The Effect of Different Soft Core/Hard Shell Ratios on the Coating Performance of Acrylic Copolymer Latexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Core–Shell Acrylic Copolymer Emulsions
2.3. Characterization of the Copolymers
2.4. Application of Latexes in Leather Finishing
3. Results and Discussion
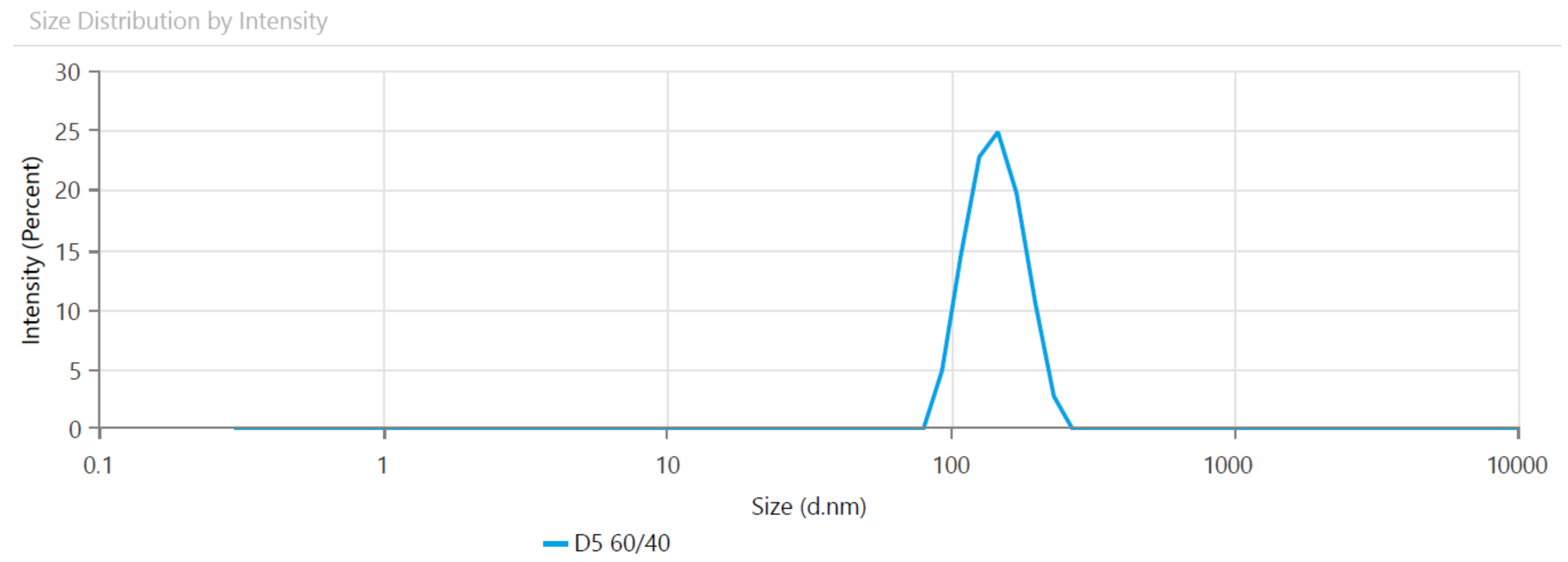
3.1. Particle Size Analysis
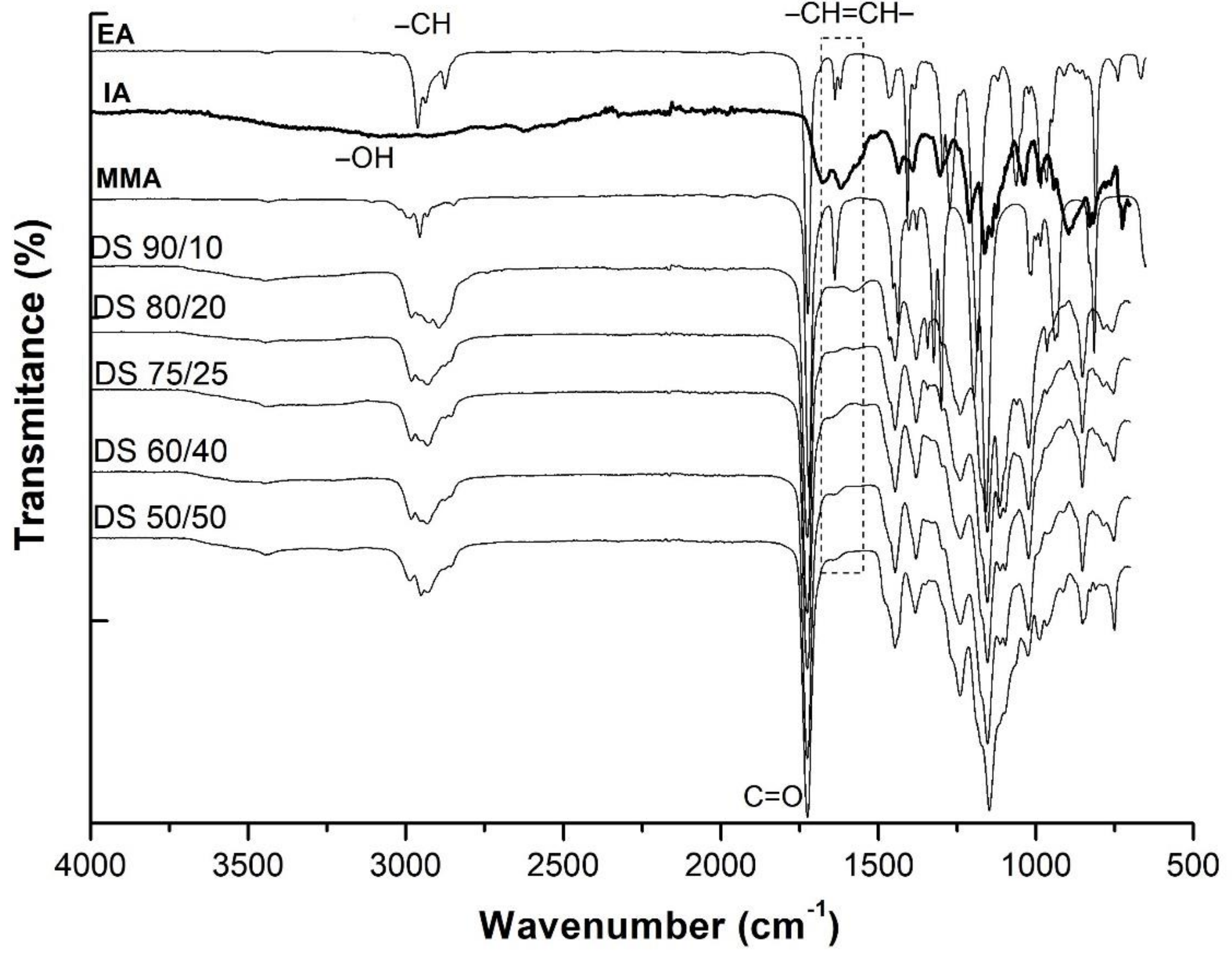
3.2. FTIR Analysis
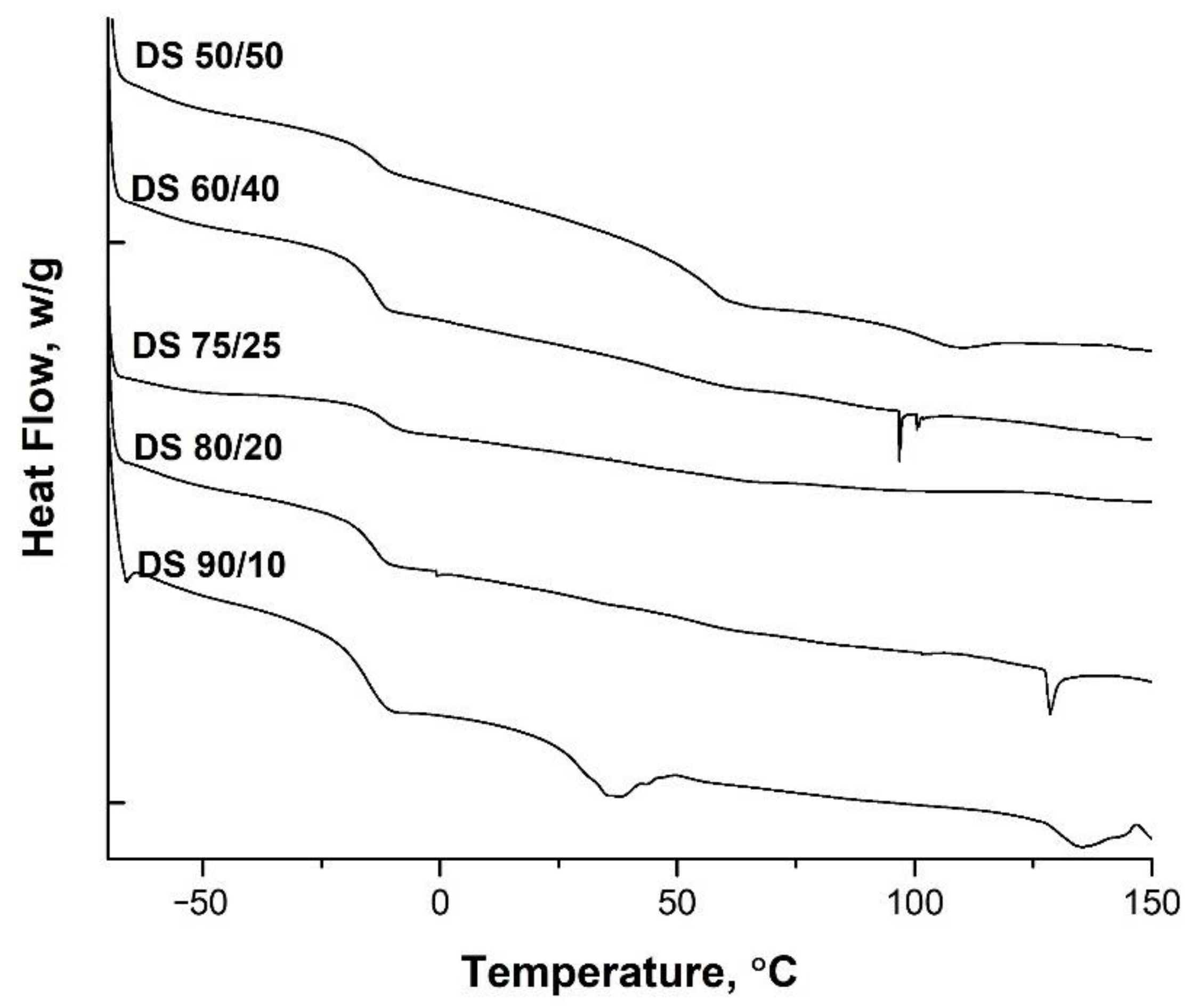
3.3. Mechanical and Thermal Analyses
3.4. Leather Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Yin, Q.; Li, X.; Hao, L.; Zhang, W.; Bao, Y.; Ma, J. A waterborne polyurethane–based leather finishing agent with excellent room temperature self-healing properties and wear-resistance. Adv. Compos. Hybrid Mater. 2021, 4, 138–149. [Google Scholar] [CrossRef]
- Ollé, L.; Frías, A.; Sorolla, S.; Cuadros, R.; Bacardit, A. Study of the impact on occupational health of the use of polyaziridine in leather finishing compared with a new epoxy acrylic self-crosslinking polymer. Prog. Org. Coat. 2021, 154, 106162. [Google Scholar] [CrossRef]
- Stanca, M.; Gaidau, C.; Alexe, C.-A.; Stanculescu, I.; Vasilca, S.; Matei, A.; Simion, D.; Constantinescu, R.-R. Multifunctional Leather Surface Design by Using Carbon Nanotube-Based Composites. Materials 2021, 14, 3003. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, Z.; Lyu, B.; Ma, J. Effect of coated and encapsulated polyacrylate/nano-SiO2 composite emulsions on the finishing performances of leather via miniemulsion polymerization. J. Mater. Sci. 2002, 55, 10070–10083. [Google Scholar] [CrossRef]
