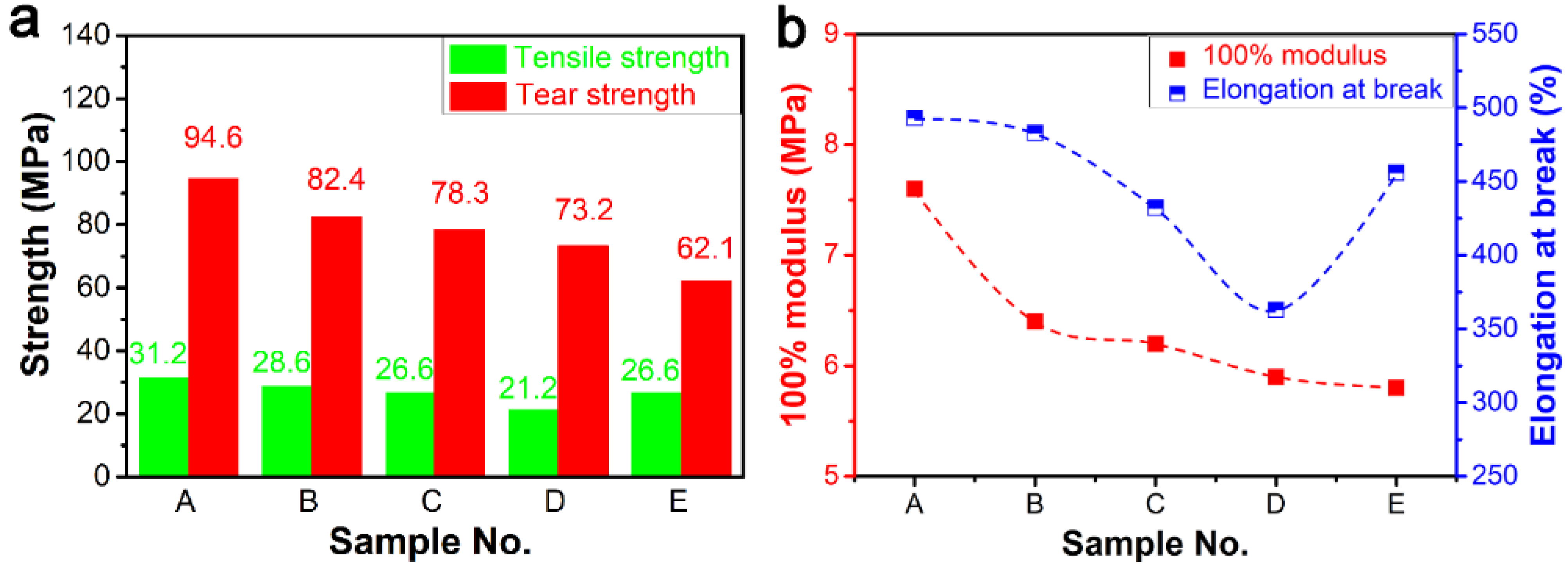
3.1.2. Mechanical Properties of ER Composites
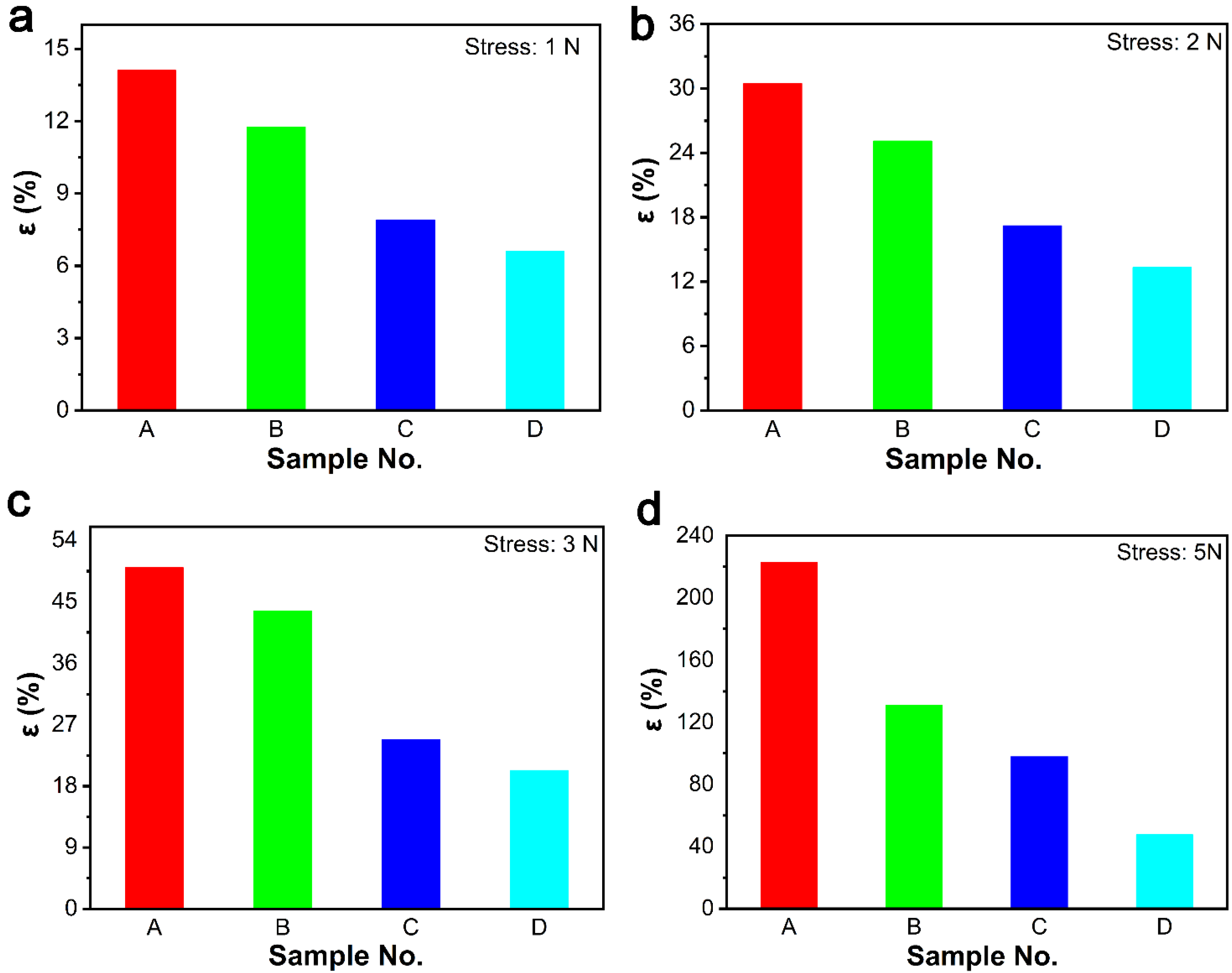
3.1.3. Swelling Cross-Link Density of ER Composites
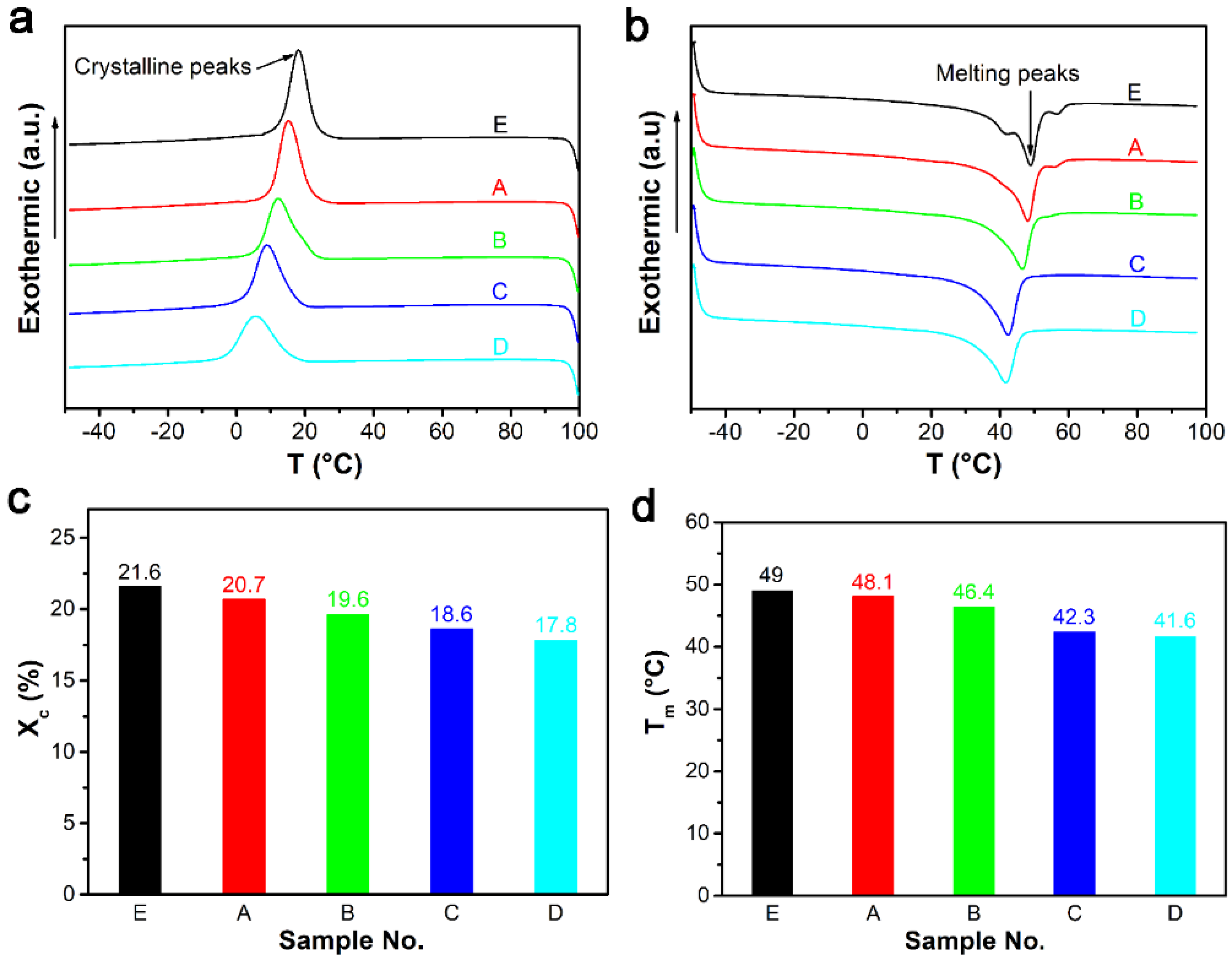
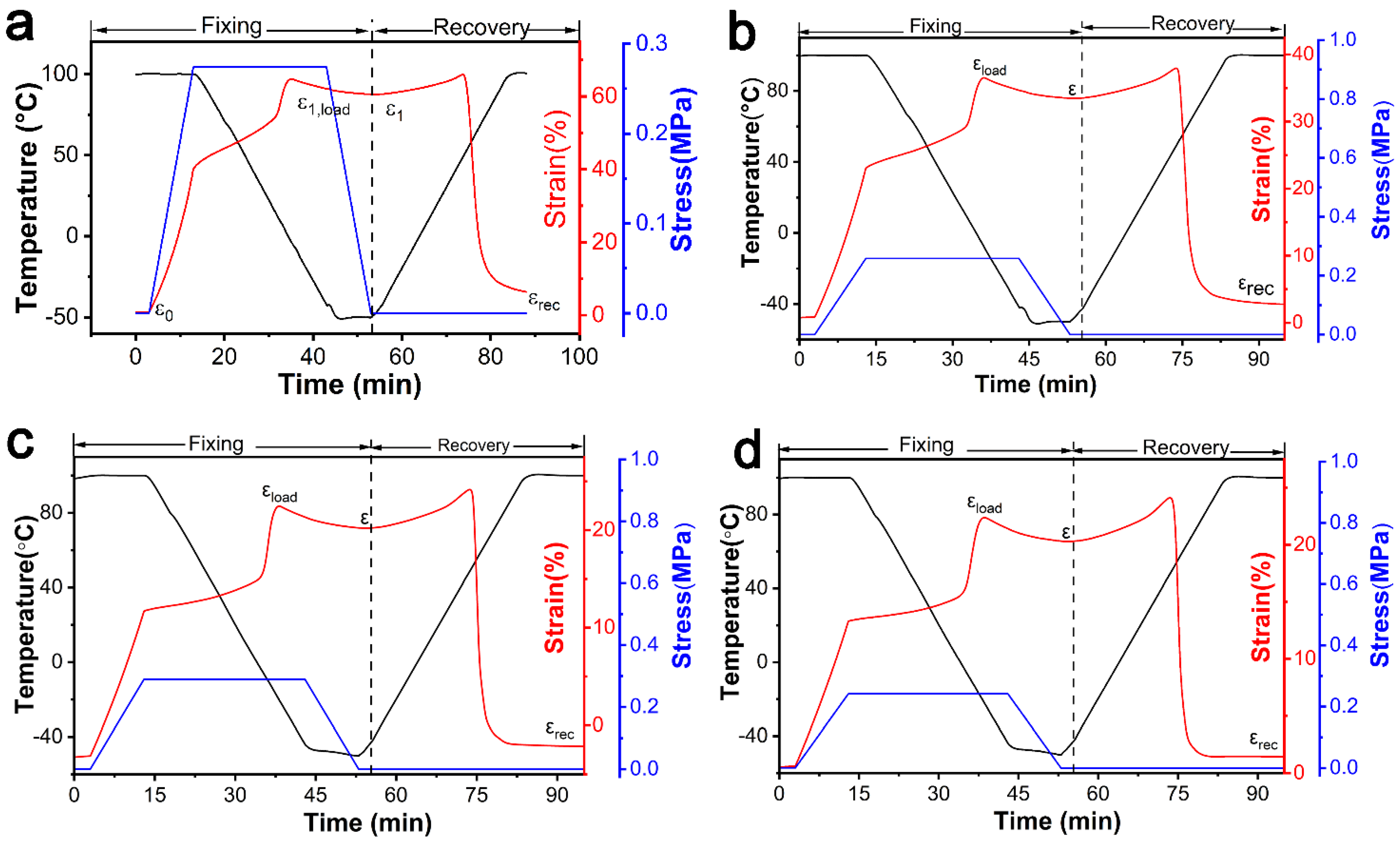
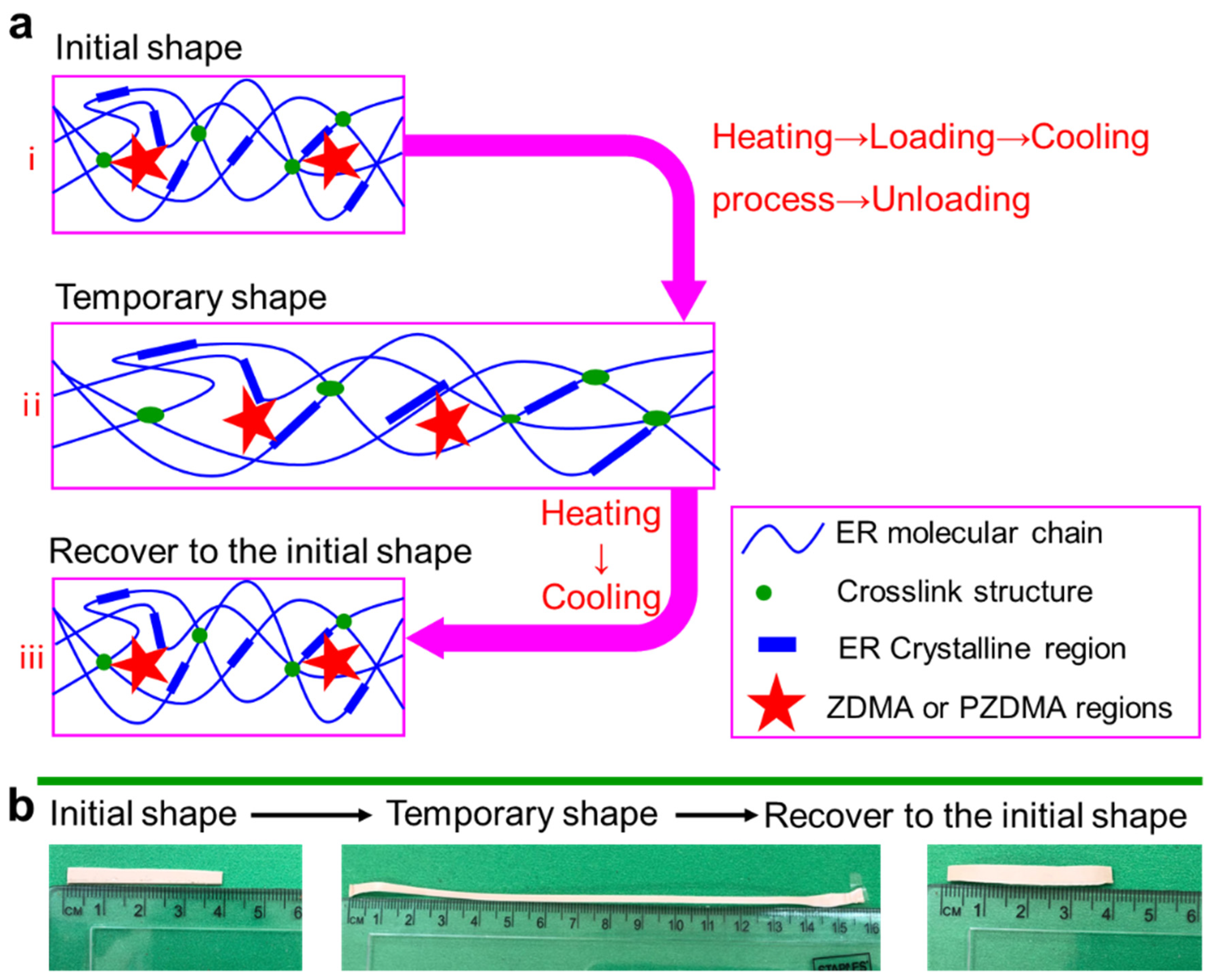
3.1.4. DSC and DMA Analysis of Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A review of shape memory polymers and composites: Mechanisms, materials, and applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Prabhakaran, M.P.; Parvin, N.; Ramakrishna, S. Thermally-induced two-way shape memory polymers: Mechanisms, structures, and applications. Chem. Eng. J. 2019, 374, 706–720. [Google Scholar] [CrossRef]
- Ze, Q.; Kuang, X.; Wu, S.; Wong, J.; Montgomery, S.M.; Zhang, R.; Kovitz, J.M.; Yang, F.Y.; Qi, H.J.; Zhao, R. Magnetic shape memory polymers with integrated multifunctional shape manipulation. Adv. Mater. 2020, 32, 1906657. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shi, C.; Wang, J.; Di, S.; Zhou, S. pH-triggered intracellular release from actively targeting polymer micelles. J. Biomater. 2013, 34, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Tekay, E. Preparation and characterization of electro-active shape memory PCL/SEBS-g-MA/MWCNT nanocomposites. Polymer 2020, 209, 122989. [Google Scholar] [CrossRef]
