Hierarchical Porous MIL-101(Cr) Solid Acid-Catalyzed Production of Value-Added Acetals from Biomass-Derived Furfural
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalyst
2.2.1. Preparation of Hierarchical Porous MIL-101(Cr)
2.2.2. Sulfonation of Hierarchical MIL-101(Cr)
2.3. Characterization of the Catalyst
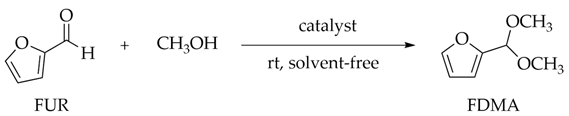
2.4. General Procedure of Catalytic Reaction and Product Analysis
3. Results and Discussion
3.1. Characterization of Catalysts
3.2. Effect of Different Catalysts on the Acetalization of FUR with Methanol
3.3. Effect of Catalyst Dosage on the Acetalization of FUR with Methanol
3.4. Effect of Temperature on the Acetalization of FUR with Methanol
3.5. Substrate Test
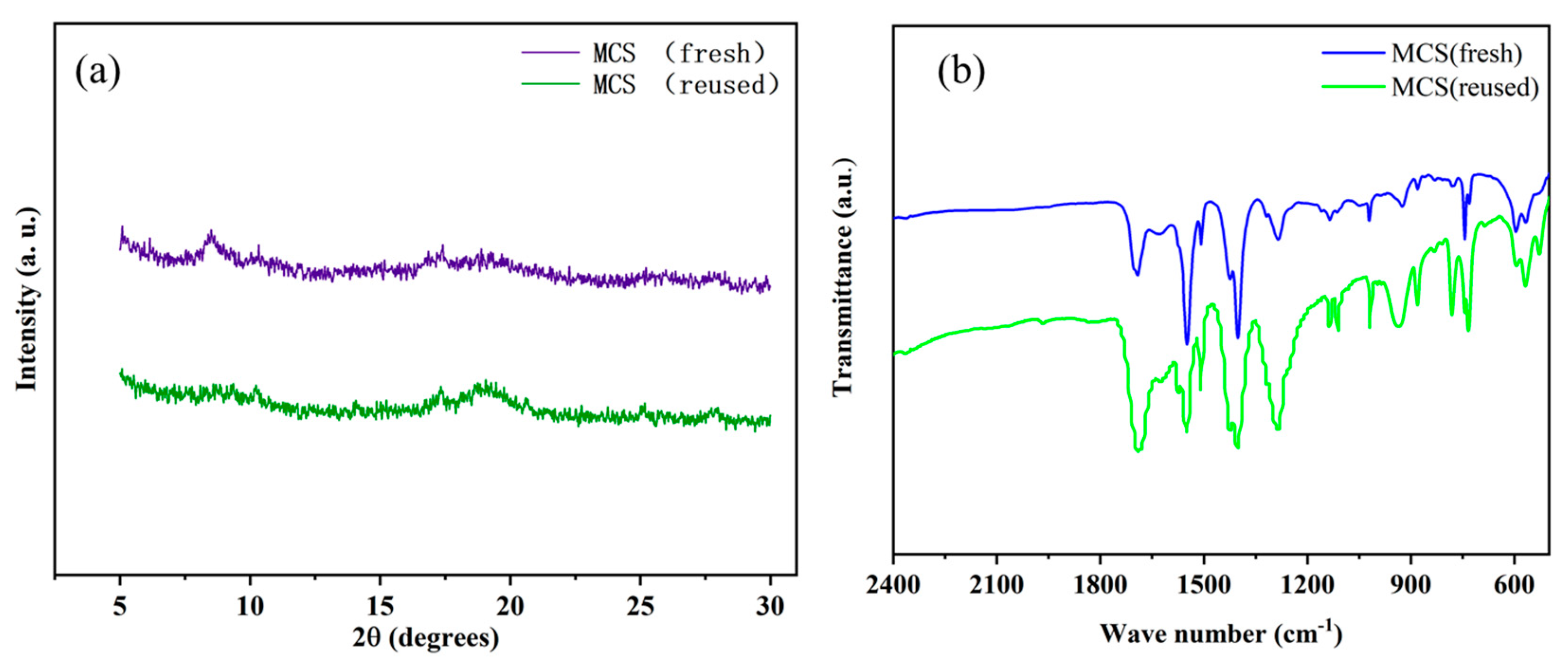
3.6. Catalyst Recycling Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Mika, L.T.; Csefalvay, E.; Nemeth, A. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Saravanamurugan, S.; Yang, S. Catalytic Upgrading of Biomass-Derived Sugars with Acidic Nanoporous Materials: Structural Role in Carbon-Chain Length Variation. ChemSusChem 2019, 12, 347–378. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Weingarten, R.; Cho, J.; Conner, J.W.C.; Huber, G.W. Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem. 2010, 12, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- Lange, J.P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural a promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Smith, R.L.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Stepan, E.; Enascuta, C.E.; Oprescu, E.E.; Radu, E.; Vasilievici, G.; Radu, A.; Stoica, R.; Velea, S.; Nicolescu, A.; Lavric, V. A versatile method for obtaining new oxygenated fuel components from biomass. Ind. Crop. Prod. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Alipour, S.; Omidvarborna, H.; Kim, D.S. A review on synthesis of alkoxymethyl furfural, a biofuel candidate. Renew. Sust. Energy Rev. 2017, 71, 908–926. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Chernyak, M.Y.; Simakova, I.L.; Kaigorodov, K.L.; Bezborodov, Y.N.; Orlovskaya, N.F. Antiknock properties of furfural derivatives. Russ. J. Appl. Chem. 2016, 88, 1778–1782. [Google Scholar] [CrossRef]
- Manzetti, S.; Andersen, O. A review of emission products from bioethanol and its blends with gasoline. Background for new guidelines for emission control. Fuel 2015, 140, 293–301. [Google Scholar] [CrossRef]
- Senthur Prabu, S.; Asokan, M.A.; Prathiba, S.; Ahmed, S.; Puthean, G. Effect of additives on performance, combustion and emission behavior of preheated palm oil/diesel blends in DI diesel engine. Renew. Energy 2018, 122, 196–205. [Google Scholar] [CrossRef]