- Elsayed, H.; Hasanin, M.; Rehan, M. Enhancement of multifunctional properties of leather surface decorated with silver nanoparticles (Ag NPs). J. Mol. Struct. 2021, 1234, 130130. [Google Scholar] [CrossRef]
- Bouca, V.; Sousa, D.; Cardoso, M.O.; Concecao, D.; Queiros, G.; Marques, E.F.; Gaiao, J.; Mourao, R.; Lopes, A.C.; Crispim, F.; et al. Evaluation of Self-cleaning Properties of Leather Surface with TiO2 Nanoparticles. J. Soc. Leather Technol. Chem. 2020, 104, 83–89. [Google Scholar]
- Li, Y.P.; Sui, Z.H.; Wang, X.; Yan, C.; Huang, S.Q.; Zu, B. Nano-TiO2/Organic Fluorine Polyacrylate Emulsion Coating for Leather. J. Soc. Leather. Technol. Chem. 2021, 105, 77–80. [Google Scholar]
- Sui, Z.H.; San, J.L.; Wu, X.D.; Zhang, J.B.; Sun, L.F. Preparation and Properties of Nano-ZnO/Organic Fluorine Modified Polyacrylate Finishing Agent. J. Am. Leather Chem. Assoc. 2020, 115, 207–214. [Google Scholar]
- Gao, D.; Zhang, J.; Lyu, B.; Ma, J.; Yang, Z. Polyacrylate crosslinked with furyl alcohol grafting bismaleimide: A self-healing polymer coating. Prog. Org. Coat. 2020, 139, 105475. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N.; Yilmaz, O. On the preparation of polyacrylic binder via mini-emulsion polymerization for leather performance coatings. Eskişehir Technical Univ. J. Sci. Tech. A Appl. Sci. Eng. 2020, 21, 389–395. [Google Scholar]
- Yilmaz, O.; Cheaburu-Yilmaz, C.N. Synthesis and Application of a Low Emulsifier Content Composite Polyacrylic Latex for Leather Finishing. J. Polytechnic 2018, 21, 19–25. [Google Scholar]
- Lai, S.Q.; Jin, Y.; Shi, L.J.; Du, W. Synthesis and Leather Application Properties of a Carboxylated Graphene Oxide Modified Waterborne Polyacrylate Leather Finishing Agent. J. Am. Leather Chem. Assoc. 2019, 114, 204–215. [Google Scholar]
