Temperature/Reduction Dual Response Nanogel Is Formed by In Situ Stereocomplexation of Poly (Lactic Acid)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
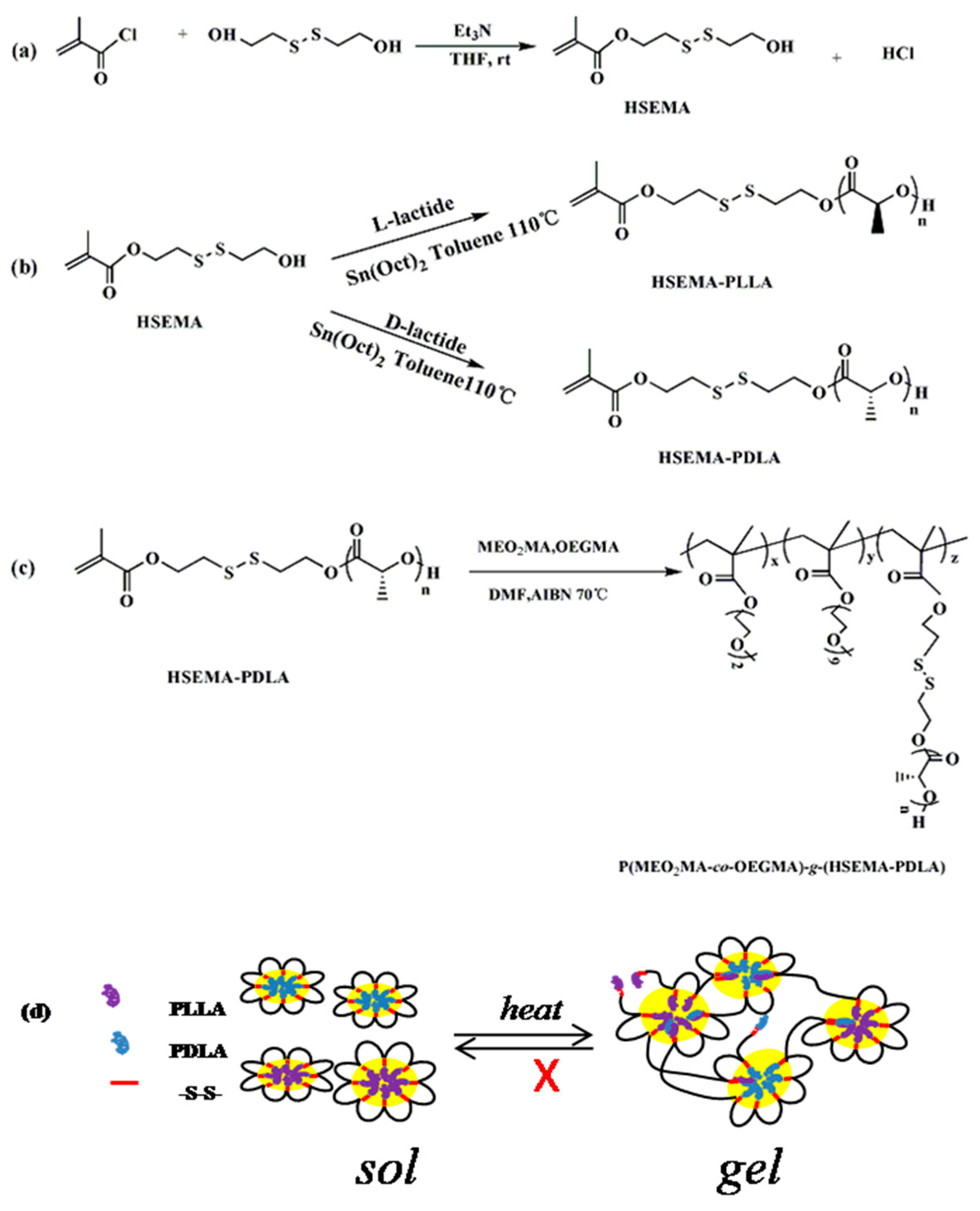
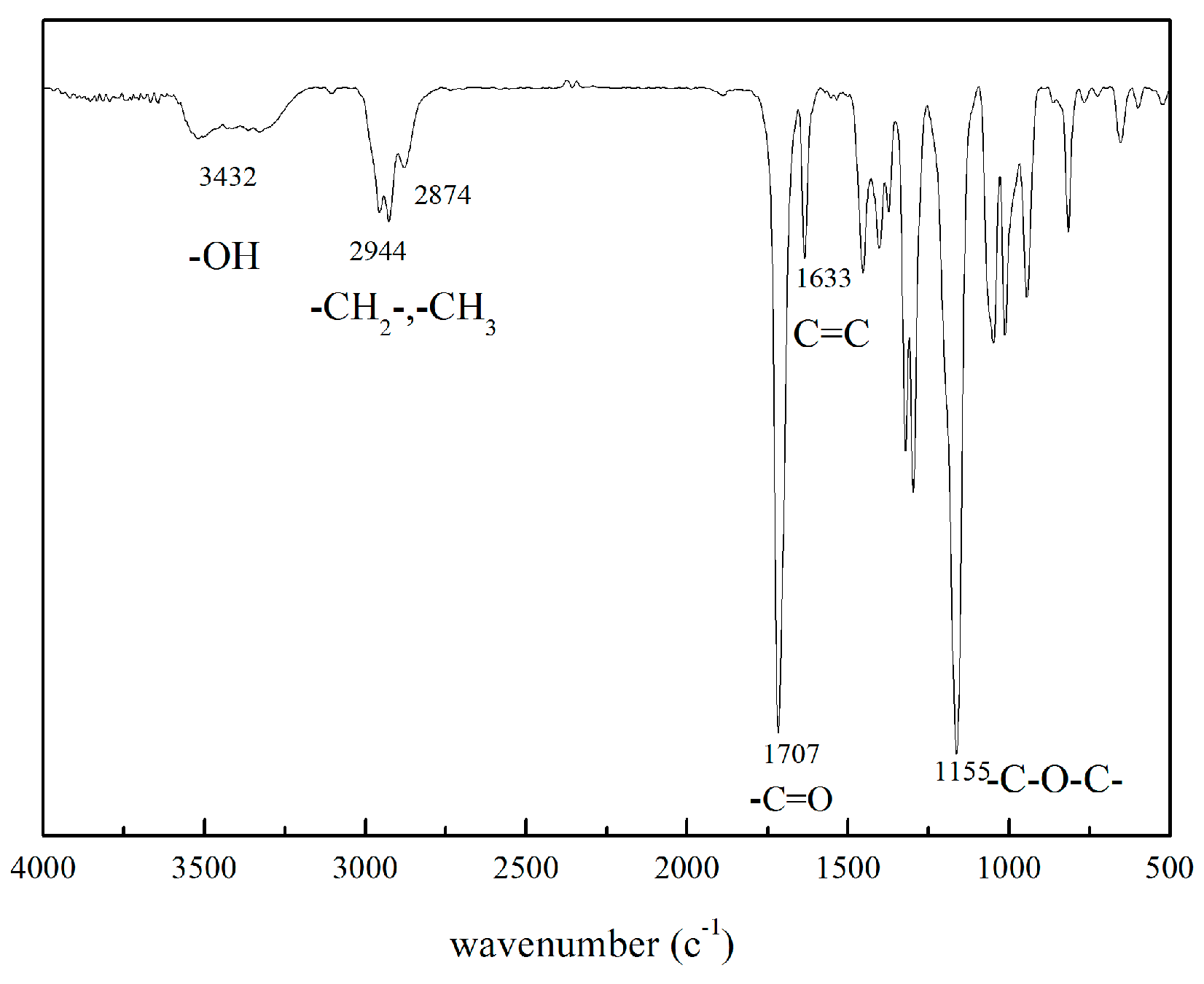
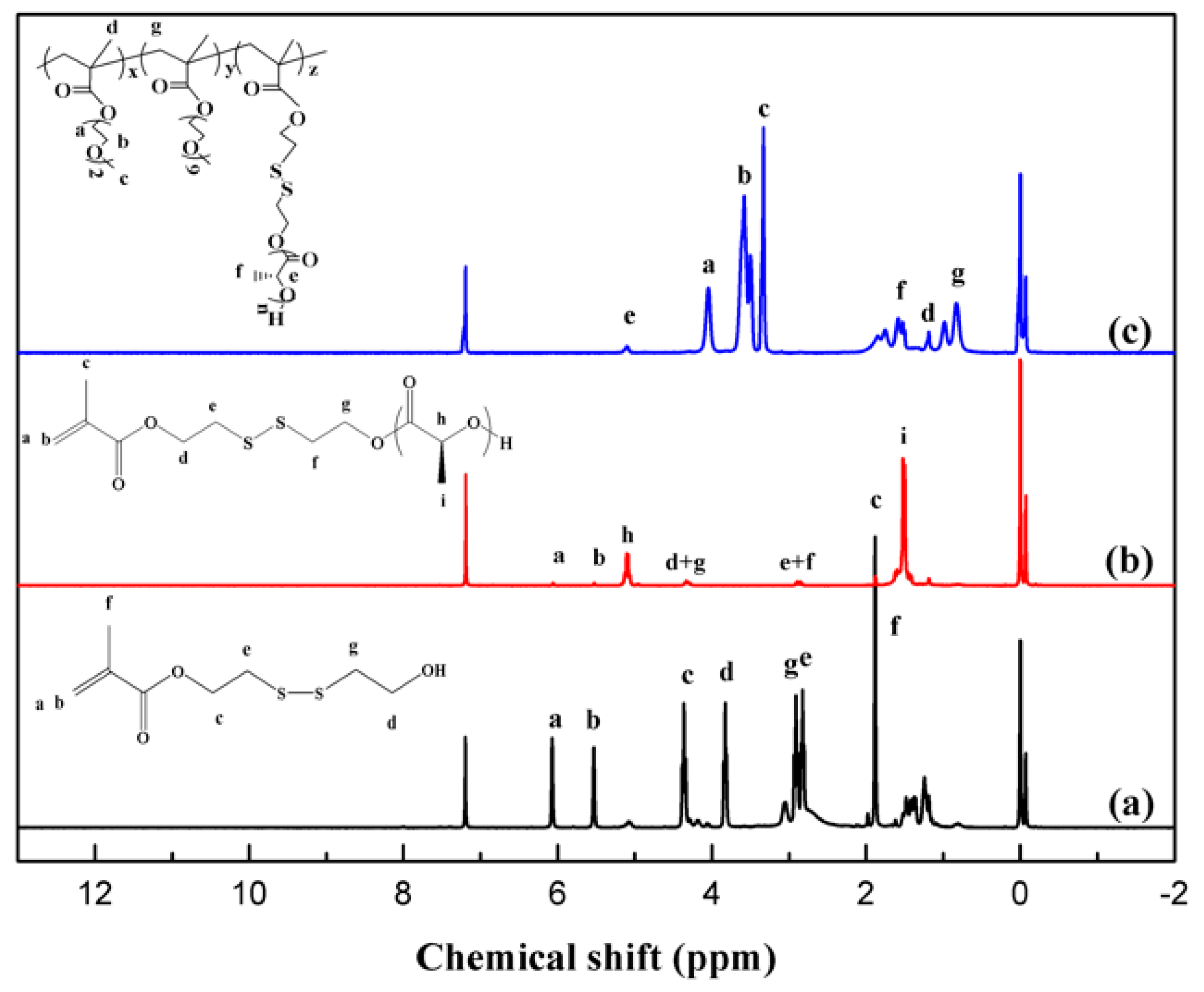
2.2. Synthesis of 2-((2-Hydroxyethyl) Disulfanyl) Ethyl Methacrylate (HSEMA)
2.3. Synthesis of Macromonomer HSEMA-PDLA
2.4. Synthesis of Graft Copolymer P(MEO2MA-co-OEGMA)-g-(HSEMA-PLLA)
2.5. Synthesis of Temperature/Reduction Nanogels
2.6. Characterization of the Samples
2.6.1. Nuclear Magnetic Resonance Spectroscopy (1H-NMR)
2.6.2. Fourier Transform Infrared Spectroscopy (FT-IR)
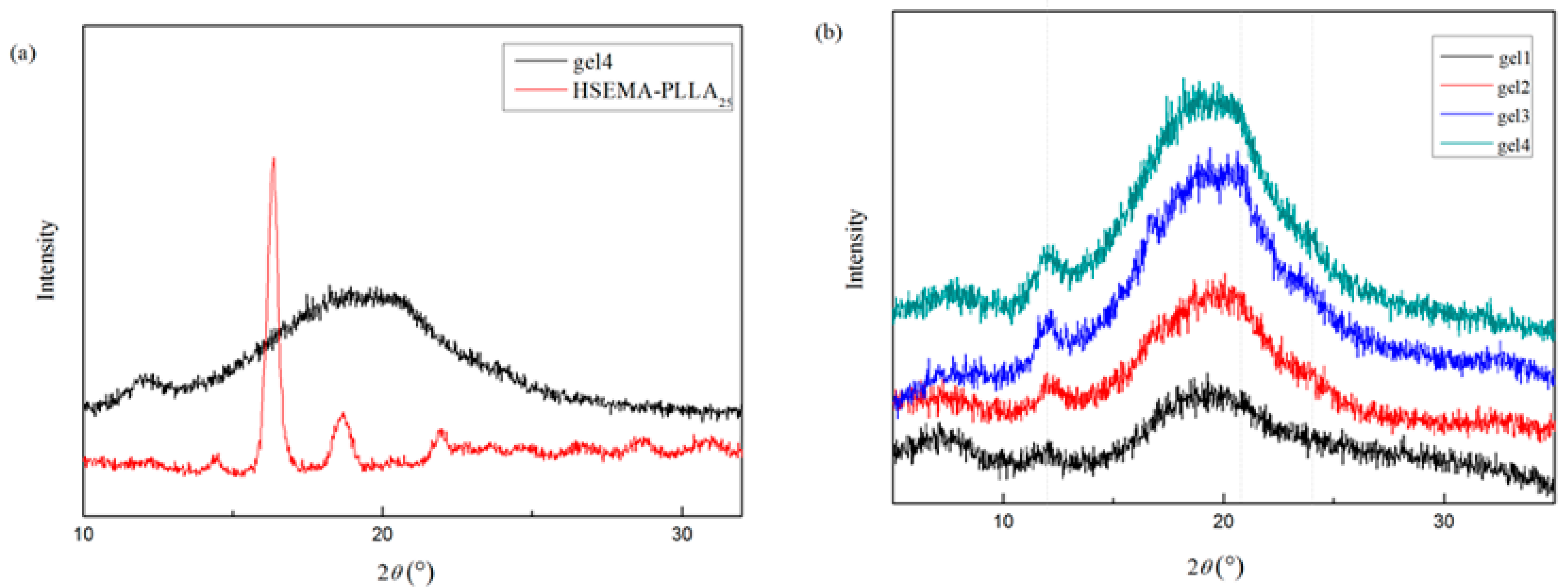
2.6.3. The Wide-Angle X-ray Diffraction (WAXD)
2.6.4. Low Critical Solution Temperature (LCST)
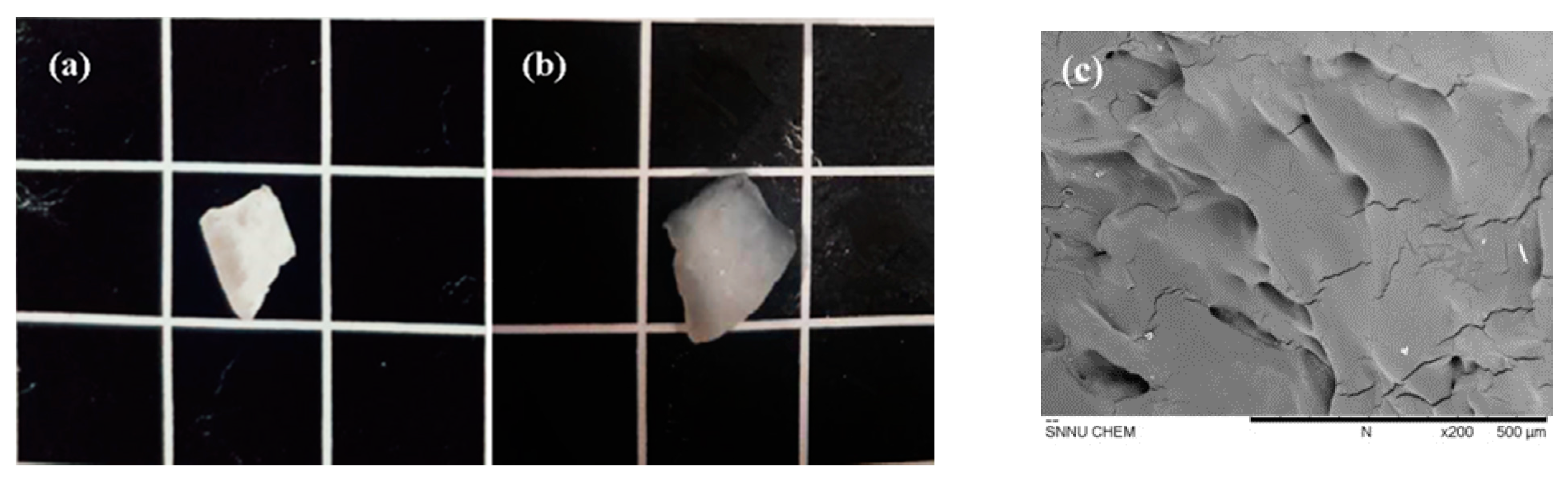
2.6.5. The Scanning Electron Microscope (SEM) Analysis
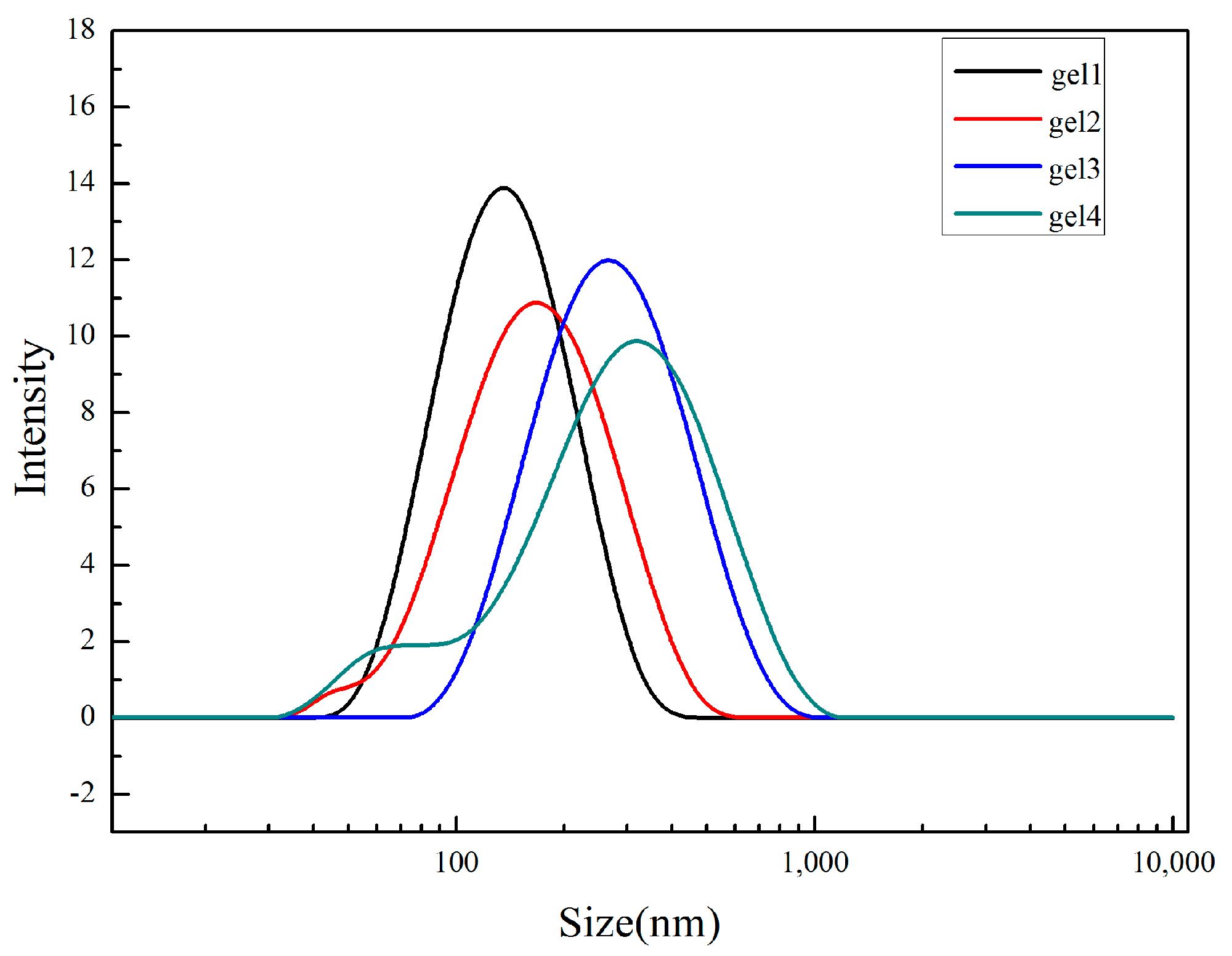
2.6.6. Dynamic Light Scattering (DLS) Analysis
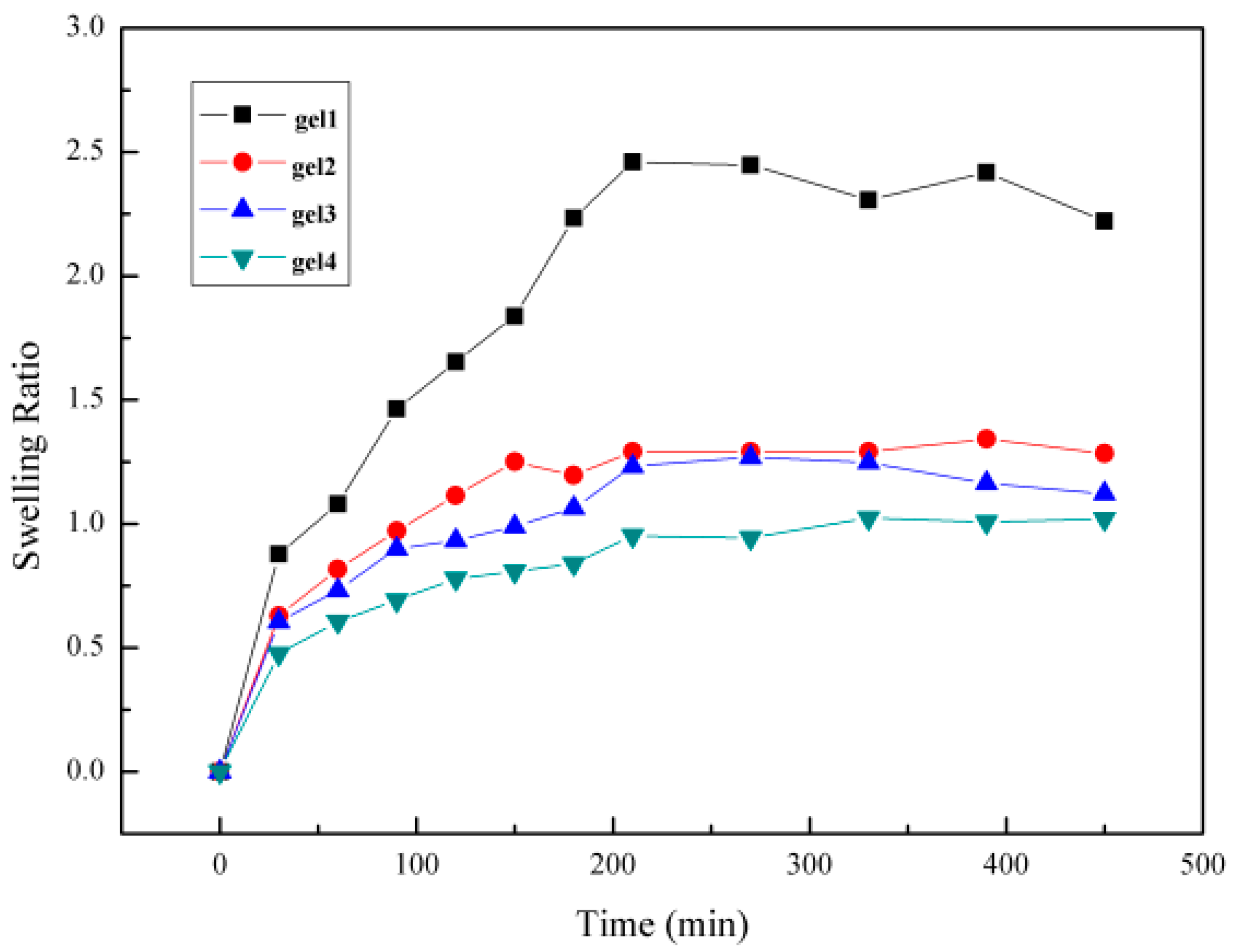
2.6.7. Swelling Kinetics of the Gel
2.6.8. Transmission Electron Microscopy (TEM) Analysis
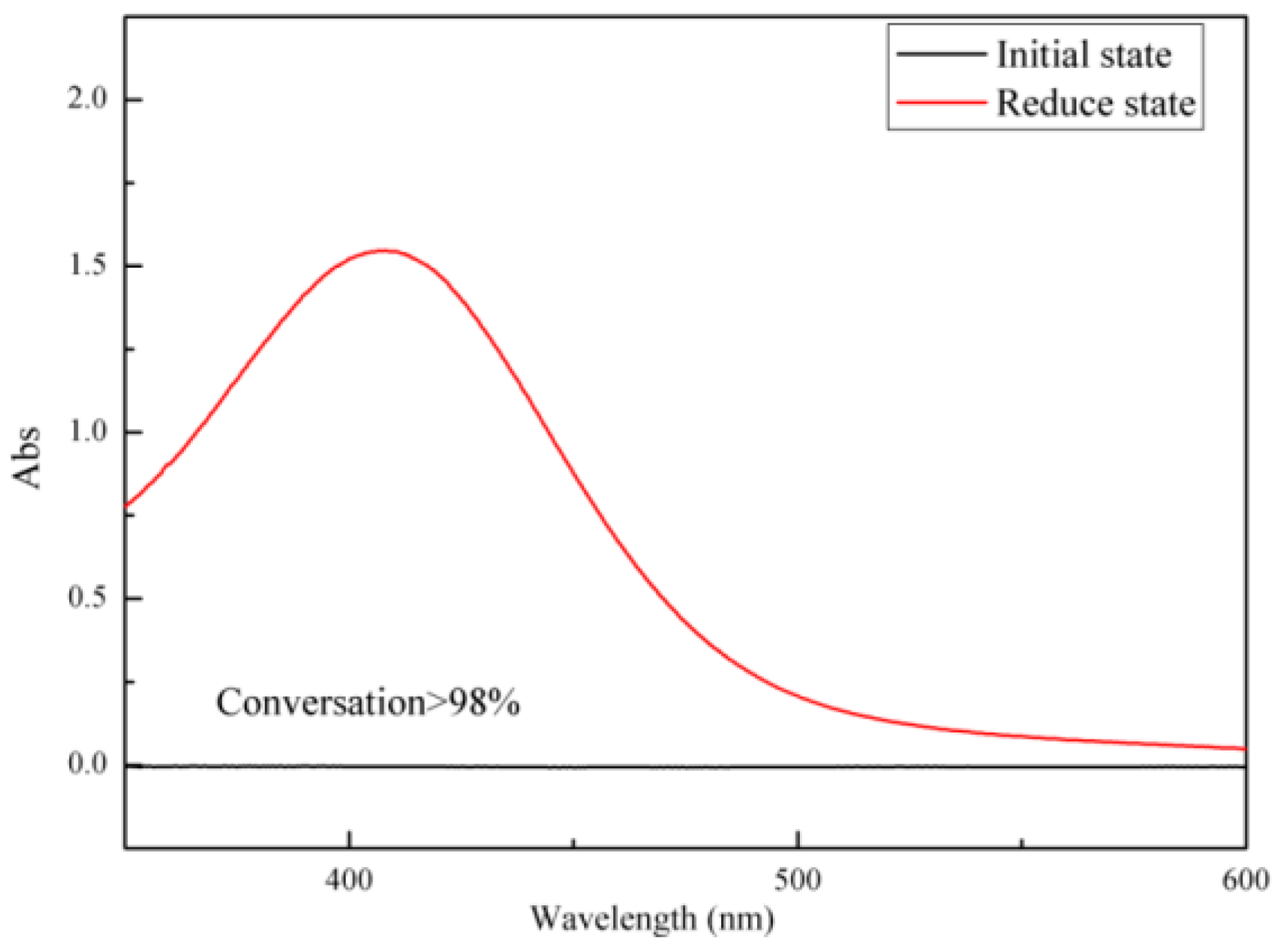
2.6.9. Determination of Disulfide Bond Reduction of Sulfhydryl in Nanohydrogel
3. Results and Discussion
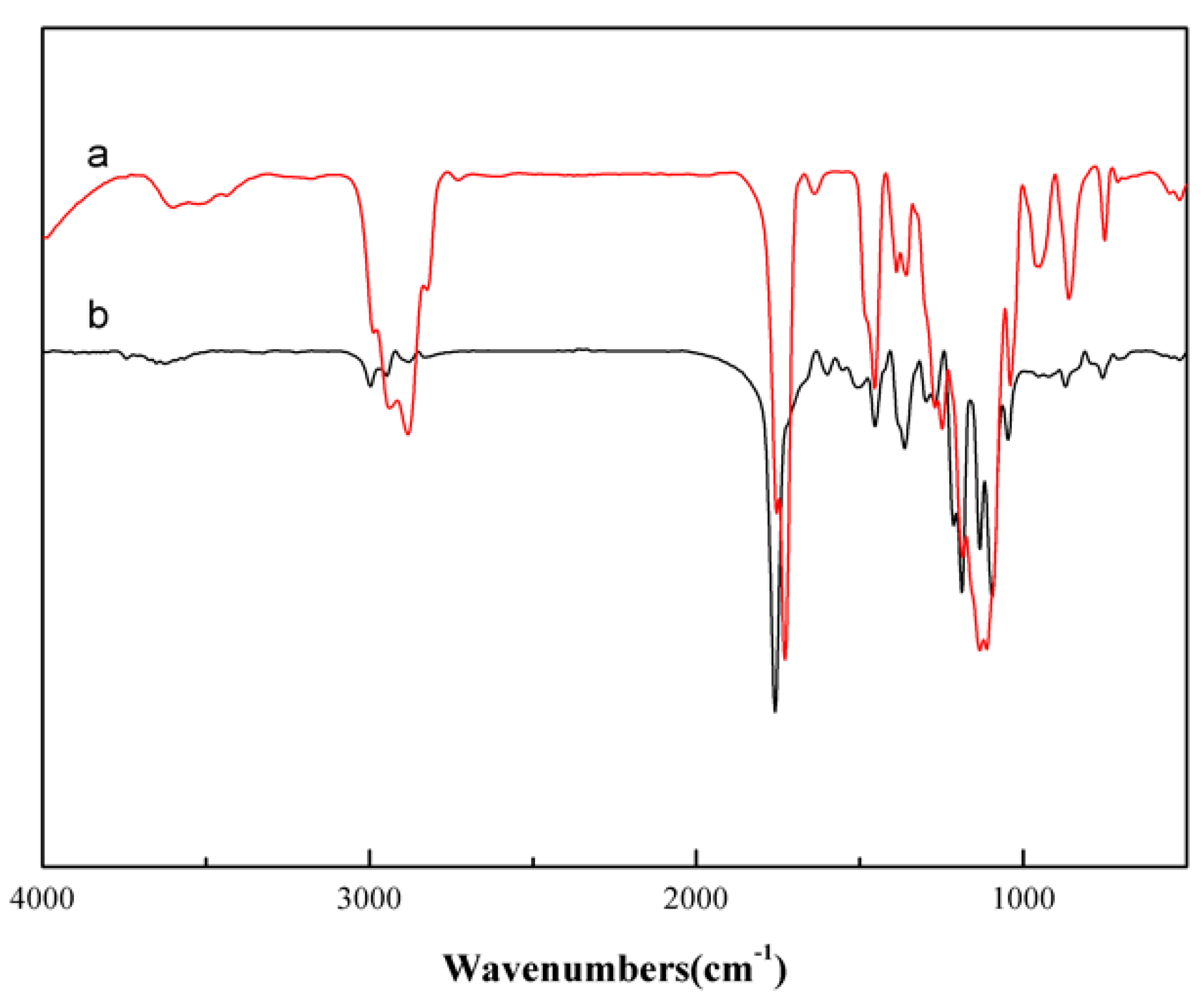
3.1. Structural Characterization of the Initiator, Macromolecular Monomers, and Branching Copolymers
