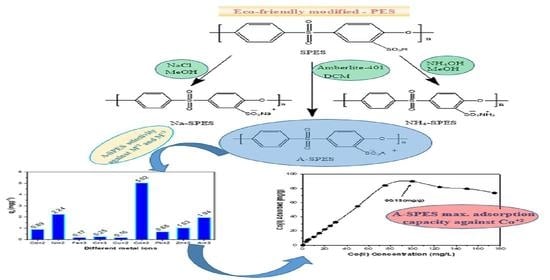
Modification of Sulfonated Polyethersulfone Membrane as a Selective Adsorbent for Co(II) Ions
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Chemical Modifications of PES
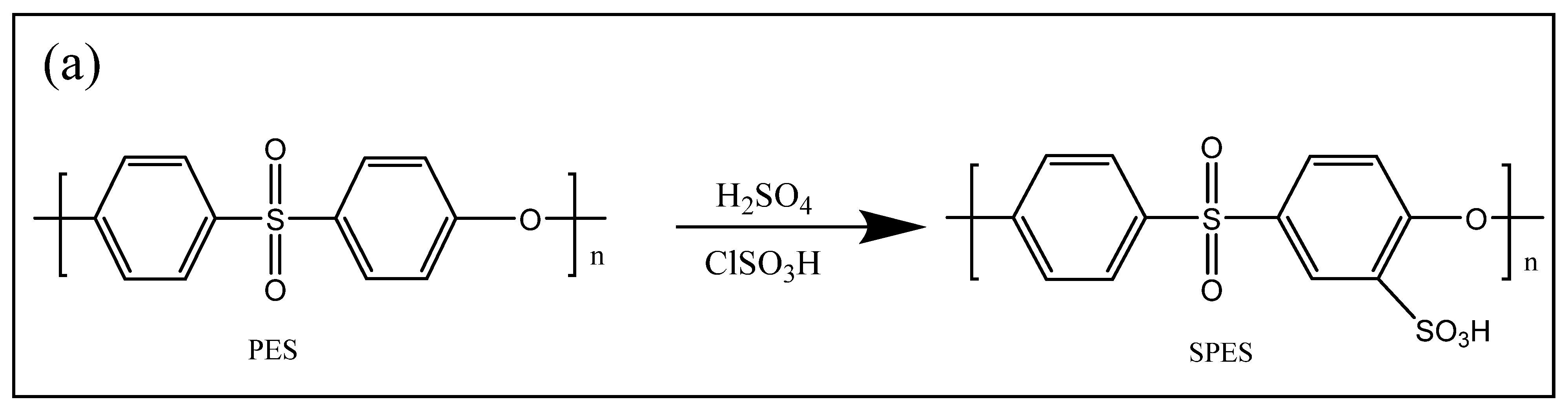
2.2.1. Preparation of the Sulfonated PES (SPES)
2.2.2. Preparation of PES Containing Pendant Sodium Sulfonate Group (Na-SPES)
2.2.3. Preparation of PES Containing Pendant Ammonium Sulfonate Group (NH4-SPES)
2.2.4. Preparation of SPES Containing Pendant Amberlite-IRA-401(A-SPES)
2.3. Adsorption Method Procedure
2.4. Instrumentation
3. Results and Discussion
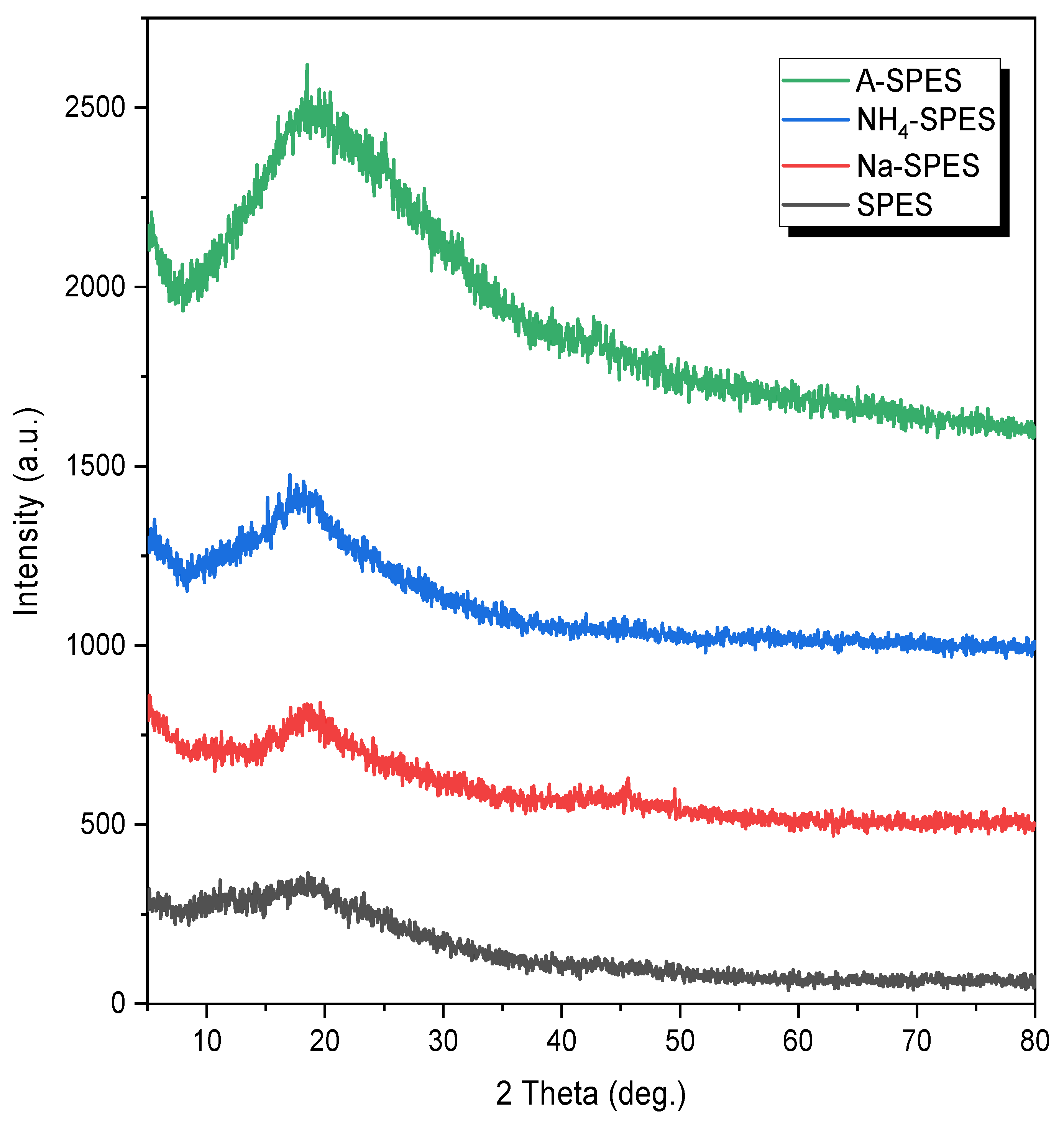
3.1. Chemical Modification and Characterization
3.2. Surface Selectivity Study
3.2.1. Effect of pH
3.2.2. Determination of Adsorption Capacity
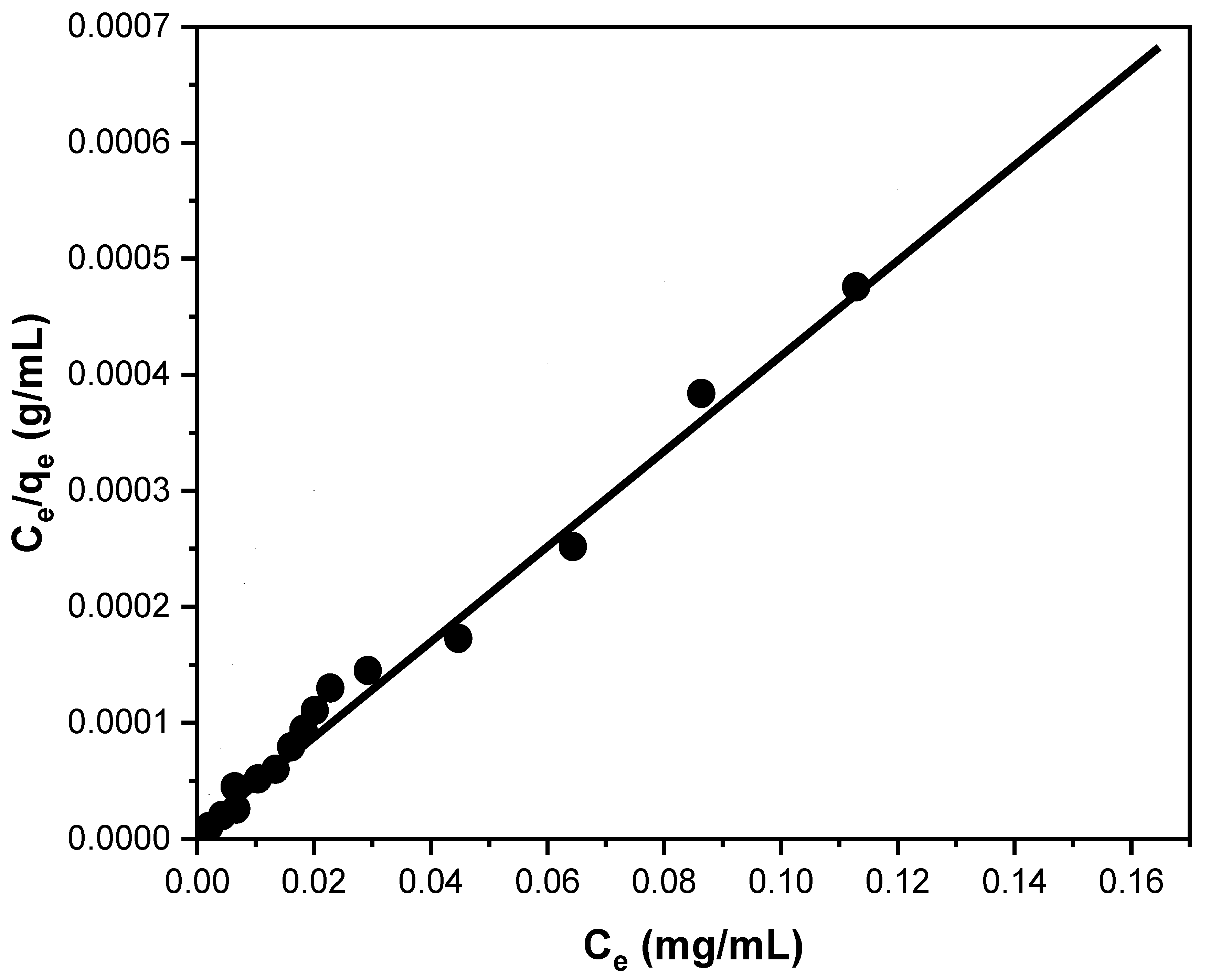
3.2.3. Adsorption Isotherm Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varma-Nair, M.; Jin, Y.; Wunderlich, B. Heat capacities of aliphatic and aromatic polysulphones. Polymer 1992, 33, 5272–5281. [Google Scholar] [CrossRef]
- Zhao, W.; Mou, Q.; Zhang, X.; Shi, J.; Sun, S.; Zhao, C. Preparation and characterization of sulfonated polyethersulfone membranes by a facile approach. Eur. Polym. J. 2013, 49, 738–751. [Google Scholar] [CrossRef]
- Pedicini, R.; Sacca, A.; Carbone, A.; Gatto, I.; Patti, A.; Passalacqua, E. Study on sulphonated polysulphone/polyurethane blend membranes for fuel cell applications. Chem. Phy. Lett. 2013, 579, 100–104. [Google Scholar] [CrossRef]
- Seetharaman, S.; Raghu, S.C.; Mahabadi, K.A. Enhancement of current density using effective membranes electrode assemblies for water electrolyser system. J. Energy Chem. 2016, 25, 77–84. [Google Scholar] [CrossRef]