- De Ferri, L.; Lorenzi, A.; Tassi, F.; Draghi, L. Organic-inorganic Hybrid Coatings via Sol-gel Route for Leather Finishing. J. Am. Leather Chem. Assoc. 2019, 114, 293–299. [Google Scholar]
- Mohamed, O.; Elsayed, H.; Attia, R.; Haroun, A.; El-Sayed, N. Preparation of acrylic silicon dioxide nanoparticles as a binder for leather finishing. Adv. Polym. Technol. 2018, 37, 3276–3286. [Google Scholar] [CrossRef]
- Sui, Z.H.; San, J.L.; Wang, X.; Zu, B.; Cao, X.Y.; Qiang, X.H. Organic Fluorosilicone Modified Polyacrylate Emulsion Finishing Agent. J. Soc. Leather. Technol. Chem. 2019, 103, 208–212. [Google Scholar]
- Liu, X.; Fan, X.-D.; Tang, M.-F.; Nie, Y. Synthesis and Characterization of Core-Shell Acrylate Based Latex and Study of Its Reactive Blends. Int. J. Mol. Sci. 2008, 9, 342–354. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.I. The effects of latex coalescence and interfacial crosslinking on the mechanical properties of latex films. Polymer 2005, 46, 1287–1293. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, L.J.; Asua, J.M. Development of Particle Morphology in Emulsion Polymerization. 1. Cluster Dynamics. Macromolecules 1995, 28, 3135–3145. [Google Scholar] [CrossRef]
- Hidalgo, M.; Cavaille, J.Y.; Guillot, J.; Guyot, A.; Pichot, C.; Rios, L.; Vassoille, R. Polystyrene(1)/poly(butyl acrylate-methacrylic acid)(2) core-shell emulsion polymers. Part II: Thermomechanical properties of latex films. Colloid Polym. Sci. 1992, 270, 1208–1221. [Google Scholar] [CrossRef]
- Ha, J.W.; Park, L.J.; Lee, S.-B.; Kim, D.K. Preparation and Characterization of Core−Shell Particles Containing Perfluoroalkyl Acrylate in the Shell. Macromolecules 2002, 35, 6811–6818. [Google Scholar] [CrossRef]
