Probing Antimicrobial Halloysite/Biopolymer Composites with Electron Microscopy: Advantages and Limitations
Abstract
:1. Introduction
2. Halloysite as a Perspective Carrier/Support for Biopolymers
3. Synthesis of Biopolymer/Halloysite Nanotube (HNT) Composites
4. Analysis of Biopolymer/HNT Composites
5. Electron Microscopy as the Ultimate Tool for Biopolymer/HNT Composites Analysis
6. Advantages and Limitations of Electron Microscopy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-biofilm activity as a health issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Sharafutdinov, I.S.; Trizna, E.Y.; Baydamshina, D.R.; Ryzhikova, M.N.; Sibgatullina, R.R.; Khabibrakhmanova, A.M.; Latypova, L.Z.; Kurbangalieva, A.R.; Rozhina, E.V.; Klinger-Strobel, M.; et al. Antimicrobial Effects of Sulfonyl Derivative of 2(5H)-Furanone Against Planktonic and Biofilm Associated Methicillin-Resistant and -Susceptible Staphylococcus Aureus. Front. Microbiol. 2017, 8, 2246. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, S.; Sen, S.K.; Pattanaik, S.; Raut, S. Review on bacterial biofilm: An universal cause of contamination. Biocatal. Agric. Biotechnol. 2016, 7, 56–66. [Google Scholar] [CrossRef]
- Wuertz, S.; Bishop, P.L.; Wilderer, P.A. (Eds.) Biofilms in Wastewater Treatment; IWA Publishing: London, UK, 2003. [Google Scholar]
- Kesaano, M.; Sims, R.C. Algal biofilm based technology for wastewater treatment. Algal Res. 2014, 5, 231–240. [Google Scholar] [CrossRef]
- Sehar, S.; Naz, I. Role of the biofilms in wastewater treatment. In Microbial Biofilms-Importance and Applications; Dhanasekaran, D., Thajuddin, N., Eds.; IntechOpen: London, UK, 2016; pp. 121–144. [Google Scholar]
- Wang, V.B.; Chua, S.L.; Cai, Z.; Sivakumar, K.; Zhang, Q.; Kjelleberg, S.; Cao, B.; Loo, J.S.C.; Yang, L. A stable synergistic microbial consortium for simultaneous azo dye removal and bioelectricity generation. Bioresour. Technol. 2014, 155, 71–76. [Google Scholar] [CrossRef]
- Erable, B.; Duţeanu, N.M.; Ghangrekar, M.M.; Dumas, C.; Scott, K. Application of electro-active biofilms. Biofouling 2010, 26, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Borole, A.P.; Reguera, G.; Ringeisen, B.; Wang, Z.W.; Feng, Y.; Kim, B.H. Electroactive biofilms: Current status and future research needs. Energy Environ. Sci. 2011, 4, 4813–4834. [Google Scholar] [CrossRef]
- Ter Heijne, A.; Pereira, M.A.; Pereira, J.; Sleutels, T. Electron storage in electroactive biofilms. Trends Biotechnol. 2021, 39, 34–42. [Google Scholar] [CrossRef]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef]
- Fitridge, I.; Dempster, T.; Guenther, J.; De Nys, R. The impact and control of biofouling in marine aquaculture: A review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef]
- Dobretsov, S.; Abed, R.M.; Teplitski, M. Mini-review: Inhibition of biofouling by marine microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Gule, N.P.; Begum, N.M.; Klumperman, B. Advances in biofouling mitigation: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 535–555. [Google Scholar] [CrossRef]
- Bannister, J.; Sievers, M.; Bush, F.; Bloecher, N. Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 2019, 35, 631–648. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, H.; Alavi, S.A.; Fotovat, M. Microbial-influenced corrosion of corten steel compared with carbon steel and stainless steel in oily wastewater by Pseudomonas aeruginosa. JOM 2015, 67, 1594–1600. [Google Scholar] [CrossRef]
- Beech, I.B. Corrosion of techniqueal materials in the presence of biofilms—current understanding and state-of-the art methods of study. Int. Biodeterior. Biodegrad. 2004, 53, 177–183. [Google Scholar] [CrossRef]
- Holmberg, A.; Rasmussen, M. Mature biofilms of Enterococcus faecalis and Enterococcus faecium are highly resistant to antibiotics. Diagn. Microbiol. Infect. Dis. 2016, 84, 19–21. [Google Scholar] [CrossRef]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef]
- Shigeta, M.; Tanaka, G.; Komatsuzawa, H.; Sugai, M.; Suginaka, H.; Usui, T. Permeation of antimicrobial agents through Pseudomonas aeruginosa biofilms: A simple method. Chemotherapy 1997, 43, 340–345. [Google Scholar] [CrossRef]
- Wilton, M.; Charron-Mazenod, L.; Moore, R.; Lewenza, S. Extracellular DNA acidifies biofilms and induces aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 544–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, S.M.; Felício, M.R.; Boas, E.V.; Gonçalves, S.; Costa, F.F.; Samy, R.P.; Santos, N.C.; Franco, O.L. New frontiers for anti-biofilm drug development. Pharmacol. Ther. 2016, 160, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Zobell, C.E. The effect of solid surfaces upon bacterial activity. J. Bacteriol. 1943, 46, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New weapons to fight old enemies: Novel strategies for the (bio) control of bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef] [Green Version]
- Ermolaeva, S.A.; Sysolyatina, E.V.; Gintsburg, A.L. Atmospheric pressure nonthermal plasmas for bacterial biofilm prevention and eradication. Biointerphases 2015, 10, 029404. [Google Scholar] [CrossRef]
- Vandervoort, K.G.; Brelles-Marino, G. Plasma-mediated inactivation of Pseudomonas aeruginosa biofilms grown on borosilicate surfaces under continuous culture system. PLoS ONE 2014, 9, e108512. [Google Scholar] [CrossRef]