- Unnikrishnan, L.; Nayak, S.K.; Mohanty, S.; Sarkhel, G. Polyethersulfone membranes: The effect of sulfonation on the properties. Polym. Plast. Technol. Eng. 2010, 49, 1419–1427. [Google Scholar] [CrossRef]
- Lu, D.; Zou, H.; Guuan, R.; Dai, H.; Li, L. Sulfonation of polyethersulfone by chlorosulfonic acid. Polym. Bull. 2005, 54, 21–28. [Google Scholar] [CrossRef]
- Kang, M.-S.; Choi, Y.J.; Choi, I.; Yoon, T.; Moon, S. Electrochemical characterization of sulfonated poly (arylene ether sulfone)(S-PES) cation-exchange membranes. J. Membr.Sci. 2003, 216, 39–53. [Google Scholar] [CrossRef]
- Klaysom, C.; Ladewig, B.P.; Lu, G.D.M.; Wang, L. Preparation and characterization of sulfonated polyethersulfone for cation-exchange membranes. J. Membr. Sci. 2011, 368, 48–53. [Google Scholar] [CrossRef]
- Chen, S.-H.; Yu, K.C.; Lin, S.S.; Chang, D.J.; Liou, R.M. Pervaporation separation of water/ethanol mixture by sulfonated polysulfone membrane. J. Membr. Sci. 2001, 183, 29–36. [Google Scholar] [CrossRef]
- Liou, R.-M.; Chen, S.H.; Lai, C.L.; Hung, M.Y.; Huang, C.H. Effect of ammonium groups of sulfonated polysulfone membrane on its pervaporation performance. Desalination 2011, 278, 91–97. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Ghorbani, S.; Shockravi, A.; Mansourpanah, Y. The influence of sulfonated polyethersulfone (SPES) on surface nano-morphology and performance of polyethersulfone (PES) membrane. Appl. Surf. Sci. 2010, 256, 1825–1831. [Google Scholar] [CrossRef]
- Lai, C.-L.; Liou, R.M.; Chen, S.H.; Shih, C.Y.; Chang, J.S.; Huang, C.H.; Hung, M.Y.; Lee, K.R. Dehydration of ethanol/water mixture by asymmetric ion-exchange membranes. Desalination 2011, 266, 17–24. [Google Scholar] [CrossRef]