- Delaey, J.; Dubruel, P.; Van Vlierberghe, S. Shape-Memory Polymers for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1909047. [Google Scholar] [CrossRef]
- Chen, H.M.; Wang, L.; Zhou, S.B. Recent progress in shape memory polymers for biomedical applications. Chin. J. Polym. Sci. 2018, 36, 905–917. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Zhang, F.; Leng, J.; Liu, Y. Shape memory polymers and their composites in biomedical applications. Mat. Sci. Eng. C Mater. 2019, 97, 864–883. [Google Scholar] [CrossRef]
- Chan, B.Q.Y.; Low, Z.W.K.; Heng, S.J.W.; Chan, S.Y.; Owh, C.; Loh, X.J. Recent advances in shape memory soft materials for biomedical applications. ACS Appl. Mater. Interfaces 2016, 8, 10070–10087. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Liu, L.; Leng, J. Shape memory polymers and their composites in aerospace applications: A review. Smart Mater. Struct. 2014, 23, 023001. [Google Scholar] [CrossRef]
- Castano, L.M.; Flatau, A.B. Smart fabric sensors and e-textile technologies: A review. Smart Mater. Struct. 2014, 23, 053001. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.; Yin, H.; Sun, B.; Gu, B.; Zhang, W. A flexible, high-strength, conductive shape memory composite fabric based on continuous carbon fiber/polyurethane yarn. Smart Mater. Struct. 2020, 29, 085044. [Google Scholar] [CrossRef]