- Kirchhecker, S.; Dell’Acqua, A.; Angenvoort, A.; Spannenberg, A.; Ito, K.; Tin, S.; Taden, A.; de Vries, J.G. HMF–glycerol acetals as additives for the debonding of polyurethane adhesives. Green Chem. 2021, 23, 957–965. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Jothi Ramalingam, R.; Al-Lohedan, H.A.; Khoerunnisa, F.; Ling, T.C.; Ng, E.P. Selective synthesis of dioxolane biofuel additive via acetalization of glycerol and furfural enhanced by MCM-41-alanine bifunctional catalyst. Fuel 2021, 288, 119573–119584. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Formation of C–C and C–Heteroatom Bonds by C–H Activation by Metal Organic Frameworks as Catalysts or Supports. ACS Catal. 2018, 9, 1081–1102. [Google Scholar] [CrossRef]
- Teixeira, M.G.; Natalino, R.; da Silva, M.J. A kinetic study of heteropolyacid-catalyzed furfural acetalization with methanol at room temperature via ultraviolet spectroscopy. Catal. Today 2020, 344, 143–149. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Hayashi, E.; Hatamachi, T.; Kodama, T.; Kitayama, Y. SO3H-functionalized silica for acetalization of carbonyl compounds with methanol and tetrahydropyranylation of alcohols. Tetrahedron Lett. 2004, 45, 5135–5138. [Google Scholar] [CrossRef]