- Cassady, H.J.; Cimino, E.C.; Kumar, M.; Hickner, M.A. Specific ion effects on the permselectivity of sulfonated poly (ether sulfone) cation exchange membranes. J. Membr. Sci. 2016, 508, 146–152. [Google Scholar] [CrossRef]
- Lyu, J.; Guo, X. Applications of Organic ion Exchange Resins in Water Treatment, in Applications of Ion Exchange Materials in the Environment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 205–225. [Google Scholar]
- Reed, B.E.; Vaughan, R.; Jiang, L. As (III), As (V), Hg, and Pb removal by Fe-oxide impregnated activated carbon. J. Environ. Eng. 2000, 126, 869–873. [Google Scholar] [CrossRef]
- Romero-González, M.E.; Williams, C.J.; Gardiner, P.H. Study of the mechanisms of cadmium biosorption by dealginated seaweed waste. Environm. Sci. Technol. 2001, 35, 3025–3030. [Google Scholar] [CrossRef]
- Mohammadi, S.; Afzali, D.; Pourtalebi, D. Flame atomic absorption spectrometric determination of trace amounts of lead, cadmium and nickel in different matrixes after solid phase extraction on modified multiwalled carbon nanotubes. Cent. Eur. J. Chem. 2010, 8, 662–668. [Google Scholar] [CrossRef]
- Cho, H.-J.; Myung, S.-W. Determination of cadmium, chromium and lead in polymers by icp-oes using a high pressure asher (hpa). Bull. Korean Chem. Soc. 2011, 32, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zheng, Q.; Yang, P.; Liu, J.; Jin, L. Sensitive voltammetric detection of trace heavy metals in real water using multi-wall carbon nanotubes/nafion composite film electrode. Chin. J. Chem. 2011, 29, 805–812. [Google Scholar] [CrossRef]