- Du, W.; Jin, Y.; Lai, S.; Shi, L.; Shen, Y.; Yang, H. Multifunctional light-responsive graphene-based polyurethane composites with shape memory, self-healing, and flame retardancy properties. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105686. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, X.; Xiong, Y.; Zhang, B.; Lu, S.; Wang, Q.; Liao, Y.; Yang, Y.; Wang, H. Multi-responsive lanthanide-based hydrogel with encryption, naked eye sensing, shape memory, self-healing, and antibacterial activity. ACS Appl. Mater. Interfaces 2020, 12, 28539–28549. [Google Scholar] [CrossRef] [PubMed]
- Hornat, C.C.; Urban, M.W. Shape memory effects in self-healing polymers. Prog. Polym. Sci. 2020, 102, 101208. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, J.; Ma, Y.; Xia, L. Thermally assisted self-healing and shape memory behaviour of natural rubber based composites. Express Polym. Lett. 2021, 15, 929–939. [Google Scholar] [CrossRef]
- Nöchel, U.; Kratz, K.; Behl, M.; Lendlein, A. Relation-between nanostructural changes and macroscopic effects during reversible temperature-memory effect under stress-free conditions in semicrystalline polymer networks. MRS Online Proc. Libr. 2015, 1718, 41–48. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Zhang, X. Ultrarobust, tough and highly stretchable self-healing materials based on cartilage-inspired noncovalent assembly nanostructure. Nat. Commun. 2021, 12, 1–10. [Google Scholar]