- Song, Q.; Yan, P.; Wang, H.; Zhu, X.; Xu, Y. Preparation and characterization of multi-layer core-shell polysiloxane/PSt/PMMA latex. Pigm. Resin Technol. 2012, 41, 339–343. [Google Scholar] [CrossRef]
- Limousin, E.; Ballard, N.; Asua, J.M. The influence of particle morphology on the structure and mechanical properties of films cast from hybrid latexes. Prog. Org. Coat. 2019, 129, 69–76. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, F.; Yang, G.; Liu, Y.; Lv, Y.; Liu, M. Preparation of cationic fluorinated acrylate copolymer latex and its application on cotton fabric. J. Coat. Technol. Res. 2020, 17, 875–885. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Li, Y.; Li, H.; Lu, K. Preparation of cellulose nanocrystal-dressed fluorinated polyacrylate latex particles via RAFT-mediated Pickering emulsion polymerization and application on fabric finishing. Cellulose 2020, 27, 6617–6628. [Google Scholar] [CrossRef]
- Gong, S.; He, T.; Zhu, D.; Sundaramurthy, A.; Li, Q.; Shu, X. Preparation of ATO-incorporated composite latex with tailored structure and controllable size for highly spectrum-selective applications. Mater. Des. 2019, 180, 107919. [Google Scholar] [CrossRef]
- Lara, B.R.; Ouzineb, K.; McKenna, T.F.L. Core–Shell Polymer Adhesive for Aluminized Coatings: From Improved Barrier Properties to Commercial Formulatio. Macromol. Mater. Eng. 2019, 304, 1900148. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, X.; Cai, Z. Preparation and application of low-temperature-curable, alkylphenol ethoxylate-free, and flame-retardant flocking adhesive. J. Text. Inst. 2019, 110, 807–814. [Google Scholar] [CrossRef]