- Tanikkul, S.; Jakmunee, J.; Lapanantnoppakhun, S.; Rayanakorn, M.; Sooksamiti, P.; Synovec, R.E.; Christian, G.D.; Grudpan, K. Flow injection in-valve-mini-column pretreatment combined with ion chromatography for cadmium, lead and zinc determination. Talanta 2004, 64, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- El-Safty, S.A. Functionalized hexagonal mesoporous silica monoliths with hydrophobic azo-chromophore for enhanced Co (II) ion monitoring. Adsorption 2009, 15, 227–239. [Google Scholar] [CrossRef]
- Gharehbaghi, M.; Shemirani, F.; Farahani, M.D. Cold-induced aggregation microextraction based on ionic liquids and fiber optic-linear array detection spectrophotometry of cobalt in water samples. J. Hazard. Mater. 2009, 165, 1049–1055. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. Highly selective colorimetric chemosensor for Co2+. Inorg. Chem. 2011, 50, 11282–11284. [Google Scholar] [CrossRef]
- Yao, Y.; Tian, D.; Li, H. Cooperative binding of bifunctionalized and click-synthesized silver nanoparticles for colorimetric Co2+ sensing. ACS Appl. Mater. Interfaces 2010, 2, 684–690. [Google Scholar] [CrossRef]
- Zhen, S.J.; Guo, F.L.; Chen, L.Q.; Li, Y.F.; Zhang, Q.; Huang, C.Z. Visual detection of cobalt (II) ion in vitro and tissue with a new type of leaf-like molecular microcrystal. Chem. Commun. 2011, 47, 2562–2564. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, H.Y.; New, E.J.; Chang, C.J. A selective reaction-based fluorescent probe for detecting cobalt in living cells. Chem. Commun. 2012, 48, 5268–5270. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhefnawy, O.; Zidan, W.I.; Abo-Aly, M.; Bakir, E.; Elsayed, G.A. Synthesis and characterization of a new surface modified Amberlite-7HP resin by nano-iron oxide (Fe3O4) and its application for uranyl ions separation. J. Radioanal. Nucl. Chem. 2014, 299, 1821–1832. [Google Scholar] [CrossRef]