3.2. Analysis of Complex Structure
3.3. Micellar Properties of Hydrogels
3.4. Analysis of the Particle Size and Micro Morphology of Nanogel in Solution
3.5. Determination of Reduced Sulfhydryl Group by Ultraviolet-Visible Spectroscopy
3.6. Analysis of Swelling Kinetics of Hydrogel
3.7. Rheological Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Y.; Akagi, T.; Akashi, M. Self-assembling stereocomplex nanoparticles by enantiomeric poly(g-glutamic acid)-poly(lactide) graft copolymers as a protein delivery carriera. Macromol. Biosci. 2014, 14, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhang, Y.Q.; Xie, Z.G.; Jing, X.B.; Bellotti, A.; Gu, Z. Stimuli-responsive polymersomes for biomedical applications. Biomacromolecules 2017, 18, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Tian, J.; Liu, T.; Zhang, G.Y.; Liu, S.Y. Photo-triggered release of caged camptothecin prodrugs from dually responsive shell cross-linked micelles. Macromolecules 2013, 46, 6243–6256. [Google Scholar] [CrossRef]
- Zhang, L.S.; Liu, Y.C.; Zhang, K.; Chen, Y.W.; Luo, X.L. Redox-responsive comparison of diselenide micelles with disulfide micelles. Colloid Polym. Sci. 2019, 297, 225–238. [Google Scholar] [CrossRef]
- Cai, M.T.; Cao, J.; Wua, Z.Z.; Cheng, F.R.; Chen, Y.W.; Luo, X.L. In vitro and in vivo anti-tumor efficiency comparison of phosphorylcholine micelles with PEG micelles. Colloids Surf. B Biointerfaces 2017, 157, 268–279. [Google Scholar] [CrossRef]
