Organosilica-Modified Multiblock Copolymers for Membrane Gas Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
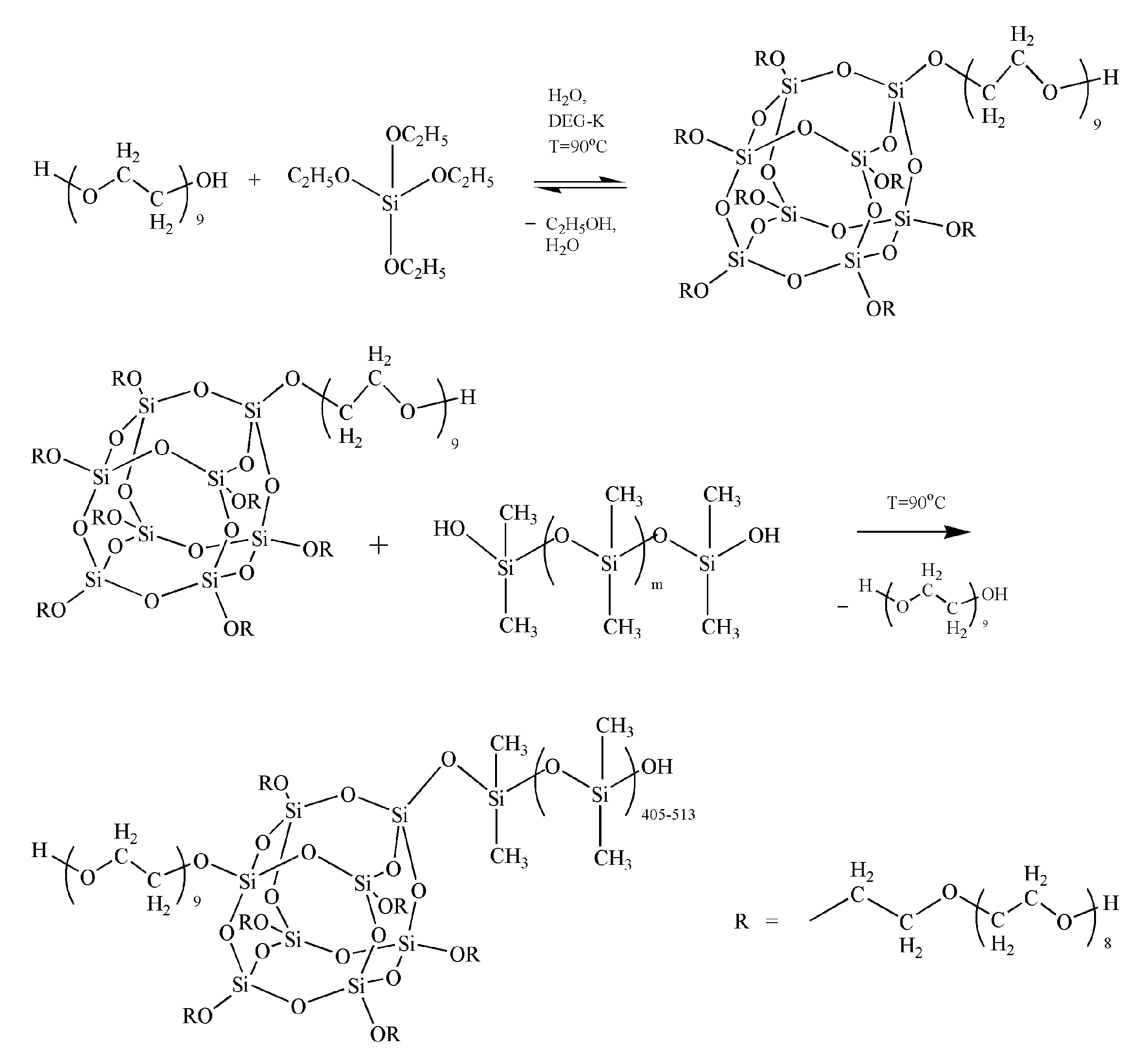