- Wu, S.; Dai, W.; Yin, S.; Li, W.; Au, C.T. Bismuth subnitrate as an efficient heterogeneous catalyst for acetalization and ketalization of carbonyl compounds with diols. Catal. Lett. 2008, 124, 127–132. [Google Scholar] [CrossRef]
- Srinivasulu, M.; Suryakiran, N.; Rajesh, K.; Malla Reddy, S.; Venkateswarlu, Y. Mild and efficient chemoselective synthesis of acetals and geminal diacetates (acylals) from aldehydes using lanthanum (III) nitrate hexahydrate. Synth. Commun. 2008, 38, 1753–1759. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Puche, M.; Fornes, V.; Garcia, H. Graphene oxide as catalyst for the acetalization of aldehydes at room temperature. ChemCatChem 2012, 4, 2026–2030. [Google Scholar] [CrossRef]
- Yang, M.; Tang, J.; Ma, Q.; Zheng, N.; Tan, L. High activity Fe-MIL-101 solid acid catalyst for acetalization of aldehydes with methanol and enamination of β-dicarbonyl compounds. J. Porous Mat. 2015, 22, 1345–1350. [Google Scholar] [CrossRef]
- Bao, Q.; Qiao, K.; Tomida, D.; Yokoyama, C. Acetalization of carbonyl compounds catalyzed by GaCl3 immobilized on imidazolium-styrene copolymers. Catal. Commun. 2009, 10, 1625–1628. [Google Scholar] [CrossRef]