- Jo, I.; Lee, S.; Zhu, J.T.; Shim, T.S.; Yi, G. Soft patchy micelles. Curr. Opin. Colloid Interface Sci. 2017, 30, 97–105. [Google Scholar] [CrossRef]
- Guo, J.X.; Kaletunç, G. Dissolution kinetics of pH responsive alginate-pectin hydrogel particles. Food Res. Int. 2016, 88, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.W.; Zhang, Y.P.; Liu, J.J.; Nie, F.Q. Integrated microfluidic device for the spherical hydrogel pH sensor fabrication. RSC Adv. 2016, 6, 11204–11210. [Google Scholar] [CrossRef]
- Cao, Q.C.; Wang, X.; Wu, D.C. Controlled cross-linking strategy for formation of hydrogels, microgels and nanogels. Chin. J. Polym. Sci. 2018, 36, 8–17. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, Y.; Sun, T.M.; Xiong, M.H.; Wu, J.; Yang, Y.Y.; Wang, J. Core–shell–corona micelle stabilized by reversible cross-linkage for intracellular drug delivery. Macromol. Rapid Commun. 2010, 31, 1201–1206. [Google Scholar] [CrossRef]
- Na, J.H.; Koo, H.; Lee, S.; Min, K.H.; Park, K.; Yoo, H.; Lee, S.H.; Park, J.H.; Kwon, I.C.; Jeong, S.Y.; et al. Real-time and non-invasive optical imaging of tumor-targeting glycol chitosan nanoparticles in various tumor models. Biomaterials 2011, 32, 5252–5261. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Nanogels for regenerative medicine. J. Control. Release 2019, 313, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Mukai, S.; Sasaki, Y.; Akiyoshi, K. Nanogel tectonics for tissue engineering: Protein delivery systems with nanogel chaperones. Adv. Healthc. Mater. 2018, 7, 1800729. [Google Scholar] [CrossRef]
- Chapla, R.; Alhaj Abed, M.; West, J. Modulating Functionalized poly(ethylene glycol) diacrylate hydrogel mechanical properties through competitive crosslinking mechanics for soft tissue applications. Polymers 2020, 12, 3000. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konieczynska, M.D.; Grinstaff, M.W. On- demand dissolution of chemically cross-linked hydrogels. Acc. Chem. Res. 2017, 50, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Fowler, E.W.; Jia, X.Q. Chemical synthesis of biomimetic hydrogels fortissue engineering. Polym. Int. 2017, 66, 1787–1799. [Google Scholar] [CrossRef]
- Chung, H.J.; Park, T.G. Self-assembled and nanostructured hydrogels for drug delivery and tissue engineering. Nano Today 2009, 4, 429–437. [Google Scholar] [CrossRef]
- Sytze, J.; Buwalda, P.J.; Dijkstra, J.F. In situ forming stereocomplexed and post-photocrosslinked acrylated star poly(ethylene glycol)-poly(lactide) hydrogels. Eur. Polym. J. 2017, 94, 152–161. [Google Scholar]
