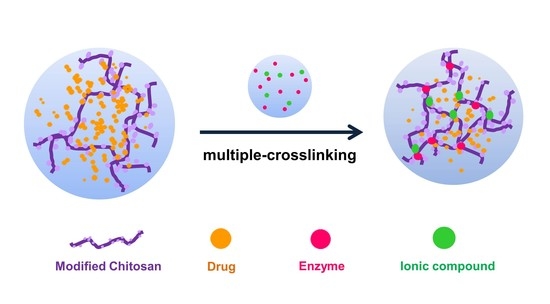
Ionic and Enzymatic Multiple-Crosslinked Nanogels for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Phenolic Hydroxyl Modified Chitosan (MC)
2.3. Preparation of Nanogels with TPP and HRP Multiple-Crosslinking
2.4. Preparation of Nanogels with Na2MoO4 and HRP Multiple Crosslinking
2.5. Measurement of the Nanogel Size
2.6. Drug Loading
2.7. Drug Release
3. Results and Discussion
3.1. Structural Characterization and Enzymatic Crosslinking of MC
3.2. Influences on the Preparations of Multiple-Crosslinked Nanogels
3.3. Drug Loading in Multiple-Crosslinked Nanogels
3.4. Drug Release from Multiple-Crosslinked Nanogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Liu, X.; Xie, Y.; Zhang, H.; Yu, W.; Xiong, Y.; Xie, W.; Ma, X. Microencapsulation using natural polysaccharides for drug delivery and cell implantation. J. Mater. Chem. 2006, 16, 3252. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Thomas, P.; Lukasiewicz, B.; Puthussery, H.; Roy, I. Polyhydroxyalkanoates, a family of natural polymers, and their applications in drug delivery. J. Chem. Technol. Biotechnol. 2015, 90, 1209–1221. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Luo, K.; Yang, Y.; Shao, Z. Physically crosslinked biocompatible silk-fibroin-based hydrogels with high mechanical performance. Adv. Funct. Mater. 2016, 26, 872–880. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng Shu, X.; Prestwich, G.D. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials 2005, 26, 4737–4746. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J. Chitosan-based membranes with different ionic crosslinking density for pharmaceutical and industrial applications. Carbohydr. Polym. 2016, 153, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Liling, G.; Di, Z.; Jiachao, X.; Xin, G.; Xiaoting, F.; Qing, Z. Effects of ionic crosslinking on physical and mechanical properties of alginate mulching films. Carbohydr. Polym. 2016, 136, 259–265. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Izzo, D.; Palazzo, B.; Scalera, F.; Gullotta, F.; lapesa, V.; Scialla, S.; Sannino, A.; Gervaso, F. Chitosan scaffolds for cartilage regeneration: Influence of different ionic crosslinkers on biomaterial properties. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 936–945. [Google Scholar] [CrossRef]
- Gupta, D.; Santoso, J.W.; McCain, M.L. Characterization of gelatin hydrogels cross-linked with microbial transglutaminase as engineered skeletal muscle substrates. Bioengineering 2021, 8, 6. [Google Scholar] [CrossRef]
- Chen, R.-N.; Ho, H.-O.; Sheu, M.-T. Characterization of collagen matrices crosslinked using microbial transglutaminase. Biomaterials 2005, 26, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.K.; Chen, T.; Williams, C.M.; Markley, K.M.; Payne, G.F. Mechanical properties of biomimetic tissue adhesive based on the microbial transglutaminase-catalyzed crosslinking of gelatin. Biomacromolecules 2004, 5, 1270–1279. [Google Scholar] [CrossRef]