- Rubio-Caballero, J.M.; Saravanamurugan, S.; Maireles-Torres, P.; Riisager, A. Acetalization of furfural with zeolites under benign reaction conditions. Catal. Today 2014, 234, 233–236. [Google Scholar] [CrossRef]
- Xu, G.; Cao, J.; Zhao, Y.; Zheng, L.; Tao, M.; Zhang, W. Phosphorylated polyacrylonitrile fibers as an efficient and greener acetalization catalyst. Chem. Asian. J. 2017, 12, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, G.; Matsuda, R.; Sato, H.; Takata, M.; Kitagawa, S. Cellulose hydrolysis by a new porous coordination polymer decorated with sulfonic acid functional groups. Adv. Mater. 2011, 23, 3294–3297. [Google Scholar] [CrossRef]
- Fang, R.; Dhakshinamoorthy, A.; Li, Y.; Garcia, H. Metal organic frameworks for biomass conversion. Chem. Soc. Rev. 2020, 49, 3638–3687. [Google Scholar] [CrossRef]
- Hong, D.Y.; Hwang, Y.K.; Serre, C.; Férey, G.; Chang, J.S. Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Nefedov, O.M.; Kustov, L.M. Metal–Organic Frameworks-Based Catalysts for Biomass Processing. Catalysts 2018, 8, 368. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, Z.; Wang, R. Functionalized Metal-Organic Framework Catalysts for Sustainable Biomass Valorization. Adv. Polym. Tech. 2020, 2020, 1201923. [Google Scholar] [CrossRef]