- Zea, L.; McLean, R.J.; Rook, T.A.; Angle, G.; Carter, D.L.; Delegard, A.; Denvir, A.; Gerlach, R.; Gorti, S.; Mcllwaine, D.; et al. Potential biofilm control strategies for extended spaceflight missions. Biofilm 2020, 2, 100026. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef] [Green Version]
- Sultana, S.T.; Babauta, J.T.; Beyenal, H. Electrochemical biofilm control: A review. Biofouling 2015, 31, 745–758. [Google Scholar] [CrossRef]
- Stewart, P.S.; Wattanakaroon, W.; Goodrum, L.; Fortun, S.M.; McLeod, B.R. Electrolytic generation of oxygen partially explains electrical enhancement of tobramycin efficacy against Pseudomonas aeruginosa biofilm. Antimicrob. Agents Chemother. 1999, 43, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Istanbullu, O.; Babauta, J.; Nguyen, H.D.; Beyenal, H. Electrochemical biofilm control: Mechanism of action. Biofouling 2012, 28, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Babauta, J.T.; Nguyen, H.D.; Istanbullu, O.; Beyenal, H. Microscale gradients of oxygen, hydrogen peroxide, and pH in freshwater cathodic biofilms. ChemSusChem 2013, 6, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.S.; Peng, S.W.; Cheng, J.Y. In vitro electrical-stimulated wound-healing chip for studying electric field-assisted wound-healing process. Biomicrofluidics 2012, 6, 034117. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Malan, S.M.; Karau, M.J.; Cede, J.; Greenwood-Quaintance, K.E.; Brinkman, C.L.; Mandrekar, J.N.; Patel, R. Antibiofilm activity of low-amperage continuous and intermittent direct electrical current. Antimicrob. Agents Chemother. 2015, 59, 4610–4615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desrousseaux, C.; Sautou, V.; Descamps, S.; Traoré, O. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. J. Hosp. Infect. 2013, 85, 87–93. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Luo, J.; Yang, J.; Wang, W.; Kennedy, J.F. A novel biopolymer/rectorite nanocomposite with antimicrobial activity. Carbohyd. Polym. 2009, 77, 449–456. [Google Scholar] [CrossRef]
- Bower, C.K.; Daeschel, M.A.; McGuire, J. Protein antimicrobial barriers to bacterial adhesion. J. Dairy Sci. 1998, 81, 2771–2778. [Google Scholar] [CrossRef]
- Malmström, E.; Mörgelin, M.; Malmsten, M.; Johansson, L.; Norrby-Teglund, A.; Shannon, O.; Schmidtchen, A.; Meijers, J.C.M.; Herwald, H. Protein C inhibitor—A novel antimicrobial agent. PLoS Pathog. 2009, 5, e1000698. [Google Scholar] [CrossRef] [Green Version]
- Marshall, K. Therapeutic applications of whey protein. Altern. Med. Rev. 2004, 9, 136–156. [Google Scholar]
- Ananbeh, H.; Rodrigo, M.A.M.; Jelinkova, P.; Strmiska, V.; Splichal, Z.; Jehmlich, N.; Michalkova, H.; Stojanović, M.; Voberkova, S.; Adam, V.; et al. Soil protein as a potential antimicrobial agent against methicillin–resistant Staphylococcus aureus. Environ. Res. 2020, 188, 109320. [Google Scholar] [CrossRef] [PubMed]
- Karwacki, M.T.; Kadouri, D.E.; Bendaoud, M.; Izano, E.A.; Sampathkumar, V.; Inzana, T.J.; Kaplan, J.B. Antibiofilm activity of Actinobacillus pleuropneumoniae serotype 5 capsular polysaccharide. PLoS ONE 2013, 8, e63844. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ Biofilms Microbiomes 2018, 4, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azemar, F.; Faÿ, F.; Réhel, K.; Linossier, I. Development of hybrid antifouling paints. Prog. Org. Coat. 2015, 87, 10–19. [Google Scholar] [CrossRef]
- Ruggiero, L.; Crociani, L.; Zendri, E.; El Habra, N.; Guerriero, P. Incorporation of the zosteric sodium salt in silica nanocapsules: Synthesis and characterization of new fillers for antifouling coatings. Appl. Surf. Sci. 2018, 439, 705–711. [Google Scholar] [CrossRef]
- Hao, X.; Wang, W.; Yang, Z.; Yue, L.; Sun, H.; Wang, H.; Guo, Z.; Cheng, F.; Chen, S. pH responsive antifouling and antibacterial multilayer films with Self-healing performance. Chem. Eng. J. 2019, 356, 130–141. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, S.; Wu, L. Self-healing underwater superoleophobic and antibiofouling coatings based on the assembly of hierarchical microgel spheres. ACS Nano 2016, 10, 1386–1394. [Google Scholar] [CrossRef]
- Selim, M.S.; Elmarakbi, A.; Azzam, A.M.; Shenashen, M.A.; EL-Saeed, A.M.; El-Safty, S.A. Eco-friendly design of superhydrophobic nano-magnetite/silicone composites for marine foul-release paints. Prog. Org. Coat. 2018, 116, 21–34. [Google Scholar] [CrossRef]
- Selim, M.S.; Shenashen, M.A.; El-Safty, S.A.; Higazy, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent progress in marine foul-release polymeric nanocomposite coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Batasheva, S.; Vinokurov, V.; Fakhrullina, G.; Sangarov, V.; Lvov, Y.; Fakhrullin, R. Antimicrobial applications of clay nanotube-based composites. Nanomaterials 2019, 9, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almajhdi, F.N.; Fouad, H.; Khalil, K.A.; Awad, H.M.; Mohamed, S.H.; Elsarnagawy, T.; Albarrag, A.M.; Al-Jassir, F.F.; Abdo, H.S. In-vitro anticancer and antimicrobial activities of PLGA/silver nanofiber composites prepared by electrospinning. J. Mater. Sci. Mater. Med. 2014, 25, 1045–1053. [Google Scholar] [CrossRef]
- Yue, J.; Zhao, P.; Gerasimov, J.Y.; van de Lagemaat, M.; Grotenhuis, A.; Rustema-Abbing, M.; van der Mei, H.; Busscher, H.J.; Herrmann, A.; Ren, Y. 3D-printable antimicrobial composite resins. Adv. Funct. Mater. 2015, 25, 6756–6767. [Google Scholar] [CrossRef]
- Moghanian, A.; Portillo-Lara, R.; Shirzaei Sani, E.; Konisky, H.; Bassir, S.H.; Annabi, N. Synthesis and characterization of osteoinductive visible light-activated adhesive composites with antimicrobial properties. J. Tissue Eng. Regen. Med. 2020, 14, 66–81. [Google Scholar] [CrossRef]