- Shevchenko, N.; Pankova, G.; Shabsels, B.; Baigildin, V.; Koshkin, A.; Ukleev, T.; Sel’kin, A. Fluorescent core-shell polymer particles containing luminophore dyes: Synthesis and optical response to acetone. J. Dispers. Sci. Technol. 2019, 40, 802–810. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, L.; Sun, J.; Li, Z.; Jia, Z.; Gu, J. Design and fabrication of PVAc-based inverted core/shell (ICS) structured adhesives for improved water-resistant wood bonding performance: II. Influence of copolymerizing-grafting sequential reaction. Int. J. Adhes. Adhes. 2020, 99, 102571. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, C.; Zhang, Q.; Zhang, M.; Zhang, H. Structure and performance of waterborne polyurethane-acrylate composite emulsions for industrial coatings: Effect of preparation methods. Colloid. Polym. Sci. 2020, 298, 139–149. [Google Scholar] [CrossRef]
- Delibas, A.; Yıldız, U.; Tauer, K. Composite latex production with high solid content. J. Appl. Polym. Sci. 2019, 136, 47423. [Google Scholar] [CrossRef]
- Sundberg, D. Structured, Composite Nanoparticles from Emulsion Polymerization-Morphological Possibilities. Biomacromolecules 2020, 21, 4388–4395. [Google Scholar] [CrossRef]
- Yilmaz, O. High performance nanocomposite coatings based on soft core-reactive shell polyacrylic latex/modified halloysite nanotubes. Prog. Org. Coat. 2019, 127, 266–275. [Google Scholar] [CrossRef]
- Roshanali, M.; Nodehi, A.; Atai, M. Synthesis and characterization of core-shell nanoparticles and their application in dental resins. J. Mech. Behav. Biomed. Mater. 2020, 110, 103926. [Google Scholar] [CrossRef]