- Roberts, J.J.; Naudiyal, P.; Lim, K.S.; Poole-Warren, L.A.; Martens, P.J. A comparative study of enzyme initiators for crosslinking phenol-functionalized hydrogels for cell encapsulation. Biomater. Res. 2016, 20, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, F.; Bae, K.H.; Kurisawa, M. Injectable hydrogel systems crosslinked by horseradish peroxidase. Biomed. Mater. 2015, 11, 14101. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.E.; Yang, Y.Y.; Gao, S.J.; Uyama, H. Injectable biodegradable hydrogels composed of hyaluronic acid–tyramine conjugates for drug delivery and tissue engineering. Chem. Commun. 2005, 4312. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Z.; Xu, C.; Fang, S.; Liu, X.; Li, X. Tough biohydrogels with interpenetrating network structure by bienzymatic crosslinking approach. Eur. Polym. J. 2015, 72, 717–725. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Y.; Fang, S.; Xu, C.; Li, X. Bienzymatically crosslinked gelatin/hyaluronic acid interpenetrating network hydrogels: Preparation and characterization. RSC Adv. 2015, 5, 1929–1936. [Google Scholar] [CrossRef]
- Naseri, N.; Deepa, B.; Mathew, A.P.; Oksman, K.; Girandon, L. Nanocellulose-Based Interpenetrating Polymer Network (IPN) hydrogels for cartilage applications. Biomacromolecules 2016, 17, 3714–3723. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.Y.; Kim, S.S.; Lee, Y.M.; Lee, K.H.; Kim, S.J. Synthesis and characteristics of interpenetrating polymer network hydrogel composed of chitosan and poly(acrylic acid). J. Appl. Polym. Sci. 1999, 73, 113–120. [Google Scholar] [CrossRef]
- Wen, C.; Lu, L.; Li, X. Mechanically robust gelatin-alginate IPN hydrogels by a combination of enzymatic and ionic crosslinking approaches. Macromol. Mater. Eng. 2014, 299, 504–513. [Google Scholar] [CrossRef]
- Jin, R.; Lin, C.; Cao, A. Enzyme-mediated fast injectable hydrogels based on chitosan-glycolic acid/tyrosine: Preparation, characterization, and chondrocyte culture. Polym. Chem. 2014, 5, 391–398. [Google Scholar] [CrossRef]
- Liu, J.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wong, C.-W.; Chang, S.-W.; Hsu, S. An injectable, self-healing phenol-functionalized chitosan hydrogel with fast gelling property and visible light-crosslinking capability for 3D printing. Acta Biomater. 2021, 122, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, G.; Zhou, H.; Ma, S.; Guo, L.; Liu, X. Temperature and H2O2-operated nano-valves on mesoporous silica nanoparticles for controlled drug release and kinetics. Colloids Surf. B Biointerfaces 2020, 187, 110643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, G.; Wang, N.; Guo, F.; Guo, L.; Liu, X. Synthesis of temperature/pH dual-sensitive supramolecular micelles from β-cyclodextrin-poly(N-isopropylacrylamide) star polymer for drug delivery. Colloids Surf. B Biointerfaces 2018, 172, 136–142. [Google Scholar] [CrossRef]
- Jin, L.; Huang, Q.J.; Zeng, H.Y.; Du, J.Z.; Xu, S.; Chen, C.R. Hydrotalcite-gated hollow mesoporous silica delivery system for controlled drug release. Microporous Mesoporous Mater. 2019, 274, 304–312. [Google Scholar] [CrossRef]
- Perioli, L.; Posati, T.; Nocchetti, M.; Bellezza, F.; Costantino, U.; Cipiciani, A. Intercalation and release of antiinflammatory drug diclofenac into nanosized ZnAl hydrotalcite-like compound. Appl. Clay Sci. 2011, 53, 374–378. [Google Scholar] [CrossRef]