- Liu, P.; Fu, K.; Zeng, X.; Chen, N.; Wen, X. Fabrication and characterization of composite meshes loaded with antimicrobial peptides. ACS Appl. Mater. Interfaces 2019, 11, 24609–24617. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Wang, S.; Wu, J.; Huang, M.; Min, H.; Liu, X. Controlled release and antibacterial activity of tetracycline hydrochloride-loaded bacterial cellulose composite membranes. Carbohydr. Polym. 2016, 145, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.B.; Jaisingh, S.J.; Sivakumar, K.; Mayakannan, A.V.; Arunprakash, V.R. Visco-elastic, thermal, antimicrobial and dielectric behaviour of areca fibre-reinforced nano-silica and neem oil-toughened epoxy resin bio composite. Silicon 2021, 13, 1703–1712. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Escárcega-González, C.E.; Castro, E.D.B.; Mendiola-Garza, G.; Marichal-Cancino, B.A.; López-Vázquez, M.A.; Morones-Ramirez, J.R. Antimicrobial and antibiofilm activity of biopolymer-Ni, Zn nanoparticle biocomposites synthesized using R. mucilaginosa UANL-001L exopolysaccharide as a capping agent. Int. J. Nanomed. 2019, 14, 2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopiraman, M.; Jatoi, A.W.; Hiromichi, S.; Yamaguchi, K.; Jeon, H.Y.; Chung, I.M.; Soo, K.I. Silver coated anionic cellulose nanofiber composites for an efficient antimicrobial activity. Carbohydr. Polym. 2016, 149, 51–59. [Google Scholar] [CrossRef]
- Vinod, A.; Sanjay, M.R.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Sothornvit, R.; Rhim, J.W.; Hong, S.I. Effect of nano-clay type on the physical and antimicrobial properties of whey protein isolate/clay composite films. J. Food Eng. 2009, 91, 468–473. [Google Scholar] [CrossRef]
- Hong, S.I.; Rhim, J.W. Antimicrobial activity of organically modified nano-clays. J. Nanosci. Nanotechnol. 2008, 8, 5818–5824. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Lee, S.H.; Choi, K.H.; Park, I. Preparation and characterization of chitosan–clay nanocomposites with antimicrobial activity. J. Phys. Chem. Solids 2010, 71, 464–467. [Google Scholar] [CrossRef]
- Mishra, R.K.; Ramasamy, K.; Lim, S.M.; Ismail, M.F.; Majeed, A.B.A. Antimicrobial and in vitro wound healing properties of novel clay based bionanocomposite films. J. Mater. Sci. Mater. Med. 2014, 25, 1925–1939. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, E.A.; Guryanov, I.D.; Yendluri, R.; Lvov, Y.M.; Fakhrullin, R.F. Clay nanotube–biopolymer composite scaffolds for tissue engineering. Nanoscale 2016, 8, 7257–7271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lvov, Y.; Guo, B.; Fakhrullin, R.F. (Eds.) Functional Polymer Composites with Nanoclays; Royal Society of Chemistry Publishing: Cambridge, UK, 2016; 433p. [Google Scholar]
- Holešová, S.; Hundáková, M.; Pazdziora, E. Antibacterial kaolinite based nanocomposites. Procedia Mater. Sci. 2016, 12, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Mohan, T.P.; Devchand, K.; Kanny, K. Barrier and biodegradable properties of corn starch-derived biopolymer film filled with nanoclay fillers. J. Plastic Film Sheeting 2017, 33, 309–336. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Liu, M.; Fakhrullin, R.; Novikov, A.; Panchal, A.; Lvov, Y. Tubule nanoclay-organic heterostructures for biomedical applications. Macromol. Biosci. 2019, 19, 1800419. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Lisuzzo, L.; Milioto, S.; Parisi, F. Filling of mater-Bi with nanoclays to enhance the biofilm rigidity. J. Funct. Biomater. 2018, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Fakhrullina, G.; Khakimova, E.; Akhatova, F.; Lazzara, G.; Parisi, F.; Fakhrullin, R. Selective antimicrobial effects of curcumin@ halloysite nanoformulation: A Caenorhabditis elegans study. ACS Appl. Mater. Interfaces 2019, 11, 23050–23064. [Google Scholar] [CrossRef]
- Targonska, S.; Rewak-Soroczynska, J.; Piecuch, A.; Paluch, E.; Szymanski, D.; Wiglusz, R.J. Preparation of a New Biocomposite Designed for Cartilage Grafting with Antibiofilm Activity. ACS Omega 2020, 5, 24546–24557. [Google Scholar] [CrossRef]
- Neji, A.B.; Jridi, M.; Nasri, M.; Sahnoun, R.D. Preparation, characterization, mechanical and barrier properties investigation of chitosan-kaolinite nanocomposite. Polymer Testing 2020, 84, 106380. [Google Scholar] [CrossRef]
- Keeling, J.L.; Pasbakhsh, P.; Churchman, G.J. The mineralogy, geology and occurrences of halloysite. In Natural Mineral Nanotubes: Properties and Applications, 1st ed.; Pasbakhsh, P., Churchman, G.J., Eds.; Apple Academic Press Inc.: Oakville, ON, Canada, 2015; pp. 95–115. [Google Scholar]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef]
- Zahidah, K.A.; Kakooei, S.; Ismail, M.C.; Raja, P.B. Halloysite nanotubes as nanocontainer for smart coating application: A review. Prog. Org. Coat. 2017, 111, 175–185. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Functional polymer-clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The application of halloysite tubule nanoclay in drug delivery. Exp. Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef]