- Qiu, L.G.; Xu, T.; Li, Z.Q.; Wang, W.; Wu, Y.; Jiang, X.; Tian, X.Y.; Zhang, L.D. Hierarchically micro- and mesoporous metal-organic frameworks with tunable porosity. Angew. Chem. Int. Ed. Engl. 2008, 47, 9487–9491. [Google Scholar] [CrossRef]
- Hasan, Z.; Jun, J.W.; Jhung, S.H. Sulfonic acid-functionalized MIL-101(Cr): An efficient catalyst for esterification of oleic acid and vapor-phase dehydration of butanol. Chem. Eng. J. 2015, 278, 265–271. [Google Scholar] [CrossRef]
- Pourreza, A.; Askari, S.; Rashidi, A.; Seif, A.; Kooti, M. Highly efficient SO3Ag-functionalized MIL-101(Cr) for adsorptive desulfurization of the gas stream: Experimental and DFT study. Chem. Eng. J. 2019, 363, 73–83. [Google Scholar] [CrossRef]
- Huang, X.X.; Qiu, L.G.; Zhang, W.; Yuan, Y.P.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Hierarchically mesostructured MIL-101 metal–organic frameworks: Supramolecular template-directed synthesis and accelerated adsorption kinetics for dye removal. CrystEngComm 2012, 14, 1613–1617. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal Organic Frameworks as Solid Acid Catalysts for Acetalization of Aldehydes with Methanol. Adv. Synth. Catal. 2010, 352, 3022–3030. [Google Scholar] [CrossRef]
- Pham, M.-H.; Vuong, G.-T.; Fontaine, F.-G.; Do, T.-O. A Route to Bimodal Micro-Mesoporous Metal–Organic Frameworks Nanocrystals. Cryst. Growth Des. 2011, 12, 1008–1013. [Google Scholar] [CrossRef]