- Negrea, P.; Gabor, A.; Davidescu, C.M.; Ciopec, M.; Negrea, A.; Duteanu, N. Kinetics and thermodynamics modeling of Nd (III) removal from aqueous solution using modified Amberlite XAD7. J. Rare Earths 2020, 38, 306–314. [Google Scholar] [CrossRef]
- Abbady, M.A.; Aly, K.I.; Mahgoub, S.A.; Hussein, M.A. New polymer synthesis part 15. synthesis and characterization of new polyketoamine polymers containing ether and thioether linkage in the main chain. J. Polym. Int. 2005, 54, 1512–1523. [Google Scholar] [CrossRef]
- Hussein, M.A. Eco-friendly polythiophene(keto-amine)s based on cyclopentanone moiety for environmental remediation. J. Polym. Environ. 2018, 26, 1194–1205. [Google Scholar] [CrossRef]
- Aly, K.I.; Hussein, M.A. New polymer syntheses part: 58. synthesis, characterization and corrosion inhibitive properties of new thiazole based polyamides containing diarylidenecyclohexanone moiety. Chin. J. Polym. Sci. 2015, 33, 1–13. [Google Scholar] [CrossRef]
- Hussein, M.A.; Alamry, K.A.; El-Shishtawy, R.M.; Elshehy, E.A.; El-Said, W.A. Nanoporous colorant sensors and captors for simultaneous recognition and recovery of gold from E-wastes. Waste Manag. 2020, 116, 166–178. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Hussein, M.A.; Abdel Rahman, M.A.; Marwani, H.M. Highly selective heteroaromatic sulfur containing polyamides for Hg+2 environmental remediation. Des. Monomers Polym. 2019, 23, 23–39. [Google Scholar] [CrossRef]