- Korshunov, D.M.; Boguslavskiy, M.A. Mineral composition and morphological features of kaolinite in ceramic clays of the Shulepovo Deposit (Ryazan Region, Central European Russia). Lithol. Miner. Resour. 2021, 56, 189–194. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes coated by chitosan for the controlled release of khellin. Polymers 2020, 12, 1766. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Minullina, R.; Abdullayev, E.; Fakhrullin, R.; Mills, D.; Lvov, Y. Enhanced efficiency of antiseptics with sustained release from clay nanotubes. RSC Adv. 2014, 4, 488–494. [Google Scholar] [CrossRef]
- Shamsi, M.H.; Geckeler, K.E. The first biopolymer-wrapped non-carbon nanotubes. Nanotechnology 2008, 19, 075604. [Google Scholar] [CrossRef]
- Shutava, T.G.; Fakhrullin, R.F.; Lvov, Y.M. Spherical and tubule nanocarriers for sustained drug release. Curr. Opin. Pharmacol. 2014, 18, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Cavallaro, G.; Lazzara, G.; Rozhina, E.; Konnova, S.; Kryuchkova, M.; Khaertdinov, N.; Fakhrullin, R. Organic-nanoclay composite materials as removal agents for environmental decontamination. RSC Adv. 2019, 9, 40553–40564. [Google Scholar] [CrossRef] [Green Version]
- Abdullayev, E.; Lvov, Y. Halloysite clay nanotubes as a ceramic “skeleton” for functional biopolymer composites with sustained drug release. J. Mater. Chem. B 2013, 1, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Aerov, A.; Fakhrullin, R. Clay nanotube encapsulation for functional biocomposites. Adv. Colloid Interface Sci. 2014, 207, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Tully, J.; Yendluri, R.; Lvov, Y. Halloysite clay nanotubes for enzyme immobilization. Biomacromolecules 2016, 17, 615–621. [Google Scholar] [CrossRef]
- Risyon, N.P.; Othman, S.H.; Basha, R.K.; Talib, R.A. Characterization of polylactic acid/halloysite nanotubes bionanocomposite films for food packaging. Food Packag. Shelf Life 2020, 23, 100450. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Parisi, F.; Lazzara, G. Thermal properties of multilayer nanocomposites based on halloysite nanotubes and biopolymers. J. Compos. Sci. 2018, 2, 41. [Google Scholar] [CrossRef] [Green Version]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K.M.; Han, S.S. Development of sodium alginate-xanthan gum based nanocomposite scaffolds reinforced with cellulose nanocrystals and halloysite nanotubes. Polymer Testing 2017, 63, 214–225. [Google Scholar] [CrossRef]
- Pierchala, M.K.; Makaremi, M.; Tan, H.L.; Pushpamalar, J.; Muniyandy, S.; Solouk, A.; Lee, S.M.; Pasbakhsh, P. Nanotubes in nanofibers: Antibacterial multilayered polylactic acid/halloysite/gentamicin membranes for bone regeneration application. Appl. Clay Sci. 2018, 160, 95–105. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Liu, M.; Chang, Y.; Yang, J.; You, Y.; He, R.; Chen, T.; Zhou, C. Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef]
- Long, Z.; Zhang, J.; Shen, Y.; Zhou, C.; Liu, M. Polyethyleneimine grafted short halloysite nanotubes for gene delivery. Mater. Sci. Eng. C 2017, 81, 224–235. [Google Scholar] [CrossRef]
- Chao, C.; Liu, J.; Wang, J.; Zhang, Y.; Zhang, B.; Zhang, Y.; Xiang, X.; Chen, R. Surface modification of halloysite nanotubes with dopamine for enzyme immobilization. ACS Appl. Mater. Interfaces 2013, 5, 10559–10564. [Google Scholar] [CrossRef]
- Long, Z.; Wu, Y.P.; Gao, H.Y.; Li, Y.F.; He, R.R.; Liu, M. Functionalization of halloysite nanotubes via grafting of dendrimer for efficient intracellular delivery of siRNA. Bioconjug. Chem. 2018, 29, 2606–2618. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, C.; Song, H.; Bai, L.; Cheng, Y.; Ba, X.; Wu, Y. A facile one-step grafting of polyphosphonium onto halloysite nanotubes initiated by Ce (IV). Chem. Commun. 2019, 55, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Yuan, P.; Liu, D.; Du, P. Surface modifications of halloysite. In Nanosized Tubualr Clay Minerals—Halloysite and Imogolite; Yuan, P., Thill, A., Bergaya, F., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 7, pp. 167–201. [Google Scholar]
- Lisuzzo, L.; Cavallaro, G.; Pasbakhsh, P.; Milioto, S.; Lazzara, G. Why does vacuum drive to the loading of halloysite nanotubes? The key role of water confinement. J. Colloid Interface Sci. 2019, 547, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Barman, M.; Mahmood, S.; Augustine, R.; Hasan, A.; Thomas, S.; Ghosal, K. Natural halloysite nanotubes/chitosan based bio-nanocomposite for delivering norfloxacin, an anti-microbial agent in sustained release manner. Int. J. Biol. Macromol. 2020, 162, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Voronin, D.V.; Demina, P.A.; Abramova, A.M.; Cherednichenko, K.A.; Vinokurov, V. Freezing-induced loading of Au nanoparticles into halloysite nanotubes. Mater. Lett. 2021, 291, 129506. [Google Scholar] [CrossRef]
- Owoseni, O.; Zhang, Y.; Su, Y.; He, J.; McPherson, G.L.; Bose, A.; John, V.T. Tuning the wettability of halloysite clay nanotubes by surface carbonization for optimal emulsion stabilization. Langmuir 2015, 31, 13700–13707. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.; Swientoniewski, L.T.; Omarova, M.; Yu, T.; Zhang, D.; Blake, D.A.; John, V.; Lvov, Y.M. Bacterial proliferation on clay nanotube Pickering emulsions for oil spill bioremediation. Colloids Surf. B Biointerfaces 2018, 164, 27–33. [Google Scholar] [CrossRef]
- Fakhrullina, G.I.; Akhatova, F.S.; Lvov, Y.M.; Fakhrullin, R.F. Toxicity of halloysite clay nanotubes in vivo: A Caenorhabditis elegans study. Environ. Sci. Nano 2015, 2, 54–59. [Google Scholar] [CrossRef]