- Seoane, B.; Dikhtiarenko, A.; Mayoral, A.; Tellez, C.; Coronas, J.; Kapteijn, F.; Gascon, J. Metal organic framework synthesis in the presence of surfactants: Towards hierarchical MOFs? CrystEngComm 2015, 17, 1693–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.X.; Chang, G.G.; Tian, G.; Pu, C.; Huang, K.X.; Ke, S.C.; Janiak, C.; Yang, X.Y. Near-Linear Controllable Synthesis of Mesoporosity in Hierarchical UiO-66 by Template-Free Nucleation-Competition. Adv. Funct. Mater. 2021, 31, 2102868–2102875. [Google Scholar] [CrossRef]
- Tateiwa, J.; Horiuchi, H.; Uemura, S. Ce3+-Exchanged Montmorillonite (Ce3+-Mont) as a Useful Substrate-Selective Acetalization Catalyst. J. Org. Chem. 1995, 60, 4039–4043. [Google Scholar] [CrossRef]
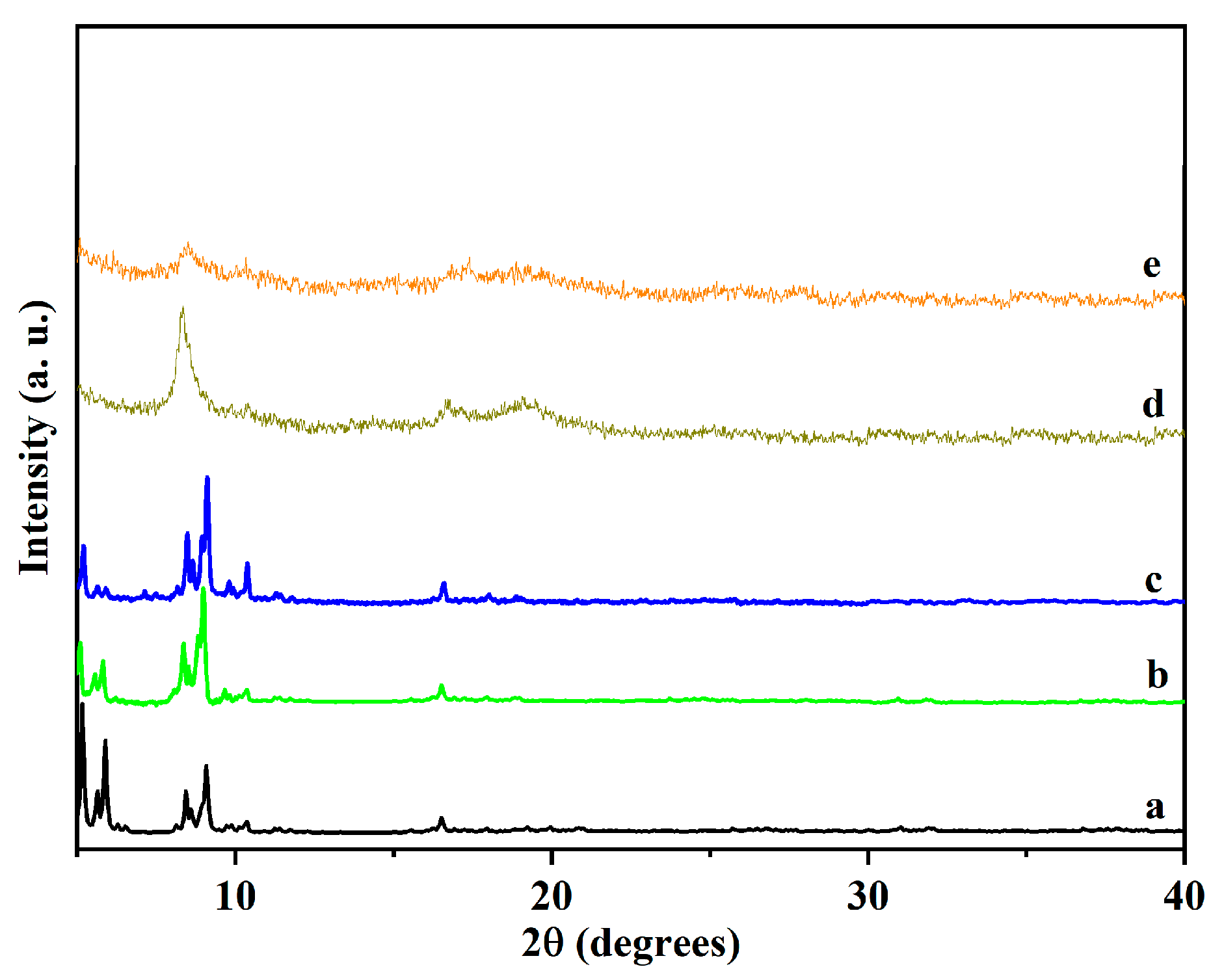
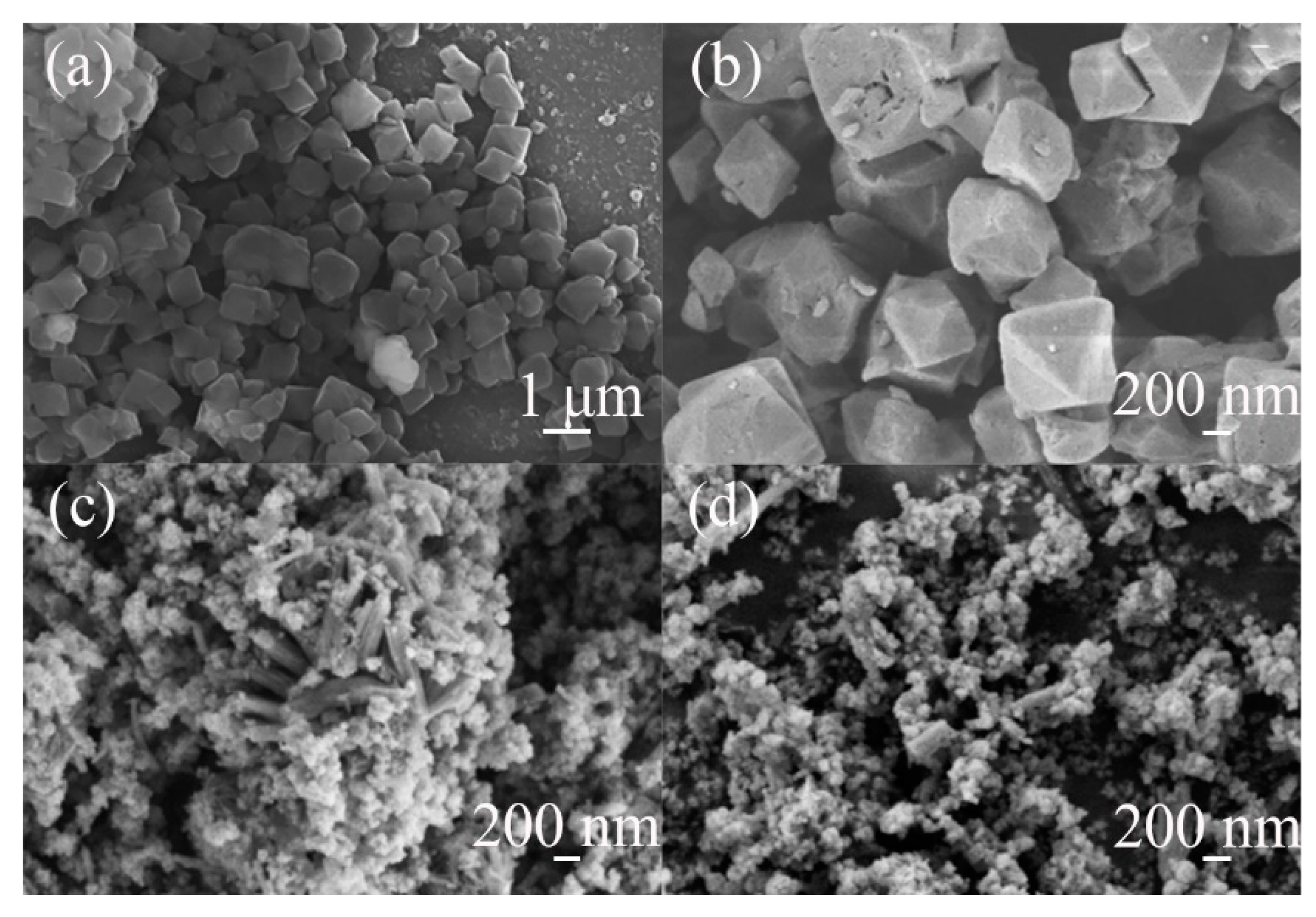
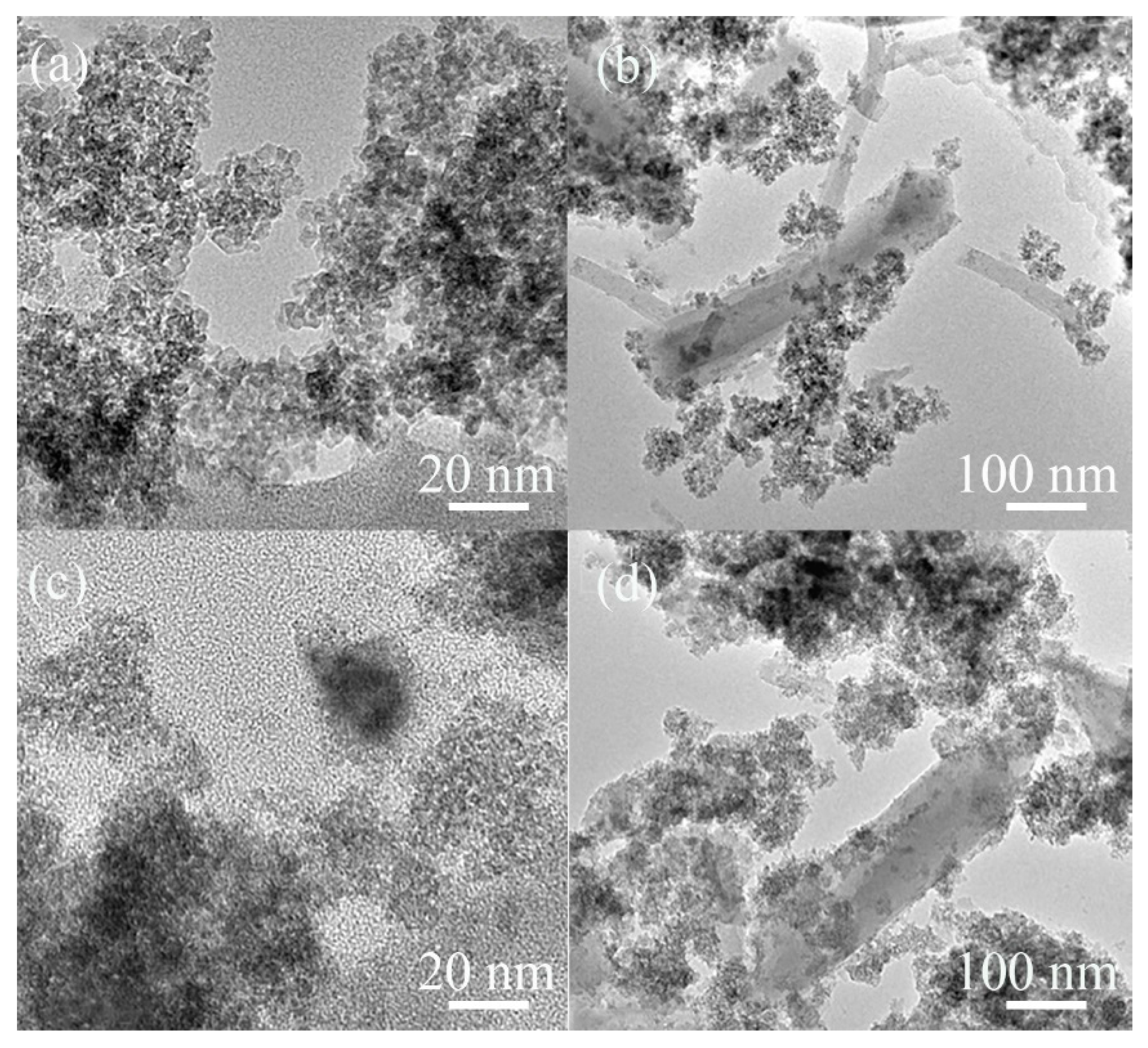
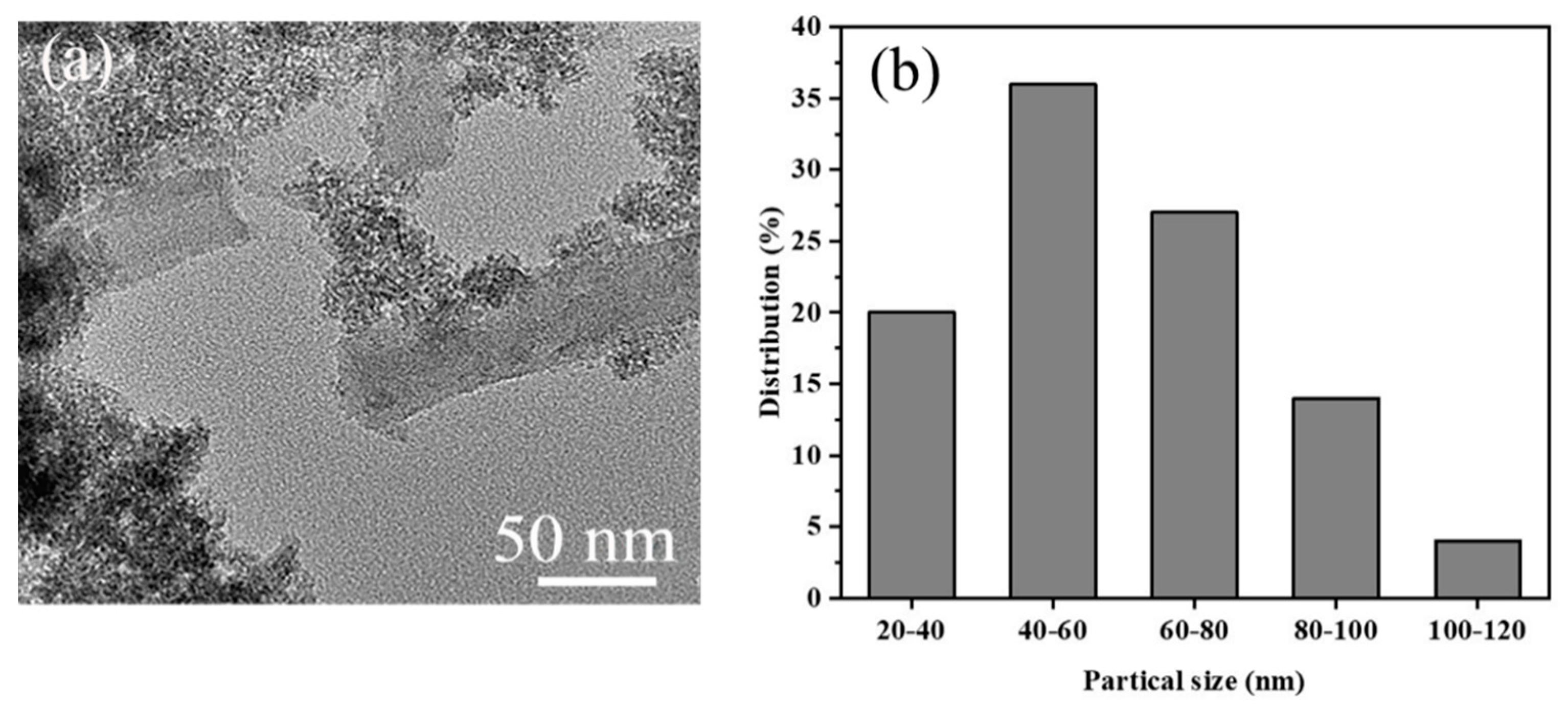




| Catalyst | Pore Size (nm) | SBET (m2/g) | Vt (cm3/g) | Vmeso (cm3/g) | Vmeso/Vmicro | Atitration (mmol(H+)/g) |
|---|---|---|---|---|---|---|
| MA | 1.71 | 2603 | 1.30 | 0.39 | 0.42 | — |
| MAS | 1.38 | 1825 | 0.90 | 0.23 | 0.34 | 0.36 |
| MC | 3.25 | 638 | 0.51 | 0.28 | 1.22 | 0.03 |
| MCS | 7.32 | 197 | 0.30 | 0.18 | 1.50 | 0.65 |