- Akhlaq, M.; Azad, A.K.; Ullah, I.; Nawaz, A.; Safdar, M.; Bhattacharya, T.; Uddin, A.B.M.H.; Abbas, S.A.; Mathews, A.; Kundu, S.K.; et al. Methotrexate-loaded gelatin and polyvinyl alcohol (Gel/PVA) hydrogel as a pH-sensitive matrix. Polymers 2021, 13, 2300. [Google Scholar] [CrossRef]
- Guo, H.; Mussault, C.; Marcellan, A.; Hourdet, D.; Sanson, N. Hydrogels with dual thermoresponsive mechanical performance. Macromol. Rapid Commun. 2017, 38, 1700287. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, X.Y.; Wang, Z.D.; Song, F.; Gao, W.L.; Liu, S.X. Stereocomplex poly(Lactic Acid) amphiphilic conetwork gel with Temperature and pH dual sensitivity. Polymers 2019, 11, 1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.Z.; Zou, C.J. Pentaerythrityl tetra-β-cyclodextrin: Synthesis, characterization and application in multiple responses hydrogel. Colloids Surfaces A 2017, 529, 571–579. [Google Scholar] [CrossRef]
- Yang, H.; Li, C.H.; Tang, J.D.; Suo, Z.G. Strong and degradable adhesion of hydrogels. ACS Appl. Bio Mater. 2019, 2, 1781–1786. [Google Scholar] [CrossRef]
- Kamata, H.; Kushiro, K.; Takai, M.; Chung, U.; Sakai, T. Non-osmotic hydrogels: A rational strategy for safely degradable hydrogels. Angew. Chem. Int. Ed. 2016, 55, 9282–9286. [Google Scholar] [CrossRef]
- Patenaude, M.; Hoare, T. Injectable, degradable thermoresponsive poly(N-isopropylacrylamide) hydrogels. ACS Macro Lett. 2012, 1, 409–413. [Google Scholar] [CrossRef]