- Wu, Y.P.; Yang, J.; Gao, H.Y.; Shen, Y.; Jiang, L.; Zhou, C.; Li, Y.F.; He, R.R.; Liu, M. Folate-conjugated halloysite nanotubes, an efficient drug carrier, deliver doxorubicin for targeted therapy of breast cancer. ACS Appl. Nano Mater. 2018, 1, 595–608. [Google Scholar] [CrossRef]
- Zhao, Y.; Cavallaro, G.; Lvov, Y. Orientation of charged clay nanotubes in evaporating droplet meniscus. J. Colloid Interface Sci. 2015, 440, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; He, R.; Yang, J.; Zhao, W.; Zhou, C. Stripe-like clay nanotubes patterns in glass capillary tubes for capture of tumor cells. ACS Appl. Mater. Interfaces 2016, 8, 7709–7719. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zou, Q.; Lei, Y.; Jia, D. Structure and performance of polyamide 6/halloysite nanotubes nanocomposites. Polym. J. 2009, 41, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Hydrophobically modified halloysite nanotubes as reverse micelles for water-in-oil emulsion. Langmuir 2015, 31, 7472–7478. [Google Scholar] [CrossRef]
- Patel, S.; Jammalamadaka, U.; Sun, L.; Tappa, K.; Mills, D.K. Sustained release of antibacterial agents from doped halloysite nanotubes. Bioengineering 2016, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Bonifacio, M.A.; Gentile, P.; Ferreira, A.M.; Cometa, S.; De Giglio, E. Insight into halloysite nanotubes-loaded gellan gum hydrogels for soft tissue engineering applications. Carbohydr. Polym. 2017, 163, 280–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Müller, E.; Meffert, M.; Gerthsen, D. On the progress of scanning transmission electron microscopy (STEM) imaging in a scanning electron microscope. Microsc. Microanal. 2018, 24, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Lema, S.; Quiles-Carrillo, L.; Garcia-Garcia, D.; Melendez-Rodriguez, B.; Balart, R.; Torres-Giner, S. Tailoring the properties of thermo-compressed polylactide films for food packaging applications by individual and combined additions of lactic acid oligomer and halloysite nanotubes. Molecules 2020, 25, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Kong, W.; Wang, W.; Liu, T.; Liu, Y.; Gong, Q.; Gao, J. Modified natural halloysite/potato starch composite films. Carbohydr. Polym. 2012, 87, 2706–2711. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Wu, C.; Xiong, S.; Zhou, C. Chitosan/halloysite nanotubes bionanocomposites: Structure, mechanical properties and biocompatibility. Int. J. Biol. Macromol. 2012, 51, 566–575. [Google Scholar] [CrossRef]
- Krepker, M.; Prinz-Setter, O.; Shemesh, R.; Vaxman, A.; Alperstein, D.; Segal, E. Antimicrobial carvacrol-containing polypropylene films: Composition, structure and function. Polymers 2018, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Niu, Y.; Gong, M.; Ye, J.; Tian, W.; Zhang, L. Antimicrobial gelatin-based elastomer nanocomposite membrane loaded with ciprofloxacin and polymyxin B sulfate in halloysite nanotubes for wound dressing. Mater. Sci. Eng. C 2018, 87, 128–138. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M.; Fateh, A.; Shahrabi, T. Electrophoretic deposition of vancomycin loaded halloysite nanotubes-chitosan nanocomposite coatings. Surf. Coat. Technol. 2018, 349, 144–156. [Google Scholar] [CrossRef]
- Cavallaro, G.; Donato, D.I.; Lazzara, G.; Milioto, S. Films of halloysite nanotubes sandwiched between two layers of biopolymer: From the morphology to the dielectric, thermal, transparency, and wettability properties. J. Phys. Chem. C 2011, 115, 20491–20498. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L.; Devine, D.M. Halloysite nanotube reinforced polylactic acid composite. Polym. Compos. 2017, 38, 2166–2173. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Dispersions of nanoclays of different shapes into aqueous and solid biopolymeric matrices. Extended physicochemical study. Langmuir 2011, 27, 1158–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, L.; Huang, W.; Zhang, Y. High-flux, antibacterial ultrafiltration membranes by facile blending with N-halamine grafted halloysite nanotubes. RSC Adv. 2015, 5, 6666–6674. [Google Scholar] [CrossRef]
- Mu, K.; Zhang, D.; Shao, Z.; Qin, D.; Wang, Y.; Wang, S. Enhanced permeability and antifouling performance of cellulose acetate ultrafiltration membrane assisted by L-DOPA functionalized halloysite nanotubes. Carbohydr. Polym. 2017, 174, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Afshar, H.A.; Ghaee, A. Preparation of aminated chitosan/alginate scaffold containing halloysite nanotubes with improved cell attachment. Carbohydr. Polym. 2016, 151, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, K.A.; Okayama, S. Penetration and energy-loss theory of electrons in solid targets. J. Phys. D Appl. Phys. 1972, 5, 43. [Google Scholar] [CrossRef]
- Rostamzadeh, T.; Islam Khan, M.S.; Riche’, K.; Lvov, Y.M.; Stavitskaya, A.V.; Wiley, J.B. Rapid and controlled in situ growth of noble metal nanostructures within halloysite clay nanotubes. Langmuir 2017, 33, 13051–13059. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Sukhorukov, G.B.; Price, R.R.; Lvov, Y.M. Halloysite nanotubes as biomimetic nanoreactors. Small 2005, 1, 510–513. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C.; Liu, J.; Zhang, Y. Development of a novel polyethersulfone ultrafiltration membrane with antibacterial activity and high flux containing halloysite nanotubes loaded with lysozyme. RSC Adv. 2015, 5, 38646–38653. [Google Scholar] [CrossRef]