- Liu, J.; Li, X.; Yang, X.; Zhang, X. Recent advances in self-Healable intelligent materials enabled by supramolecular crosslinking design. Adv. Intell. Syst. 2021, 3, 2000183. [Google Scholar] [CrossRef]
- Liu, X.; Su, G.; Guo, Q.; Lu, C.; Zhou, T.; Zhou, C.; Zhang, X. Hierarchically structured self-healing sensors with tunable positive/negative piezoresistivity. Adv. Funct. Mater. 2018, 28, 1706658. [Google Scholar] [CrossRef]
- Liu, X.; Lu, C.; Wu, X.; Zhang, X. Self-healing strain sensors based on nanostructured supramolecular conductive elastomers. J. Mater. Chem. A 2017, 5, 9824–9832. [Google Scholar] [CrossRef]
- Shinohara, K.I.; Kodera, N.; Oohashi, T. Single-molecule imaging of photodegradation reaction in a chiral helical π-conjugated polymer chain. J. Polym. Sci. Pol. Chem. 2010, 48, 4103–4107. [Google Scholar] [CrossRef]
- Mather, P.T.; Luo, X.; Rousseau, I.A. Shape memory polymer research. Annu. Rev. Mater. Res. 2009, 39, 445–471. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Shandas, R.; Safranski, D.; Ortega, A.M.; Sassaman, K.; Gall, K. Strong, tailored, biocompatible shape-memory polymer networks. Adv. Funct. Mater. 2008, 18, 2428–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.J. Shape Memory Polymer. Angew. Chem. 2017, 41, 2035. [Google Scholar]