- Pei, Y.; Molley, T.G.; Kilian, K.A. Enzyme responsive inverse opal hydrogels. Macromol. Rapid Commun. 2020, 41, 1900555. [Google Scholar] [CrossRef] [PubMed]
- Peschke, T.; Bitterwolf, P.; Gallus, S.; Hu, Y.; Oelschlaeger, C.; Willenbacher, N.; Rabe, K.S.; Niemeyer, C.M. Self-assembling all-enzyme hydrogels for flow biocatalysis. Angew. Chem. Int. Ed. 2018, 57, 17028–17032. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Samanta, P.; Dhara, D. Temperature, pH and redox responsive cellulose based hydrogels for protein delivery. Int. J. Biol. Macromol. 2016, 87, 92–100. [Google Scholar] [CrossRef]
- Yildirim, T.; Traeger, A.; Preussger, E.; Stumpf, S.; Fritzsche, C.; Hoeppener, S.; Schubert, S.; Schubert, U.S. Dual responsive nanoparticles from a RAFT copolymer library for the controlled delivery of doxorubicin. Macromolecules 2016, 49, 3856–3868. [Google Scholar] [CrossRef]
- An, X.N.; Zhu, A.J.; Luo, H.H.; Ke, H.T.; Chen, H.B.; Zhao, Y.L. Rational design of multi-stimuli-responsive nanoparticles for precise cancer therapy. ACS Nano 2016, 10, 5947–5958. [Google Scholar] [CrossRef]
- Hu, X.L.; Hu, J.M.; Tian, J.; Ge, Z.S.; Zhang, G.Y.; Luo, K.F.; Liu, S.Y. Polyprodrug amphiphiles: Hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J. Am. Chem. Soc. 2013, 135, 17617–17629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xu, L.; Liu, F.; Zhang, W.A. Construction of reduction-responsive photosensitizers based on amphiphilic block copolymers and their application for photodynamic therapy. Polymer 2016, 97, 323–334. [Google Scholar] [CrossRef]