- Batasheva, S.; Kryuchkova, M.; Fakhrullin, R.; Cavallaro, G.; Lazzara, G.; Akhatova, F.; Nigamatzyanova, L.; Evtugyn, V.; Rozhina, E.; Fakhrullin, R. Facile fabrication of natural polyelectrolyte-nanoclay composites: Halloysite nanotubes, nucleotides and DNA study. Molecules 2020, 25, 3557. [Google Scholar] [CrossRef]
- Jang, S.H.; Jang, S.R.; Lee, G.M.; Ryu, J.H.; Park, S.I.; Park, N.H. Halloysite nanocapsules containing thyme essential oil: Preparation, characterization, and application in packaging materials. J. Food Sci. 2017, 82, 2113–2120. [Google Scholar] [CrossRef]
- Dzamukova, M.R.; Naumenko, E.A.; Lvov, Y.M.; Fakhrullin, R.F. Enzyme-activated intracellular drug delivery with tubule clay nanoformulation. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khodzhaeva, V.; Makeeva, A.; Ulyanova, V.; Zelenikhin, P.; Evtugyn, V.; Hardt, M.; Rozhina, E.; Lvov, Y.; Fakhrullin, R.; Ilinskaya, O. Binase immobilized on halloysite nanotubes exerts enhanced cytotoxicity toward human colon adenocarcinoma cells. Front. Pharmacol. 2017, 8, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Evtugyn, V.; Rozhina, E.; Fakhrullin, R. Nanohydrogel formation within the halloysite lumen for triggered and sustained release. ACS Appl. Mater. Interfaces 2018, 10, 8265–8273. [Google Scholar] [CrossRef]
- Liu, M.; Wu, C.; Jiao, Y.; Xiong, S.; Zhou, C. Chitosan–halloysite nanotubes nanocomposite scaffolds for tissue engineering. J. Mater. Chem. B 2013, 1, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, V.I.; Schulz, P.C.; Gschaider, M.E.; Andreucetti, N. Chitosan structure in aqueous solution. Colloid Polym. Sci. 2003, 282, 100–102. [Google Scholar] [CrossRef]
- Anal, A.K.; Tobiassen, A.; Flanagan, J.; Singh, H. Preparation and characterization of nanoparticles formed by chitosan–caseinate interactions. Colloids Surf. B Biointerfaces 2008, 64, 104–110. [Google Scholar] [CrossRef]
- Mady, M.M.; Darwish, M.M.; Khalil, S.; Khalil, W.M. Biophysical studies on chitosan-coated liposomes. Eur. Biophys. J. 2009, 38, 1127–1133. [Google Scholar] [CrossRef]
- Abdou, E.S.; Galhoum, G.F.; Mohamed, E.N. Curcumin loaded nanoemulsions/pectin coatings for refrigerated chicken fillets. Food Hydrocoll. 2018, 83, 445–453. [Google Scholar] [CrossRef]
- Lentzen, M. Progress in aberration-corrected high-resolution transmission electron microscopy using hardware aberration correction. Microsc. Microanal. 2006, 12, 191–205. [Google Scholar] [CrossRef] [Green Version]
- Milne, J.L.; Borgnia, M.J.; Bartesaghi, A.; Tran, E.E.; Earl, L.A.; Schauder, D.M.; Lengyel, J.; Pierson, J.; Patwardhan, A.; Subramaniam, S. Cryo-electron microscopy–a primer for the non-microscopist. FEBS J. 2013, 280, 28–45. [Google Scholar] [CrossRef]
- Suga, M.; Asahina, S.; Sakuda, Y.; Kazumori, H.; Nishiyama, H.; Nokuo, T.; Alfredsson, V.; Kjellman, T.; Stevens, S.M.; Cho, H.S.; et al. Recent progress in scanning electron microscopy for the characterization of fine structural details of nano materials. Prog. Solid State Chem. 2014, 42, 1–21. [Google Scholar] [CrossRef]
- Takeda, H.; Yoshida, H. Atomic-resolution environmental TEM for quantitative in-situ microscopy in materials science. Microscopy 2013, 62, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Yao, N. Advances in windowed gas cells for in-situ TEM studies. Nano Energy 2015, 13, 735–756. [Google Scholar] [CrossRef]
- Wu, J.; Shan, H.; Chen, W.; Gu, X.; Tao, P.; Song, C.; Shang, W.; Deng, T. In situ environmental TEM in imaging gas and liquid phase chemical reactions for materials research. Adv. Mater. 2016, 28, 9686–9712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, Y.; Han, Z.; Gao, S.; Wang, M.; Wang, P. Electrochemical and structural analysis in all-solid-state lithium batteries by analytical electron microscopy: Progress and perspectives. Adv. Mater. 2020, 32, 1903747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Firestein, K.L.; Fernando, J.F.S.; Siriwardena, D.; von Treifeldt, J.E.; Golberg, D. Recent progress of in situ transmission electron microscopy for energy materials. Adv. Mater. 2020, 32, 1904094. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Fujita, T.; Takahashi, Y.; Yamasaki, J.; Murata, K.; Arai, S. Progress in environmental high-voltage transmission electron microscopy for nanomaterials. Philos. Trans. R. Soc. A 2020, 378, 20190602. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, R.A. Advances in crystal orientation mapping with the SEM and TEM. Ultramicroscopy 1997, 67, 19–24. [Google Scholar] [CrossRef]
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, S.; Harris, J.R. Negative staining and cryo-negative staining of macromolecules and viruses for TEM. Micron 2011, 42, 117–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.R.; De Carlo, S. Negative staining and cryo-negative staining: Applications in biology and medicine. In Electron Microscopy. Methods in Molecular Biology (Methods and Protocols); Kuo, J., Ed.; Humana Press: Totowa, NJ, USA, 2014; Volume 1117, pp. 215–258. [Google Scholar]
- Barreto-Vieira, D.F.; Barth, O.M. Negative and positive staining in transmission electron microscopy for virus diagnosis. In Microbiology in Agriculture and Human Health; Shah, M.M., Ed.; IntechOpen: London, UK, 2015; pp. 45–56. [Google Scholar]
- Franken, L.E.; Boekema, E.J.; Stuart, M.C. Transmission electron microscopy as a tool for the characterization of soft materials: Application and interpretation. Adv. Sci. 2017, 4, 1600476. [Google Scholar] [CrossRef]
- Weisman, J.A.; Jammalamadaka, U.; Tappa, K.; Mills, D.K. Doped halloysite nanotubes for use in the 3D printing of medical devices. Bioengineering 2017, 4, 96. [Google Scholar] [CrossRef] [Green Version]