- Made in China 2025 Issued by the State Council. Available online: http://www.gov.cn/zhengce/content/2015-05/19/content_9784.htm (accessed on 19 May 2015).
- Xu, C.; Cao, L.; Huang, X.; Chen, Y.; Lin, B.; Fu, L. Self-healing natural rubber with tailorable mechanical properties based on ionic supramolecular hybrid network. ACS Appl. Mater. Interfaces 2017, 9, 29363–29373. [Google Scholar] [CrossRef]
- Xia, L.; Wang, Y.; Lu, N.; Xin, Z. Facile fabrication of shape memory composites from natural Eucommia rubber and high density polyethylene. Polym. Int. 2017, 66, 653–658. [Google Scholar] [CrossRef]
- Xia, L.; Xian, J.; Geng, J.; Wang, Y.; Shi, F.; Zhou, Z.; Lu, N.; Du, A.; Xin, Z. Multiple shape memory effects of trans-1,4-polyisoprene and low-density polyethylene blends. Polym. Int. 2017, 66, 1382–1388. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, J.; Xia, L.; Xin, Z. Multiple shape memory behaviors of natural Eucommia ulmoides rubber and polybutene-1 composites. Polym. Int. 2018, 67, 901–908. [Google Scholar] [CrossRef]
- Xian, J.; Geng, J.; Wang, Y.; Xia, L. Quadruple-shape-memory effect of TPI/LDPE/HDPE composites. Polym. Advan. Technol. 2018, 29, 982–988. [Google Scholar] [CrossRef]