- Khan, A.; Hussein, M.A.; Alshehri, F.M.; Alamry, K.A.; Asiri, A.M. A new class of polyethylene glycol-grafted graphene carbon nanotube composite as a selective adsorbent for Au(III). Waste Biomass Valori. 2021, 12, 937–946. [Google Scholar] [CrossRef]
- Al-Ghamdi, Y.O.; Alamry, K.A.; Hussein, M.A.; Marwani, H.M.; Asiri, A.M. Sulfone-modified chitosan as selective adsorbent for the extraction of toxic Hg(II) metal ions. Adsor. Sci. Technol. 2019, 37, 139–159. [Google Scholar] [CrossRef] [Green Version]
- Hussein, M.A.; Marwany, H.; Alamry, K.; Asiri, A.M.; El-Daly, S.A. Surface selectivity competition of newly synthesized polyarylidene(keto-amine)s polymers toward different metal ions. J. Appl. Polym. Sci. 2014, 131, 40873. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, X.; Zhai, Y.; He, Q.; Huang, X.; Hu, Z.; Jiang, N. Selective solid phase extraction of trace Sc (III) from environmental samples using silica gel modified with 4-(2-morinyldiazenyl)-N-(3-(trimethylsilyl) propyl) benzamide. Anal. Chim. Acta 2008, 629, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Proc. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Mckay, G. The adsorption of basic dye onto silica from aqueous solution-solid diffusion model. Chem. Eng. Sci. 1984, 39, 129–138. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef] [Green Version]
- Mckay, G.; Blair, H.; Gardner, J. Adsorption of dyes on chitin. I. Equilibrium studies. J. Appl. Polym. Sci. 1982, 27, 3043–3057. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Chen, C.; Ren, X.; Hu, J.; Wang, X. Removal of cobalt from aqueous solution by magnetic multiwalled carbon nanotube/iron oxide composites. Chem. Eng. J. 2011, 174, 126–133. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Hu, J.; Wang, J.L. Removal of Co2+ from radioactive wastewater by polyvinyl alcohol (PVA)/chitosan magnetic composite. Prog. Nucl. Energy 2014, 71, 172–178. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhuang, S.; Wang, J. Biosorptive removal of cobalt(II) from aqueous solutions using magnetic cyanoethyl chitosan beads. J. Environ. Chem. Eng. 2020, 8, 104531. [Google Scholar] [CrossRef]
- Chen, Y.W.; Wang, J.L. The characteristics and mechanism of Co(II) removal from aqueous solution by a novel xanthate-modified magnetic chitosan. Nucl. Eng. Des. 2012, 242, 452–457. [Google Scholar] [CrossRef]
- da Fonseca, M.G.; de Oliveira, M.M.; Arakaki, L.N.H.; Espinola, J.G.P.; Airoldi, C. Natural vermiculite as an exchanger support for heavy cations in aqueous solution. J. Colloid Interface Sci. 2005, 285, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lu, J.; Zhang, L. Magnesium hydroxide with higher adsorption capacity for effective removal of Co(II) from aqueous solutions. J. Taiwan Inst. Chem. E 2013, 44, 630–636. [Google Scholar] [CrossRef]



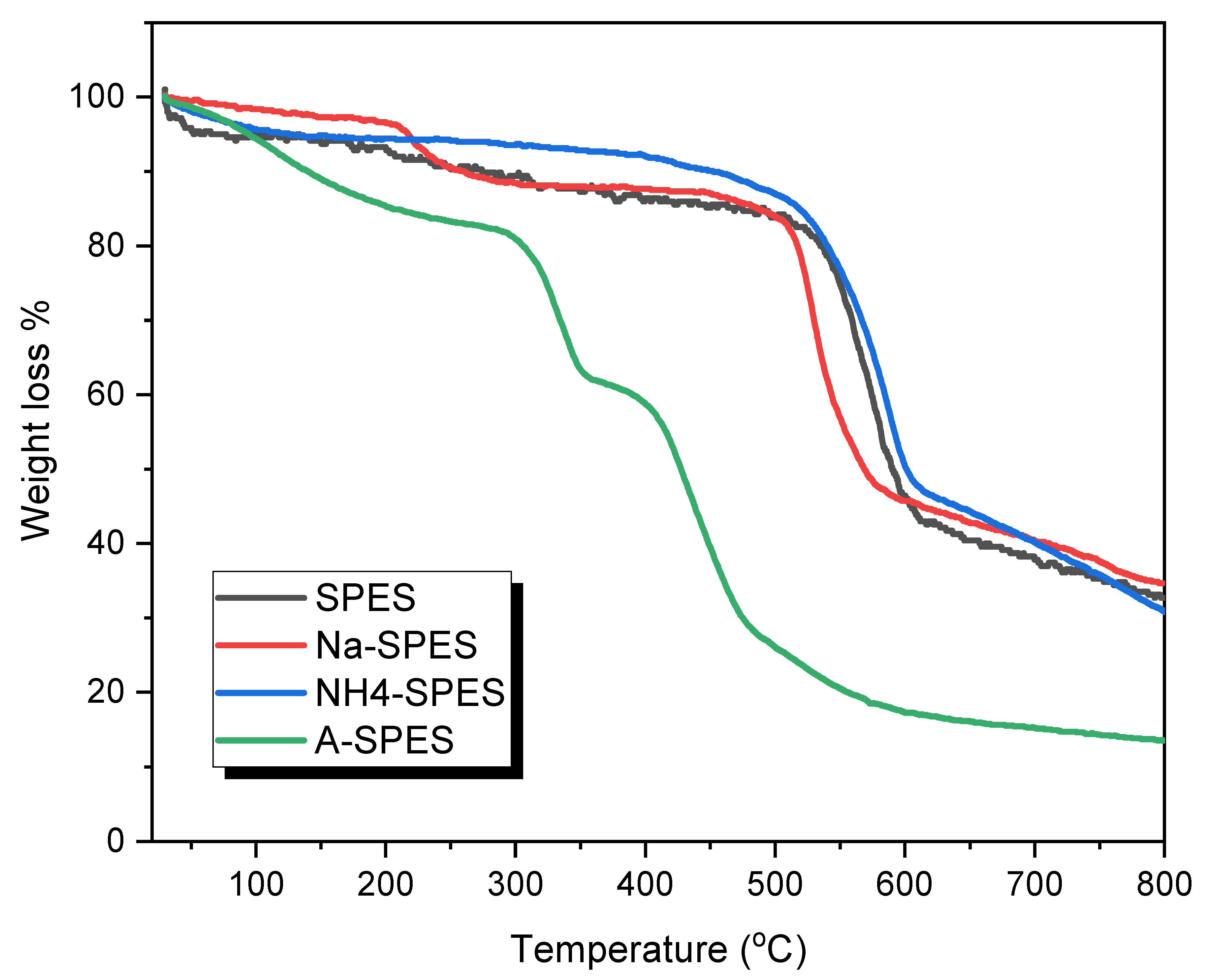
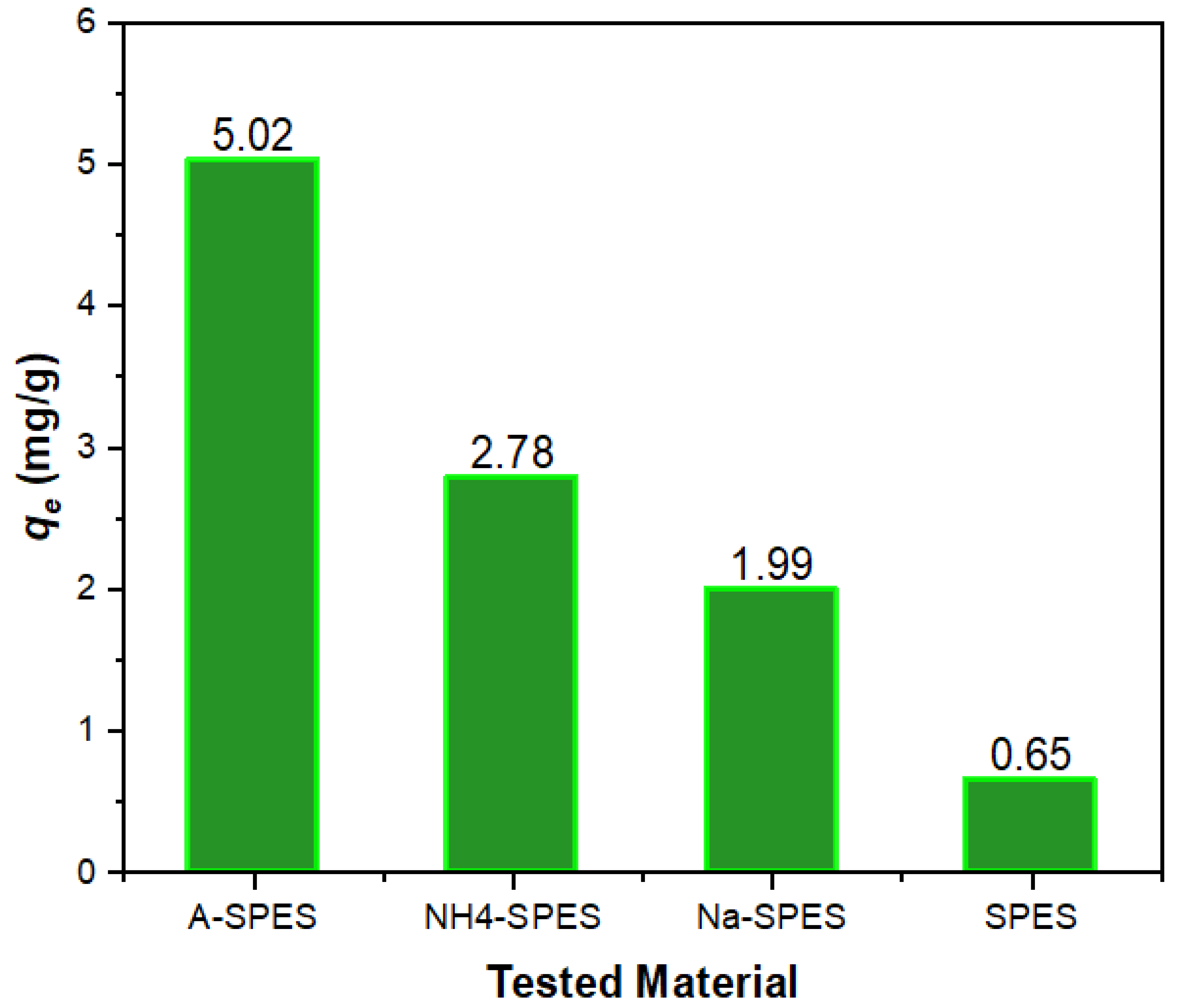
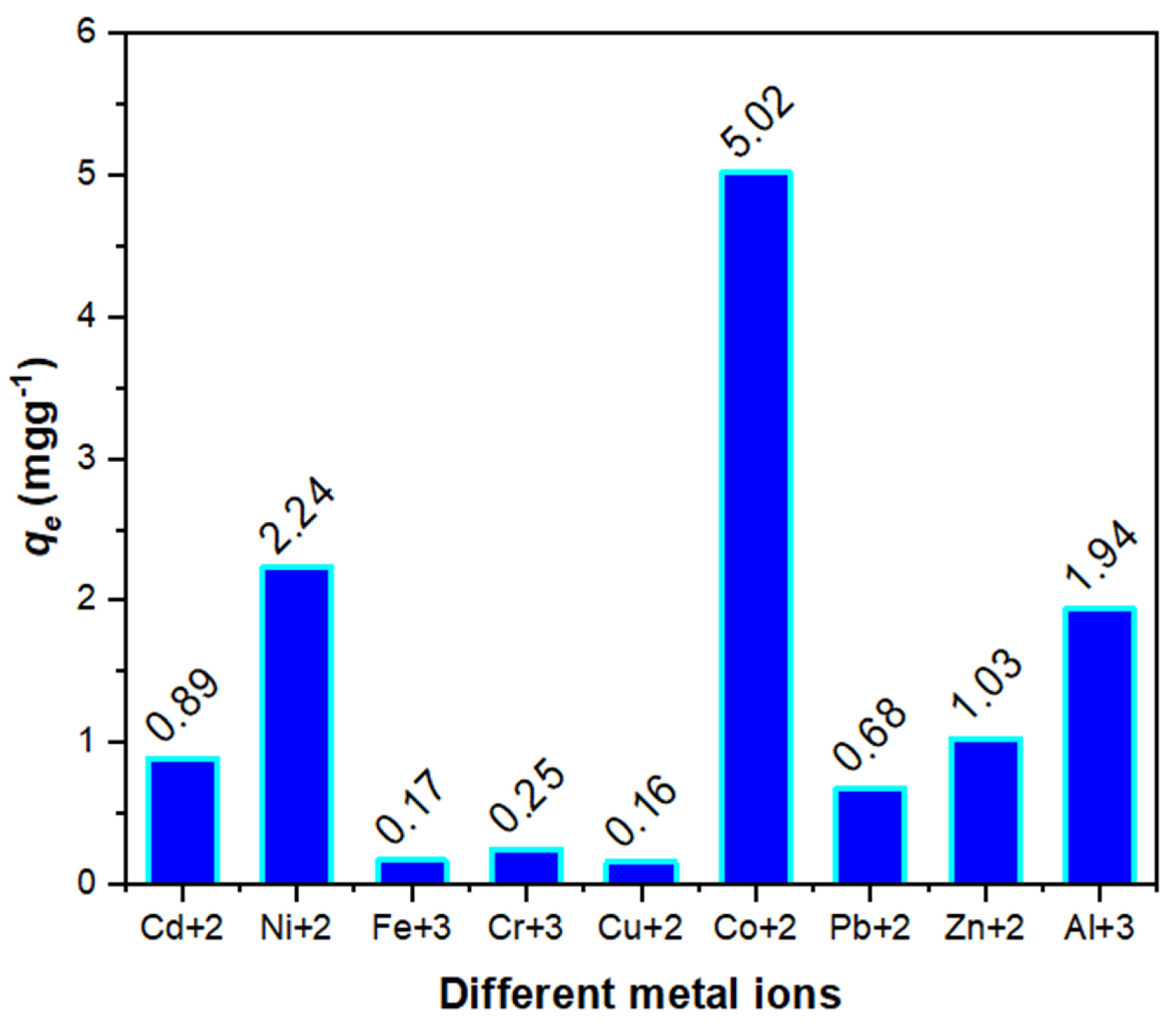


| Material | Temperature (°C) for Various % Decompositions * | ||||
|---|---|---|---|---|---|
| 10% | 20% | 30% | 40% | 50% | |
| SPES | 258 | 535 | 560 | 542 | 592 |
| Na-SPES | 256 | 517 | 529 | 575 | 569 |
| NH4-SPES | 445 | 540 | 566 | 584 | 601 |
| A-SPES | 136 | 304 | 335 | 390 | 426 |
| Metalions | qe (mg/g) | Kd (mL/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| A-SPES | NH4-SPES | Na-SPES | SPES | A-SPES | NH4-SPES | Na-SPES | SPES | |
| Cd+2 | 0.89 | - | - | - | 368.31 | - | - | - |