- Ul-Hamid, A. A Beginners’ Guide to Scanning Electron Microscopy; Springer International Publishing: Cham, Switzerland, 2018; 402p. [Google Scholar]
- Makhlouf, A.S.H.; Aliofkhazraei, M. (Eds.) Handbook of Materials Failure Analysis with Case Studies from the Aerospace and Automotive Industries; Butterworth-Heinemann: Oxford, United Kingdom, 2015; p. 524. [Google Scholar]
- Terracio, L.; Schwabe, K.G. Freezing and drying of biological tissues for electron microscopy. J. Histochem. Cytochem. 1981, 29, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franks, F. Freeze-drying of bioproducts: Putting principles into practice. Eur. J. Pharm. Biopharm. 1998, 45, 221–229. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Ciurzynska, A.; Lenart, A. Freeze-drying-application in food processing and biotechnology—A review. Polish J. Food Nutr. Sci. 2011, 61, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Schatten, H. (Ed.) Scanning Electron Microscopy for the Life Sciences; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Stokes, D.J. Recent advances in electron imaging, image interpretation and applications: Environmental scanning electron microscopy. Philos. Trans. A Math. Phys. Eng. Sci. 2003, 361, 2771–2787. [Google Scholar] [CrossRef] [PubMed]
- Bogner, A.; Jouneau, P.H.; Thollet, G.; Basset, D.; Gauthier, C. A history of scanning electron microscopy developments: Towards “wet-STEM” imaging. Micron 2007, 38, 390–401. [Google Scholar] [CrossRef]
- James, B. Advances in “wet” electron microscopy techniques and their application to the study of food structure. Trends Food Sci. Technol. 2009, 20, 114–124. [Google Scholar] [CrossRef]
- Kirk, S.E.; Skepper, J.N.; Donald, A.M. Application of environmental scanning electron microscopy to determine biological surface structure. J. Microsc. 2009, 233, 205–224. [Google Scholar] [CrossRef]
- Danilatos, G.D. Theory of the gaseous detector device in the environmental scanning electron microscope. In Advances in Electronics and Electron Physics; Hawkes, P.W., Ed.; Academic Press: Cambridge, MA, USA, 1990; pp. 1–102. [Google Scholar]
- Conforto, E.; Joguet, N.; Buisson, P.; Vendeville, J.E.; Chaigneau, C.; Maugard, T. An optimized methodology to analyze biopolymer capsules by environmental scanning electron microscopy. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.; Nelson, E.M.; Tanaka, T.; Damiano, J.; Timp, G. Live bacterial physiology visualized with 5 nm resolution using scanning transmission electron microscopy. ACS Nano 2016, 10, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, A.; Wu, H.; van Rijt, M.M.; Vena, M.P.; Keizer, A.D.; Esteves, A.C.C.; Tuinier, R.; Friedrich, H.; Sommerdijk, N.A.J.M.; Patterson, J.P. Liquid–liquid phase separation during amphiphilic self-assembly. Nat. Chem. 2019, 11, 320–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchello, G.; De Pace, C.; Gutierrez, S.A.; Vasquez, C.L.; Wilkinson, N.; Cervasio, F.L.; Ruiz-Perez, L.R.; Battaglia, G. 4D imaging of soft matter in liquid water. bioRxiv 2021. [Google Scholar] [CrossRef]
- Liao, H.G.; Zheng, H. Liquid cell transmission electron microscopy. Annu. Rev. Phys. Chem. 2016, 67, 719–747. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. Monitoring chemical reactions in liquid media using electron microscopy. Nat. Rev. Chem. 2019, 3, 624–637. [Google Scholar] [CrossRef]
- Sutter, P.; Sutter, E. Real-time electron microscopy of nanocrystal synthesis, transformations, and self-assembly in solution. Acc. Chem. Res. 2021, 54, 11–21. [Google Scholar] [CrossRef]
- Pu, S.; Gong, C.; Robertson, A.W. Liquid cell transmission electron microscopy and its applications. R. Soc. Open Sci. 2020, 7, 191204. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.L.; Wu, C.C.; Huang, Y.J.; Peng, H.L.; Chang, H.Y.; Chang, P.; Hsu, L.; Yew, T.R. Novel microchip for in situ TEM imaging of living organisms and bio-reactions in aqueous conditions. Lab Chip 2008, 8, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Watanabe, T.; Pinkowitz, A.; Duquette, D.J.; Hull, R.; Steingart, D.A.; Ross, F.M. In situ EC-TEM studies of metal thin film corrosion in liquid solutions at elevated temperatures. Microsc. Microanal. 2018, 24, 254–255. [Google Scholar] [CrossRef] [Green Version]
- Hickey, C.D.; Sheehan, J.J.; Wilkinson, M.G.; Auty, M.A. Growth and location of bacterial colonies within dairy foods using microscopy techniques: A review. Front. Microbiol. 2015, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Kaledhonkar, S.; Borg, A.; Sun, M.; Chen, B.; Grassucci, R.A.; Ehrenberg, M.; Frank, J. Key intermediates in ribosome recycling visualized by time-resolved cryoelectron microscopy. Structure 2016, 24, 2092–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaguchi, T.; Kilcrease, J.; Igarashi, K.; Wakui, A.; Tamura, K. Advantages of low-kV TEM in the study of beam sensitive materials. Microsc. Microanal. 2020, 26, 554–555. [Google Scholar] [CrossRef]
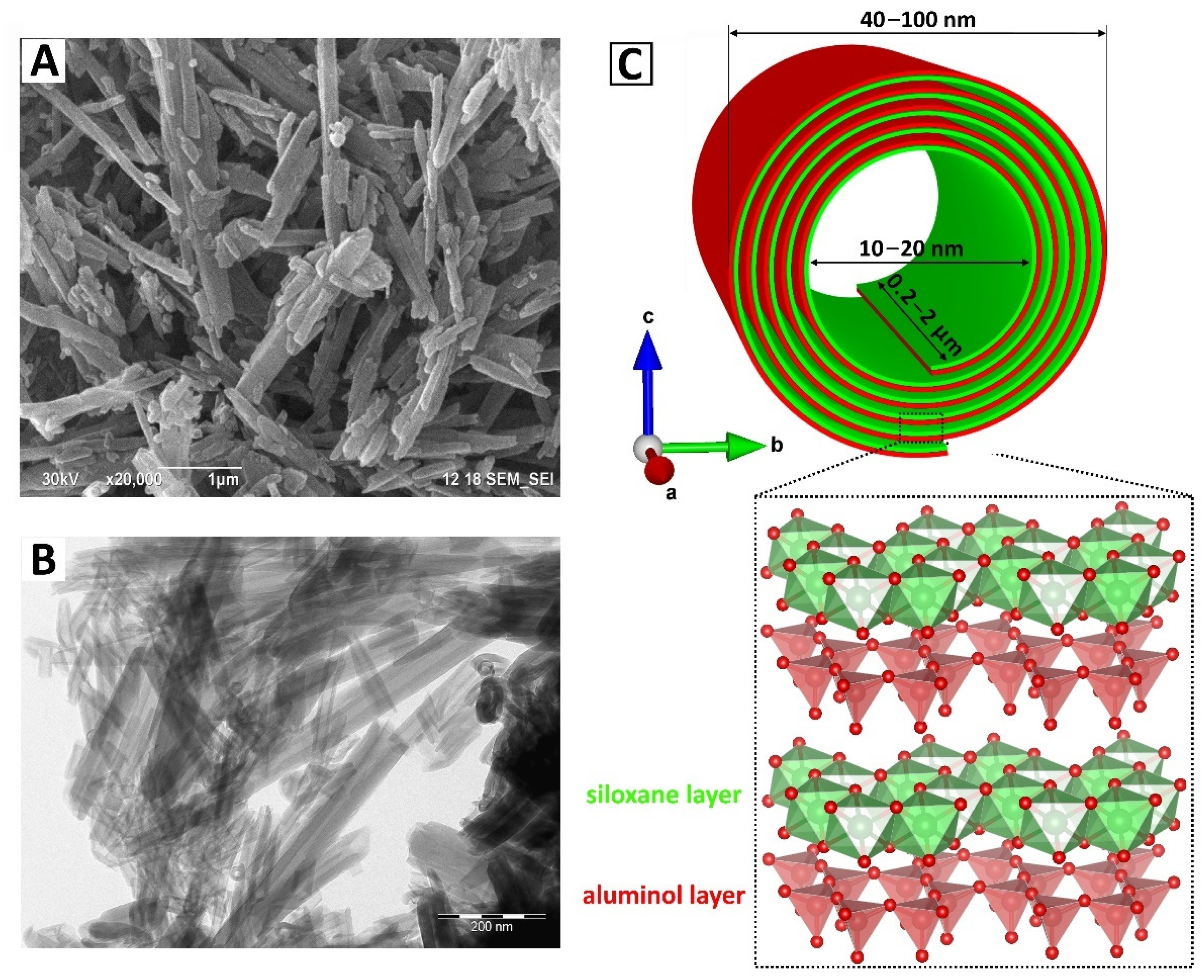

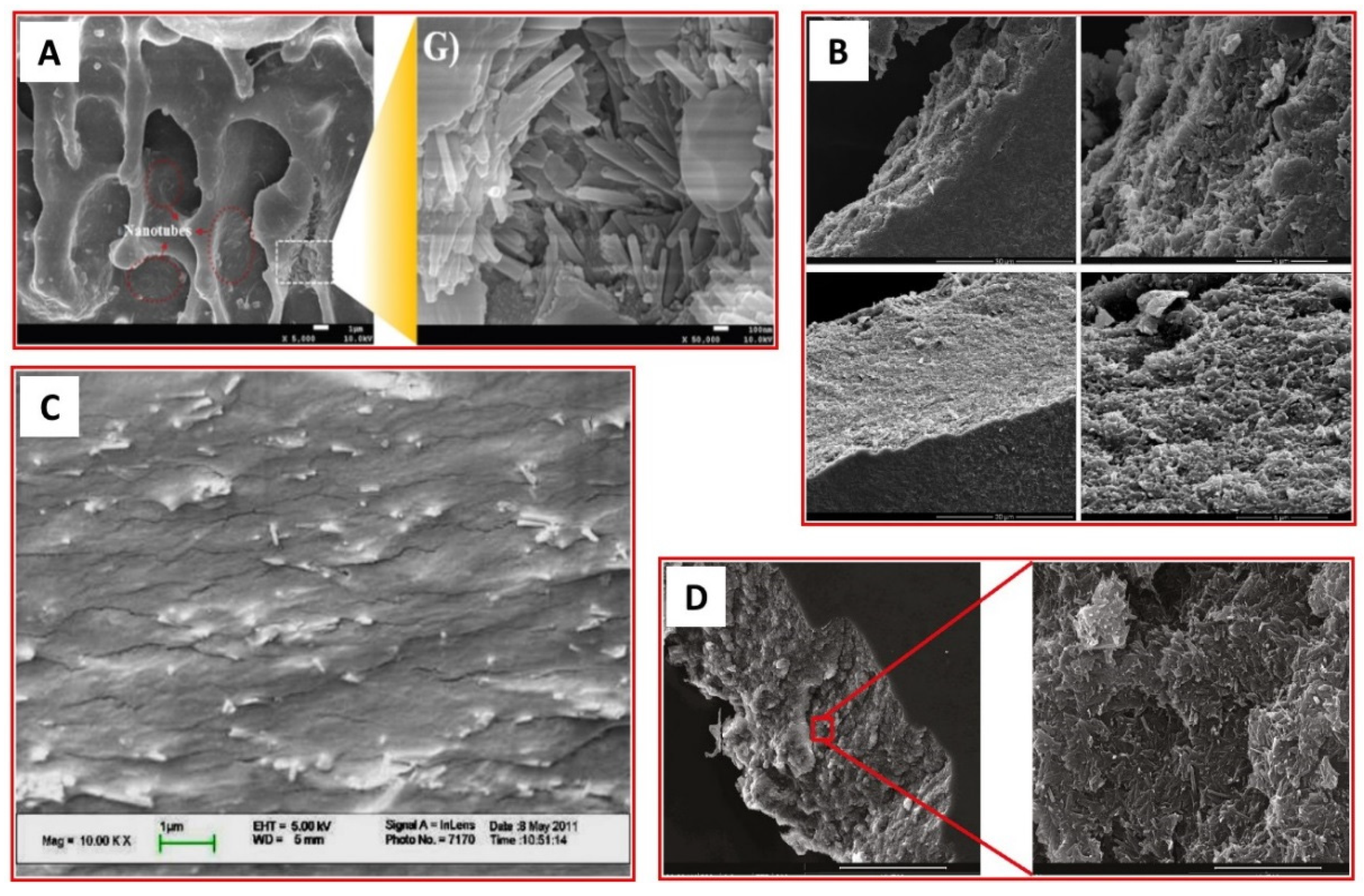
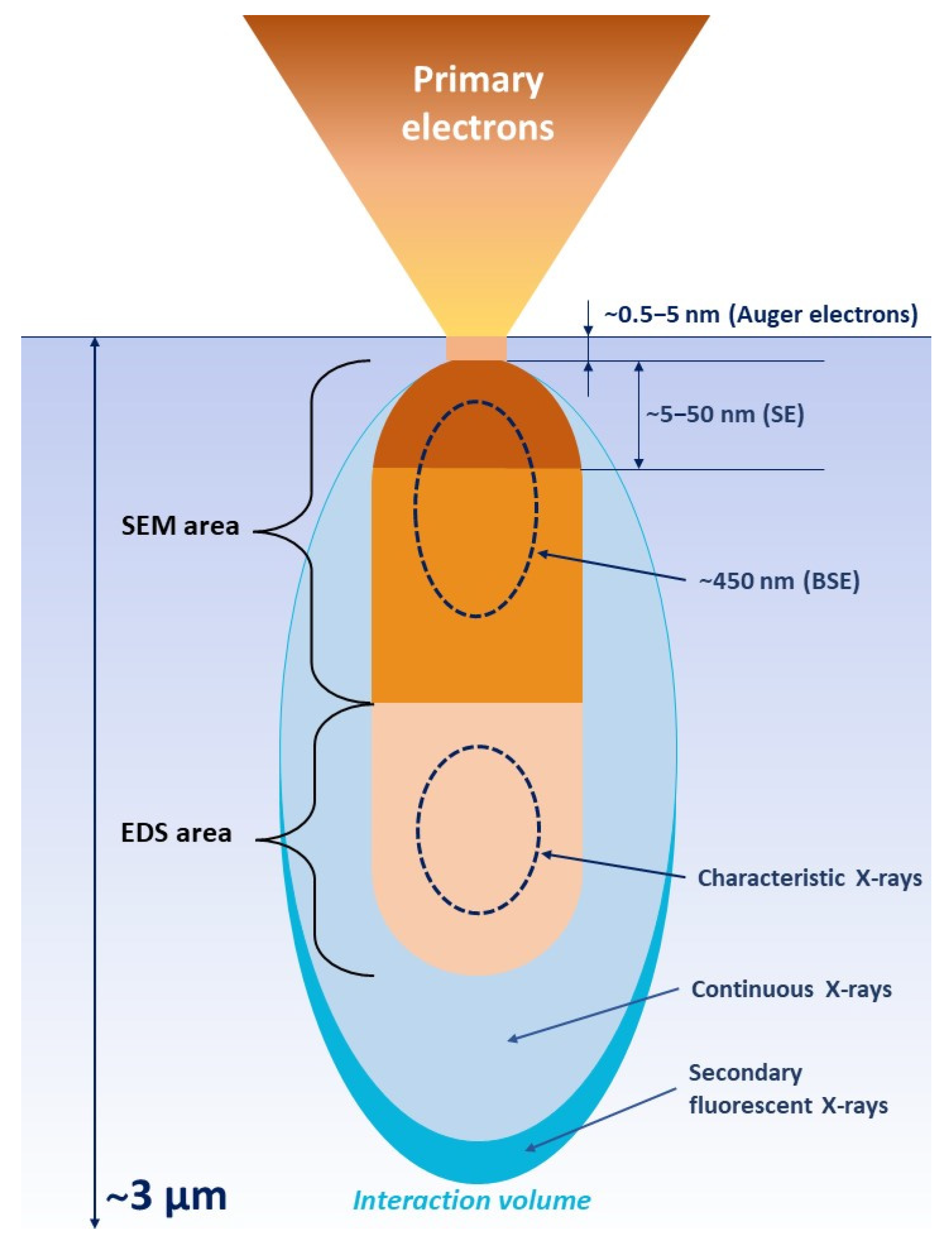

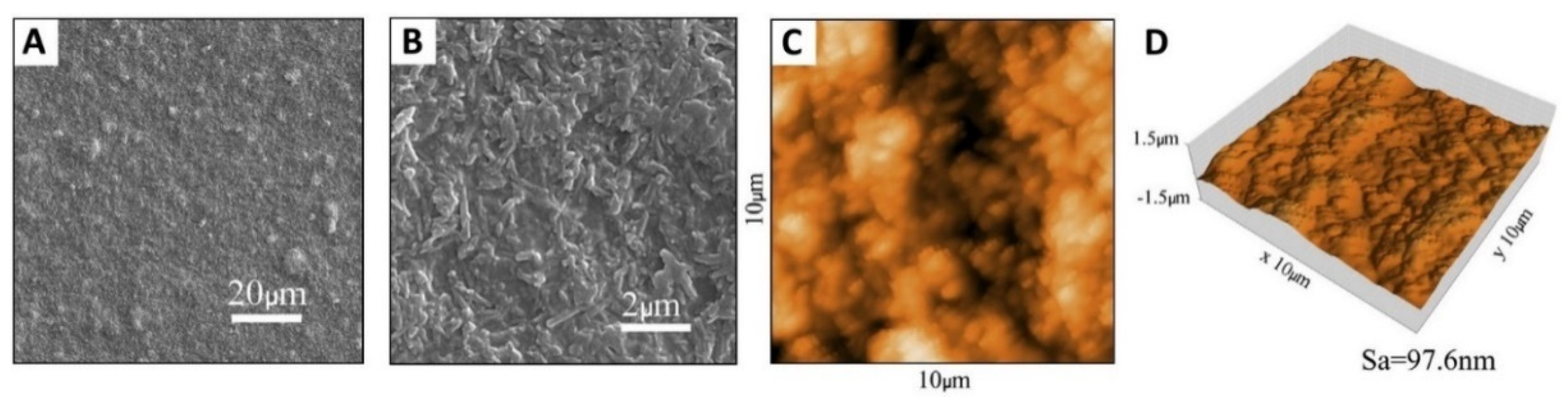



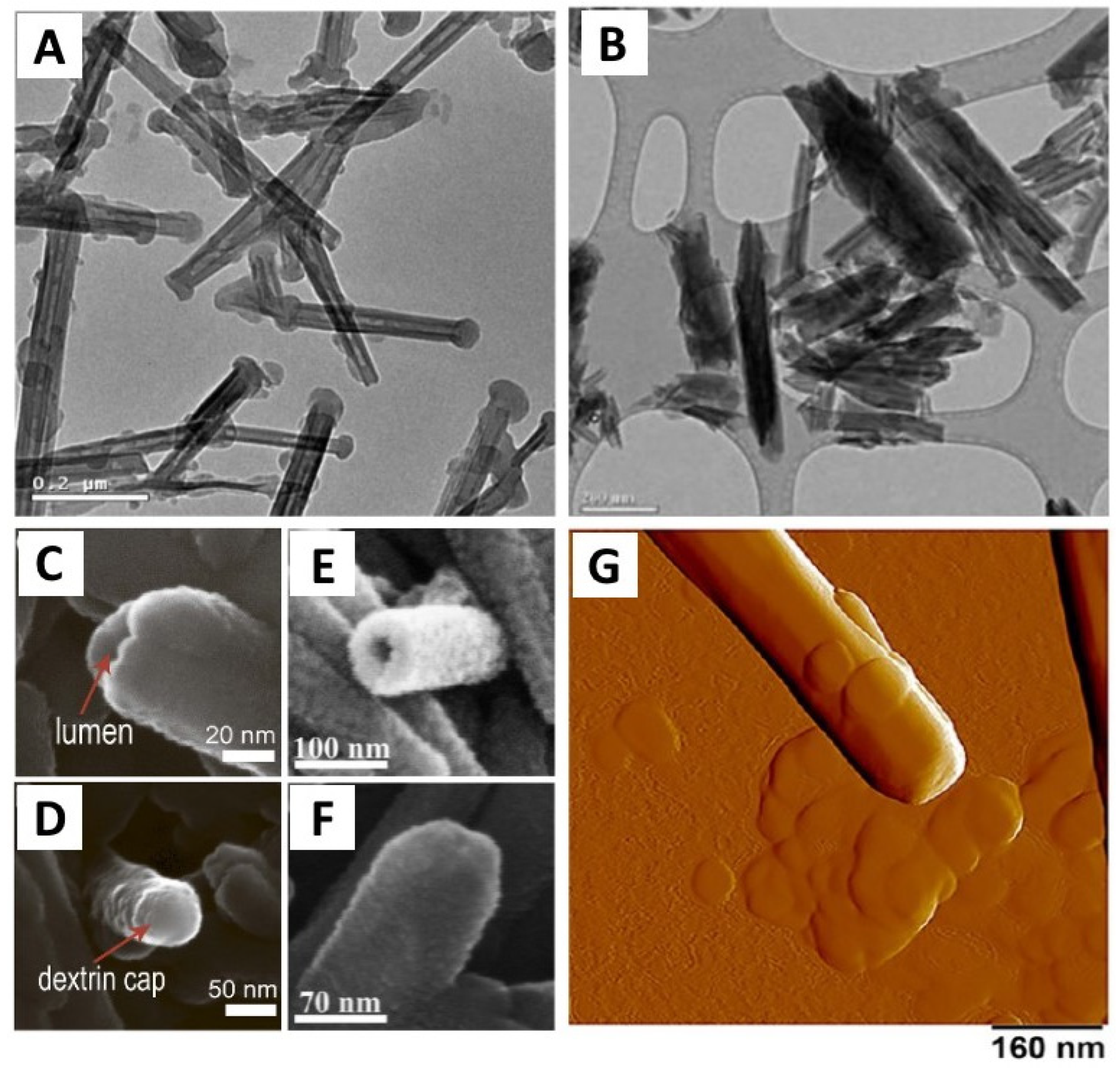
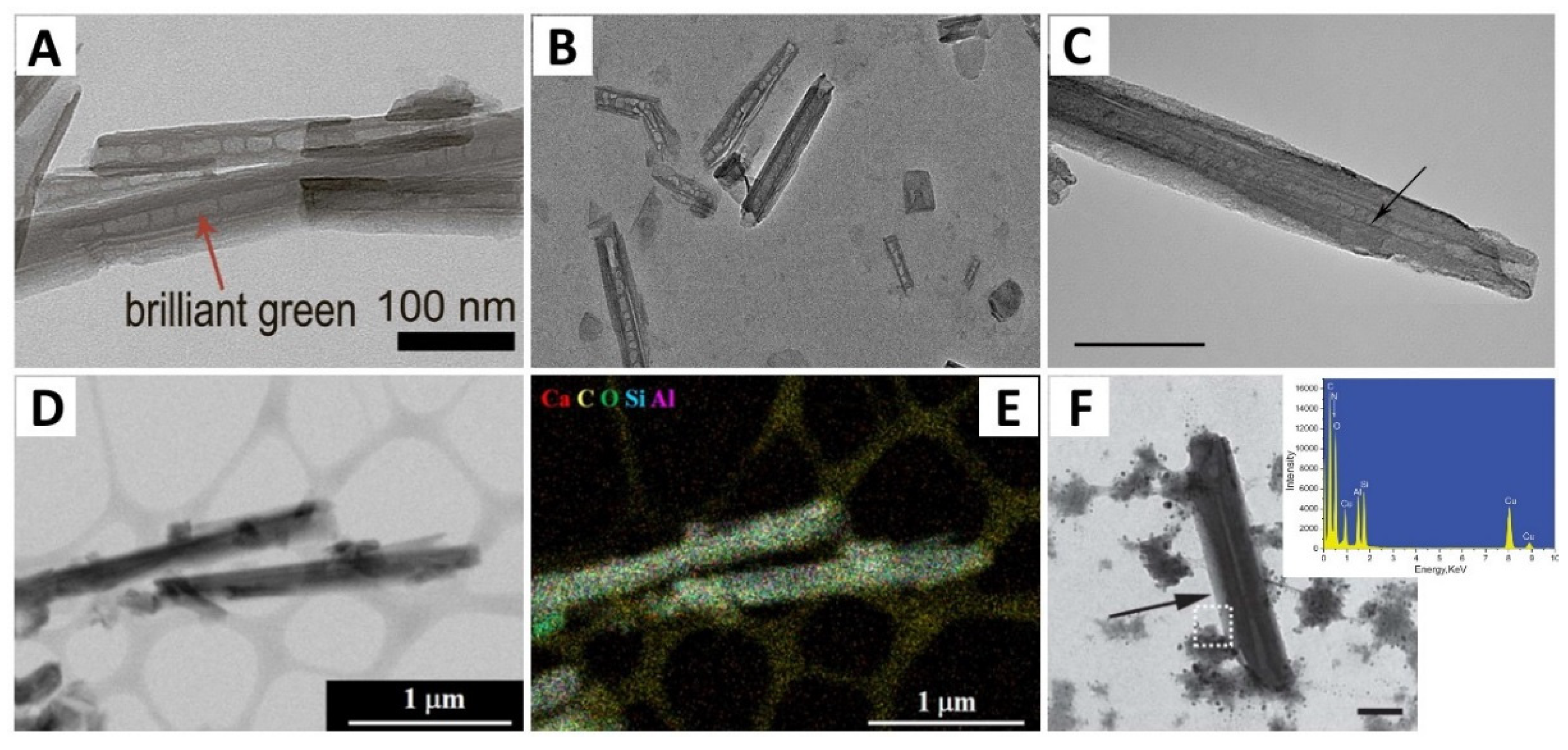

| Analytical Technique | Ref. | Information Obtained | |||||
|---|---|---|---|---|---|---|---|
| Chemical Structure/ Composition | Morphology/ Bulk Information | Crystal Structure | Particles Size | HNT Surface Modification Confirmation | HNT Loading Efficiency | ||