- Nie, Y.; Huang, G.; Qu, L.; Zhang, P.; Weng, G.; Wu, J. Cure kinetics and morphology of natural rubber reinforced by the in situ polymerization of zinc dimethacrylate. J. Appl. Polym. Sci. 2010, 115, 99–106. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C. Specific nonlinear viscoelasticity behaviors of natural rubber and zinc dimethacrylate composites due to multi-crosslinking bond interaction by using rubber process analyzer 2000. Polym. Compos. 2011, 32, 1593–1600. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C. Crosslink network evolution of nature rubber/zinc dimethacrylate composite during peroxide vulcanization. Polym. Compos. 2011, 32, 1505–1514. [Google Scholar] [CrossRef]
- Kang, H.; Xu, M.; Wang, H.; Li, L.; Li, J.; Fang, Q.; Zhang, J. Heat-responsive shape memory Eucommia ulmoides gum composites reinforced by zinc dimethacrylate. J. Appl. Polym. Sci. 2020, 137, 49133. [Google Scholar] [CrossRef]
- Quitmann, D.; Reinders, F.M.; Heuwers, B.; Katzenberg, F.; Tiller, J.C. Programming of shape memory natural rubber for near-discrete shape transitions. Acs Appl. Mater. Interfaces 2015, 7, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Hoeher, R.; Raidt, T.; Rose, M.; Katzenberg, F.; Tiller, J.C. Recoverable strain storage capacity of shape memory polyethylene. J. Polym. Sci. Pol. Phys. 2013, 51, 1033–1040. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Tian, M.; Geng, H.; Zhang, L. Study on mechanical properties of elastomers reinforced by zinc dimethacrylate. Eur. Polym. J. 2005, 41, 589–598. [Google Scholar] [CrossRef]