- Limousin, E.; Ballard, N.; Asua, J.M. Soft core–hard shell latex particles for mechanically strong VOC-freepolymer films. J. Appl. Polym. Sci. 2019, 136, 47608. [Google Scholar] [CrossRef]
- Steiger, M.G.; Blumhoff, M.L.; Mattanovich, D.; Sauer, M. Biochemistry of microbial itaconic acid production. Front. Microbiol. 2013, 4, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-J.; Tian, R.-X.; Li, Q.; Liu, Y.-P. Synthesis and Characterization of Large Dimension PBA-P(MMA-ITA) Latex Particles with Core-Shell Morphology. J. Macromol. Sci. Part A 2013, 50, 562–573. [Google Scholar] [CrossRef]
- Kang, K.; Kan, C.; Du, Y.; Liu, D. Study on soap-free P(MMA–EA–AA or MAA) latex particles with narrow size distribution. Polym. Adv. Technol. 2006, 17, 193–198. [Google Scholar] [CrossRef]
- Loginova, E.V.; Mikheev, I.V.; Volkov, D.S.; Proskurnin, M.A. Quantification of copolymer composition (methyl acrylate and itaconic acid) in polyacrylonitrile carbon-fiber precursors by FTIR-spectroscopy. Anal. Methods 2016, 8, 371–380. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Yu, H. Why tand of poly (butyl acrylate) and poly (ethyl acrylate) with little double bonds are becoming asymmetric? Polymer 2014, 55, 4720–4727. [Google Scholar] [CrossRef]
- Schroffenegger, M.; Reimhult, E. Thermoresponsive Core-Shell Nanoparticles and Their Potential Applications. Compr. Nanosci. Nanotechnol. 2019, 2, 145–170. [Google Scholar]
- Xu, C.; Qiu, T.; Deng, J.; Meng, Y.; He, L.; Li, X. Dynamic mechanical study on multilayer core–shell latex for damping applications. Prog. Org. Coat. 2012, 74, 233–239. [Google Scholar] [CrossRef]
- poly(ethyl acrylate). Available online: https://polymerprocessing.com/polymers/PEA.html (accessed on 10 September 2021).