- Tan, D.; Yuan, P.; Annabi-Bergaya, F.; Liu, D.; Wang, L.; Liu, H.; He, H. Loading and in vitro release of ibuprofen in tubular halloysite. Appl. Clay Sci. 2014, 96, 50–55. [Google Scholar] [CrossRef]
- Kong, B.J.; Kim, A.; Park, S.N. Properties and in vitro drug release of hyaluronic acid-hydroxyethyl cellulose hydrogels for transdermal delivery of isoliquiritigenin. Carbohydr. Polym. 2016, 147, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]





| Sample | MC (mg mL−1) | TPP (mg mL−1) | HRP ((U mL−1) | H2O2 (mM) |
|---|---|---|---|---|
| MC/T/H-A1 | 1.0 | 0.20 | 0.5 | 0.08 |
| MC/T/H-A2 | 1.0 | 0.10 | 0.5 | 0.08 |
| MC/T/H-A3 | 1.0 | 0.05 | 0.5 | 0.08 |
| MC/T/H-A4 | 1.0 | 0.04 | 0.5 | 0.08 |
| MC/T/H-B1 | 1.0 | 0.20 | 0.5 | 0.08 |
| MC/T/H-B2 | 1.0 | 0.10 | 0.5 | 0.08 |
| MC/T/H-B3 | 1.0 | 0.05 | 0.5 | 0.08 |
| MC/T/H-B4 | 1.0 | 0.04 | 0.5 | 0.08 |
| Sample | MC (mg mL−1) | Na2MoO4 (mg mL−1) | HRP (U mL−1) | H2O2 (mM) |
|---|---|---|---|---|
| MC/M/H-A1 | 1.0 | 0.20 | 0.5 | 0.08 |
| MC/M/H-A2 | 1.0 | 0.10 | 0.5 | 0.08 |
| MC/M/H-A3 | 1.0 | 0.05 | 0.5 | 0.08 |
| MC/M/H-A4 | 1.0 | 0.04 | 0.5 | 0.08 |
| MC/M/H-B1 | 1.0 | 0.20 | 0.5 | 0.08 |
| MC/M/H-B2 | 1.0 | 0.10 | 0.5 | 0.08 |
| MC/M/H-B3 | 1.0 | 0.05 | 0.5 | 0.08 |
| MC/M/H-B4 | 1.0 | 0.04 | 0.5 | 0.08 |
| m0/m (%) | |||
|---|---|---|---|
| 50 | 100 | 150 | |
| DLC (%) | 23.55 ± 4.68 | 32.30 ± 7.04 | 35.01 ± 5.19 |
| EE (%) | 66.82 ± 7.92 | 64.60 ± 4.09 | 58.35 ± 8.66 |
| Models | m0/m (%) | pH = 5.5 | pH = 6.5 | pH = 7.5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | R2 | R2 | ||||||||
| Zero-order | 50 | 0.9108 | 0.8786 | 0.9365 | ||||||
| F = kt | 100 | 0.8967 | 0.8655 | 0.7143 | ||||||
| 150 | 0.8902 | 0.7734 | 0.647 | |||||||
| R2 | R2 | R2 | ||||||||
| First-order | 50 | 0.9496 | 0.9081 | 0.9538 | ||||||
| F = 1 − e−kt | 100 | 0.9314 | 0.8881 | 0.7322 | ||||||
| 150 | 0.9077 | 0.7902 | 0.6562 | |||||||
| R2 | R2 | R2 | ||||||||
| Higuchi | 50 | 0.9856 | 0.9647 | 0.9874 | ||||||
| F = kt0.5 | 100 | 0.9768 | 0.9661 | 0.8765 | ||||||
| 150 | 0.982 | 0.9149 | 0.8276 | |||||||
| R2 | n | R2 | n | R2 | n | |||||
| Korsmeyer-Peppas | 50 | 0.9908 | 0.6106 | 0.9798 | 0.6502 | 0.9767 | 0.4823 | |||
| F = ktn | 100 | 0.9849 | 0.4493 | 0.9893 | 0.3026 | 0.9536 | 0.2738 | |||
| 150 | 0.9947 | 0.3302 | 0.9687 | 0.2369 | 0.9444 | 0.2199 | ||||
| R2 | k1 | k2 | R2 | k1 | k2 | R2 | k1 | k2 | ||
| Peppas-Sahlin | 50 | 0.9947 | 0.3034 | −0.0286 | 0.9853 | 0.2361 | −0.0206 | 0.9851 | 0.1933 | −0.0208 |
| F = k1tm + k2t2m | 100 | 0.9717 | 0.3067 | −0.0385 | 0.9809 | 0.1161 | −0.0094 | 0.9764 | 0.1333 | −0.0174 |
| 150 | 0.9901 | 0.1568 | −0.0194 | 0.9542 | 0.0686 | −0.0062 | 0.9565 | 0.0935 | −0.0132 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Q.; Zhong, J.; Wang, R.; Huang, Y. Ionic and Enzymatic Multiple-Crosslinked Nanogels for Drug Delivery. Polymers 2021, 13, 3565. https://doi.org/10.3390/polym13203565
Tao Q, Zhong J, Wang R, Huang Y. Ionic and Enzymatic Multiple-Crosslinked Nanogels for Drug Delivery. Polymers. 2021; 13(20):3565. https://doi.org/10.3390/polym13203565
Chicago/Turabian StyleTao, Qian, Julong Zhong, Rui Wang, and Yuzhu Huang. 2021. "Ionic and Enzymatic Multiple-Crosslinked Nanogels for Drug Delivery" Polymers 13, no. 20: 3565. https://doi.org/10.3390/polym13203565