- Peng, Z.; Liang, X.; Zhang, Y.; Zhang, Y. Reinforcement of EPDM by in situ prepared zinc dimethacrylate. J. Appl. Polym. Sci. 2002, 84, 1339–1345. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Z. A comparative study on the properties of Eucommia ulmoides gum and synthetic trans-1, 4-polyisoprene. Polym. Test. 2011, 30, 753–759. [Google Scholar] [CrossRef]
- Çavdar, S.; Özdemir, T.; Usanmaz, A. Comparative study on mechanical, thermal, viscoelastic and rheological properties of vulcanised EPDM rubber. Plast. Rubber Compos. 2010, 39, 277–282. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, Z.; Wu, W.; Wang, Z.; Fu, L. Dynamically vulcanized PP/EPDM blends with balanced stiffness and toughness via in-situ compatibilization of MAA and excess ZnO nanoparticles: Preparation, structure and properties. Compos. Part B Eng. 2019, 160, 147–157. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Yang, K.; Zhao, S.G.; Guo, L.N. Self-healing behavior of ethylene propylene diene rubbers based on ionic association. Chin. J. Polym. Sci. 2019, 37, 700–707. [Google Scholar] [CrossRef]
- Sasikumar, K.; Jayesh, P.; Manoj, N.R.; Mukundan, T.; Khastgir, D. Effect of pristine multiwalled carbon nanotubes on tensile hysteresis in carboxylated nitrile rubber composites. Polym. Compos. 2018, 39, 1269–1279. [Google Scholar] [CrossRef]