- Fytas, G.; Patkowski, A.; Meier, G.; Dorfmueller, T. Pressure- and temperature-dependent photon correlation study of bulk poly(ethyl acrylate) above the glass transition temperature. Macromolecules 1982, 15, 870–874. [Google Scholar] [CrossRef]
- Mu, Y.; Qiu, T.; Li, X.; Guan, Y.; Zhang, S.; Li, X. Layer-by-Layer Synthesis of Multilayer Core-Shell Latex and the Film Formation Properties. Langmuir 2011, 27, 4968–4978. [Google Scholar] [CrossRef] [PubMed]



| Sample Code | Experimental Set-Up | Results | |||||
|---|---|---|---|---|---|---|---|
| Seed (g) (EA + IA) | Core (g) (EA) | Shell (g) (MMA) | APS (g) | Solid wt % | Conversion % | ||
| DS 90/10 | 3.5 | 1 | 36.00 | 4.50 | 0.35 | 25 | 99 |
| DS 80/20 | 3.5 | 1 | 31.50 | 9.00 | 0.35 | 25 | 99 |
| DS 75/25 | 3.5 | 1 | 29.25 | 11.25 | 0.35 | 25 | 99 |
| DS 60/40 | 3.5 | 1 | 22.50 | 18.00 | 0.35 | 25 | 99 |
| DS 50/50 | 3.5 | 1 | 18.00 | 22.50 | 0.35 | 25 | 99 |
| Components | Application Steps | Descriptions | |
|---|---|---|---|
| Basecoat (I) (Parts Per Unit) | Topcoat (II) (Parts Per Unit) | ||
| Water | 35 | 20 | Spray I × 3 times |
| Pigment | 10 | Hot plate 90 °C/100 bar | |
| Wax | 5 | ||
| Core–shell Latex | 30 | Spray I × 2 times | |
| Polyurethane Emulsion | 5 | Hot plate 90 °C/70 bar | |
| Casein Emulsion | 5 | ||
| Isopropyl alcohol | 0.5 | ||
| Aqueous NC Lacquer | 10 | Spray II × 1 time | |
| Sample Code | Size (nm) | Polydispersity Index (PDI) |
|---|---|---|
| DS 90/10 | 113.9 ± 2.35 | 0.0570 ± 0.008 |
| DS 80/20 | 119.7 ± 2.37 | 0.1280 ± 0.013 |
| DS 75/25 | 83.1 ± 5.56 | 0.0148 ± 0.005 |
| DS 60/40 | 140.4 ± 4.53 | 0.0021 ± 0.0011 |
| DS 50/50 | 173.3 ± 2.75 | 0.0580 ± 0.006 |
| Leather Sample. | Flexing Endurance (×200,000) | Fastness Level—Grey Scale | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 Rubs (Dry) | 500 Rubs (Dry) | 10 Rubs (Wet) | 25 Rubs (Wet) | Water Spotting (16 h) | ||||||
| Leather | Felt | Leather | Felt | Leather | Felt | Leather | Felt | Leather | ||
| DS 50/50 | Large Grain Cracks | 5 | 5 | 4/5 | 4 | 2 | 1 | 1 | 1 | 5 |
| DS 60/40 | Fine Grain Cracks | 5 | 5 | 5 | 5 | 4/5 | 5 | 4 | 4 | 5 |
| DS 75/25 | Fine Grain Cracks | 5 | 5 | 4/5 | 5 | 4/5 | 4/5 | 3 | 2 | 5 |
| DS 80/20 | Fine Grain Cracks | 5 | 5 | 4/5 | 5 | 4/5 | 4 | 3 | 2/3 | 5 |
| DS 90/10 | Excellent | 5 | 5 | 5 | 5 | 1 | 3 | 1 | 1/2 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheaburu-Yilmaz, C.N.; Yilmaz, O.; Darie-Nita, R.N. The Effect of Different Soft Core/Hard Shell Ratios on the Coating Performance of Acrylic Copolymer Latexes. Polymers 2021, 13, 3521. https://doi.org/10.3390/polym13203521
Cheaburu-Yilmaz CN, Yilmaz O, Darie-Nita RN. The Effect of Different Soft Core/Hard Shell Ratios on the Coating Performance of Acrylic Copolymer Latexes. Polymers. 2021; 13(20):3521. https://doi.org/10.3390/polym13203521
Chicago/Turabian StyleCheaburu-Yilmaz, Catalina Natalia, Onur Yilmaz, and Raluca Nicoleta Darie-Nita. 2021. "The Effect of Different Soft Core/Hard Shell Ratios on the Coating Performance of Acrylic Copolymer Latexes" Polymers 13, no. 20: 3521. https://doi.org/10.3390/polym13203521