| Ni+2 | 2.24 | - | - | - | 447.68 | - | - | - |
| Fe+3 | 0.17 | - | - | - | 52.82 | - | - | - |
| Cr+3 | 0.25 | - | - | - | 128.35 | - | - | - |
| Cu+2 | 0.16 | - | - | - | 51.74 | - | - | - |
| Co+2 | 5.02 | 2.78 | 1.99 | 0.65 | 51,489.60 | 566.09 | 218.43 | 268.87 |
| Pb+2 | 0.68 | - | - | - | 303.09 | - | - | - |
| Zn+2 | 1.03 | - | - | - | 155.36 | - | - | - |
| Al+3 | 1.94 | - | - | - | 202.45 | - | - | - |
| Adsorbents | Adsorption Capacity (mg/g) | References |
|---|---|---|
| Magnetic multiwalled carbon nanotube/iron oxide composites | 10.61 | [43] |
| Polyvinyl alcohol/chitosan magnetic composite | 14.39 | [44] |
| Magnetic cyanoethyl chitosan beads | 17.92 | [45] |
| Xanthate-modified magnetic chitosan | 18.50 | [46] |
| Natural vermiculite | 49.50 | [47] |
| Magnesium hydroxide powders | 125.0 | [48] |
| A-SPES | 89.46 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashour, G.R.; Hussein, M.A.; Sobahi, T.R.; Alamry, K.A.; Alqarni, S.A.; Rafatullah, M. Modification of Sulfonated Polyethersulfone Membrane as a Selective Adsorbent for Co(II) Ions. Polymers 2021, 13, 3569. https://doi.org/10.3390/polym13203569
Ashour GR, Hussein MA, Sobahi TR, Alamry KA, Alqarni SA, Rafatullah M. Modification of Sulfonated Polyethersulfone Membrane as a Selective Adsorbent for Co(II) Ions. Polymers. 2021; 13(20):3569. https://doi.org/10.3390/polym13203569
Chicago/Turabian StyleAshour, Gadeer R., Mahmoud A. Hussein, Tariq R. Sobahi, Khalid A. Alamry, Sara A. Alqarni, and Mohd Rafatullah. 2021. "Modification of Sulfonated Polyethersulfone Membrane as a Selective Adsorbent for Co(II) Ions" Polymers 13, no. 20: 3569. https://doi.org/10.3390/polym13203569