| Chemical (phr) a | A | B | C | D | E |
|---|---|---|---|---|---|
| ER | 100 | 100 | 100 | 100 | 100 |
| ZDMA | 5 | 5 | 5 | 5 | 0 |
| Antioxidant 264 | 2 | 2 | 2 | 2 | 2 |
| DCP | 0.2 | 0.4 | 0.8 | 1.2 | 0.2 |
| Properties | A | B | C | D | E |
|---|---|---|---|---|---|
| Rf (%) | 93.51 ± 0.30 | 91.65 ± 0.25 | 89.98 ± 0.28 | 90.66 ± 0.27 | 95.30 ± 0.26 |
| Rr (%) | 93.93 ± 0.28 | 94.33 ± 0.11 | 94.65 ± 0.20 | 95.58 ± 0.18 | 90.12 ± 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Meng, J.; Ma, Y.; Zhao, P. Facile Fabrication of Eucommia Rubber Composites with High Shape Memory Performance. Polymers 2021, 13, 3479. https://doi.org/10.3390/polym13203479
Xia L, Meng J, Ma Y, Zhao P. Facile Fabrication of Eucommia Rubber Composites with High Shape Memory Performance. Polymers. 2021; 13(20):3479. https://doi.org/10.3390/polym13203479
Chicago/Turabian StyleXia, Lin, Jiafeng Meng, Yuan Ma, and Ping Zhao. 2021. "Facile Fabrication of Eucommia Rubber Composites with High Shape Memory Performance" Polymers 13, no. 20: 3479. https://doi.org/10.3390/polym13203479
APA StyleXia, L., Meng, J., Ma, Y., & Zhao, P. (2021). Facile Fabrication of Eucommia Rubber Composites with High Shape Memory Performance. Polymers, 13(20), 3479. https://doi.org/10.3390/polym13203479