- Abebe, D.G.; Fujiwara, T. Controlled thermoresponsive hydrogels by stereocomplexed PLA-PEG-PLA prepared via hybrid micelles of pre-mixed copolymers with different PEG lengths. Biomacromolecules 2012, 13, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Pholharn, D.; Cheerarot, O.; Baimark, Y. Stereocomplexation and mechanical properties of polylactide-b-poly(propylene glycol)-b-polylactide blend films: Effects of polylactide block length and blend ratio. Chin. J. Polym. Sci. 2017, 35, 1391–1401. [Google Scholar] [CrossRef]
- Che, Y.J.; Zschoche, S.; Obst, F.; Appelhans, D.; Voit, B. Double-crosslinked reversible redox-responsive hydrogels based on disulfide-thiol interchange. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2590–2601. [Google Scholar] [CrossRef]
- Baimark, Y.; Pasee, S.; Rungseesantivanon, W.; Prakymoramas, N. Flexible and high heat-resistant stereocomplex PLLA-PEG-PLLA/PDLA blends prepared by melt process: Effect of chain extension. J. Polym. Res. 2019, 26, 218. [Google Scholar] [CrossRef]
- Hiemstra, C.; Zhong, Z.Y.; Li, L.B.; Dijkstra, P.J.; Feijen, J. In-situ formation of biodegradable hydrogels by stereocomplexation of PEG-(PLLA)8 and PEG-(PDLA)8 star block copolymers. Biomacromolecules 2006, 7, 2790–2795. [Google Scholar] [CrossRef]