| FTIR | [92,96,97,99,100,101,102,106,117] | + (the finger-print bands of the corresponding functional groups) | – | – | – | + (appearance of new bands relating to chemical bonds/phase formation) | – |
| XRD | [96,98,100,102,106,118] | – | – | + | – | + (at condition new phase is crystalline) | – |
| TGA | [92,93,96,97,100,101,103] | + (by loss of organic content) | – | – | – | + | + (high accuracy) |
| XPS | [102,103,117] | + | – | – | – | + | – |
| DLS | [102,111] | – | – | – | + (dispersions of spherical particles 0.1 nm–10 μm) | ± (small change in size) | – |
| Zeta potential | [100,102] | – | – | – | – | + (significant change) | + (rough estimation by distribution) |
| NMR | [103] | + | – | – | – | ± (not universal method) | ± (not universal method) |
| UV-Vis- near IR spectroscopy | [97,100,116] | – | – | – | – | ± (not universal method) | + (in solution after antimicrobial agent release) |
| Optical microscopy | [108,109,110] | – | + (relatively low resolution) | – | + (particles > 1 μm) | – | – |
| Confocal microscopy | [82,93,101] | – | + (fluorescent tag or staining are necessary) | – | + (particles > 0.5 μm) | ± (not universal method) | ± (not universal method) |
| SEM | [86,87,88,89,90,91,96,103,106,117,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133] | + (EDS or WDS) | + (FIB, electron tomography for bulk information) | + (EBSD) | + | + | – |
| TEM | [81,86,99,100,101,102,106,131,133,134,135,136,137,138,139,140,141,142,143,144] | + (EDS) | + | + (SAED, HRTEM) | + | + | + |
| DENS Solutions | Protochips | ||||
|---|---|---|---|---|---|
| Wildfire (in situ TEM heating) | Heating control | 4-point probe heating | Fusion Select (in situ TEM heating & biasing) | Temperature Range | RT to 1200 °C (900 °C for Electrothermal) |
| Temperature range | RT to 1300 °C | ||||
| Temperature accuracy | ≥95% | Temperature Accuracy | 95% | ||
| Temperature Homogeneity | ≥99.5% | Temperature Stability | <0.01 °C | ||
| Viewable area | 850 µm2 | Maximum Voltage | 55 V (inquire for higher voltages) | ||
| Ligthning (in situ TEM biasing & heating) | Heating & biasing control | Closed 4-point probe feedback loop | |||
| Temperature range | RT to 1300 °C | Heating and Cooling Rate | programmable, any rate up to 1000 °C/ms | ||
| Membrane breakdown voltage | ≥150 V at RT/900 °C | ||||
| Attainable E-fields | ≥300 kV/cm at RT/900 °C | Current Range | Electrical Standard Resolution (ESR) 100 pA to 100 mA | ||
| Detectable current range | 1 pA to 100 mA | ||||
| AC measurement | Up to 100 Hz | ||||
| Temperature accuracy | ≥95% | Electrical High Resolution (EHR) 2 pA to 100 mA | |||
| Temperature homogeneity | ≥99.5% | ||||
| Stream (in situ TEM liquid/biasing or heating) | Liquid thickness | <500 nm | Poseidon Select (in situ electrochemistry/liquid heating | Configurable E-chip Spacer | microwells, 0 nm, 50 nm, 150 nm, 500 nm, 1 μm or 5 μm |
| Resolution | ≤3 Å | Resolution | 2 ± 1 nm or better | ||
| Liquid pressure range (accuracy) | 200–4000 mbar (±2 mbar) | Flow Ports | 3 (static, flow or mixing) | ||
| Liquid modes | Static, flow (infusion, withdrawal) | ||||
| Liquid flow range | 0 to 8 µL/min | ||||
| Flow control: Direct closed loop feedback control | <10 s | E-chip Sealing Method | 1 gasket | ||
| Temperature range | RT to >100 °C | ||||
| Temperature stability | ±0.01 °C | Heating | RT to 100 °C closed-loop control | ||
| Voltage range | −10 V to +10 V | Vapor Introduction | Software-controlled introduction of water, | ||
| Current range | From pA to mA | ||||
| AC impedance frequency range | 10 uHz–1 M Hz | ||||
| Climate (in situ TEM gas & heating) | Resolution | ≤100 pm | Atmosphere (in situ TEM gas & heating) | Resolution | <1.5 Å |
| Gas mixing method | Continuous | Gas Mixing | 0.01–99.99% mixtures of up to 3 gases via volumetric blending methanol, ethanol, hexane, naphtha, etc. | ||
| Gas switching | ≤15 s | ||||
| Gas input lines | 3 | ||||
| Gas flow range (normalised) | 0–1 mL/min | ||||
| Flow Rate | 0.005–1.000 mL/min | ||||
| Pressure range | 0–2000 mbar | Holder Base Pressure | 3.0 × 10−2 Torr | ||
| Heating range | RT–1000 °C | Operating pressure range | 1.0–760.0 Torr | ||
| Temperature stability | ≤±0.01 °C | Temperature | 25–1000 °C | ||
| Heating control mode | 4-point probe resistive feedback | ||||
| Heating & cooling rate | Up to 150 °C/s | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherednichenko, K.; Kopitsyn, D.; Batasheva, S.; Fakhrullin, R. Probing Antimicrobial Halloysite/Biopolymer Composites with Electron Microscopy: Advantages and Limitations. Polymers 2021, 13, 3510. https://doi.org/10.3390/polym13203510
Cherednichenko K, Kopitsyn D, Batasheva S, Fakhrullin R. Probing Antimicrobial Halloysite/Biopolymer Composites with Electron Microscopy: Advantages and Limitations. Polymers. 2021; 13(20):3510. https://doi.org/10.3390/polym13203510
Chicago/Turabian StyleCherednichenko, Kirill, Dmitry Kopitsyn, Svetlana Batasheva, and Rawil Fakhrullin. 2021. "Probing Antimicrobial Halloysite/Biopolymer Composites with Electron Microscopy: Advantages and Limitations" Polymers 13, no. 20: 3510. https://doi.org/10.3390/polym13203510
APA StyleCherednichenko, K., Kopitsyn, D., Batasheva, S., & Fakhrullin, R. (2021). Probing Antimicrobial Halloysite/Biopolymer Composites with Electron Microscopy: Advantages and Limitations. Polymers, 13(20), 3510. https://doi.org/10.3390/polym13203510