| Entry | Catalyst | SBET [m2/g] | Pore Volume [cm3/g] | Atitration [mmol(H+)/g] | Catalyst Amount (mg) | FDMA Yield (%) |
|---|---|---|---|---|---|---|
| 1 | No catalyst | — | — | — | — | 1 |
| 2 | MA | 2594 | 1.30 | — | 20 | 9 |
| 3 | MC | 638 | 0.51 | 0.03 | 20 | 25 |
| 4 | MCS | 197 | 0.30 | 0.65 | 20 | 91 |
| 5 | Amberlyst-15 | 50 | — | 4.70 | 5 | 63 |
| 6 | H-USY(Si/Al = 6) | 37.6 | 0.08 | 1.40 | 5 | 71 |
| 7 b | Cu3(BTC)2 | 1019 | — | — | 100 | 86 |
| Entry | Aldehyde | Acetal | Yield (%) |
|---|---|---|---|
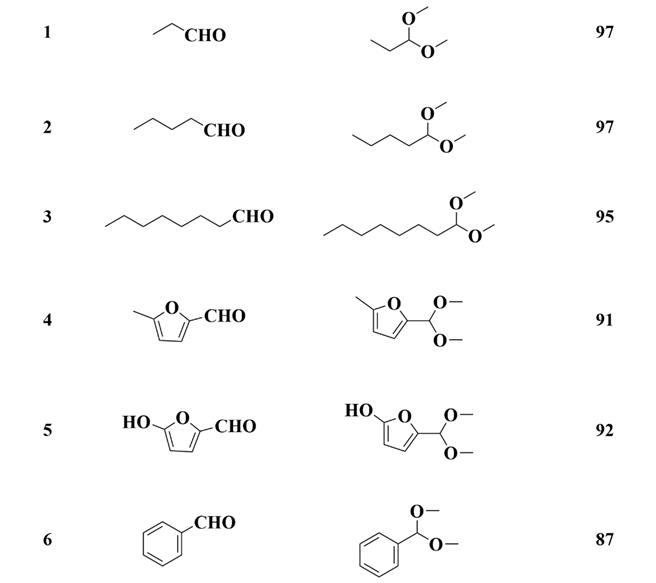 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Meng, Y.; Li, H.; Yang, S. Hierarchical Porous MIL-101(Cr) Solid Acid-Catalyzed Production of Value-Added Acetals from Biomass-Derived Furfural. Polymers 2021, 13, 3498. https://doi.org/10.3390/polym13203498
Liu S, Meng Y, Li H, Yang S. Hierarchical Porous MIL-101(Cr) Solid Acid-Catalyzed Production of Value-Added Acetals from Biomass-Derived Furfural. Polymers. 2021; 13(20):3498. https://doi.org/10.3390/polym13203498
Chicago/Turabian StyleLiu, Shengqi, Ye Meng, Hu Li, and Song Yang. 2021. "Hierarchical Porous MIL-101(Cr) Solid Acid-Catalyzed Production of Value-Added Acetals from Biomass-Derived Furfural" Polymers 13, no. 20: 3498. https://doi.org/10.3390/polym13203498