| Sample | [HSEMA]:[PLLA] (n:n) | Mna (Theory) | Mnb (1H NMR) | [M]:[O] c (n:n) | [P(MOL)]:[P(MOD)] (n:n) |
|---|---|---|---|---|---|
| gel1 | 1:13 | 1158 | 1230 | 95:5 | 1:1 |
| gel2 | 1:16 | 1374 | 1518 | 95:5 | 1:1 |
| gel3 | 1:20 | 1662 | 1878 | 95:5 | 1:1 |
| gel4 | 1:25 | 2022 | 2310 | 95:5 | 1:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Wang, Z.; Song, F.; Fu, Y.; Wu, Q.; Liu, S. Temperature/Reduction Dual Response Nanogel Is Formed by In Situ Stereocomplexation of Poly (Lactic Acid). Polymers 2021, 13, 3492. https://doi.org/10.3390/polym13203492
Gao W, Wang Z, Song F, Fu Y, Wu Q, Liu S. Temperature/Reduction Dual Response Nanogel Is Formed by In Situ Stereocomplexation of Poly (Lactic Acid). Polymers. 2021; 13(20):3492. https://doi.org/10.3390/polym13203492
Chicago/Turabian StyleGao, Wenli, Zhidan Wang, Fei Song, Yu Fu, Qingrong Wu, and Shouxin Liu. 2021. "Temperature/Reduction Dual Response Nanogel Is Formed by In Situ Stereocomplexation of Poly (Lactic Acid)" Polymers 13, no. 20: 3492. https://doi.org/10.3390/polym13203492
APA StyleGao, W., Wang, Z., Song, F., Fu, Y., Wu, Q., & Liu, S. (2021). Temperature/Reduction Dual Response Nanogel Is Formed by In Situ Stereocomplexation of Poly (Lactic Acid). Polymers, 13(20), 3492. https://doi.org/10.3390/polym13203492