2.2. Synthesis of the ASiP–AGM/the ASiP
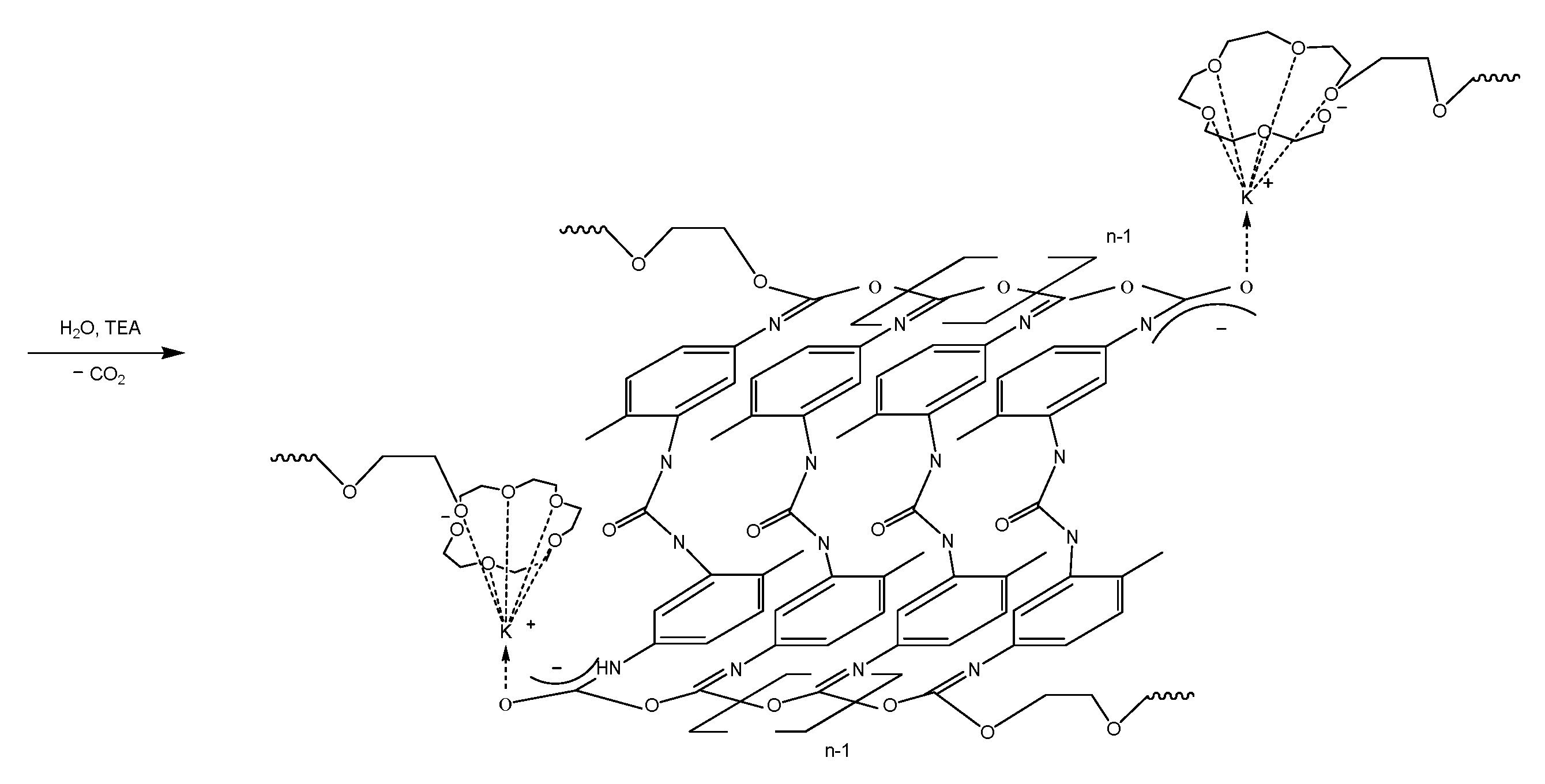
2.3. Synthesis of Modified by the ASiP–AGM/ASiP Multiblock Copolymers Based on PPEG and TDI
2.4. Methods
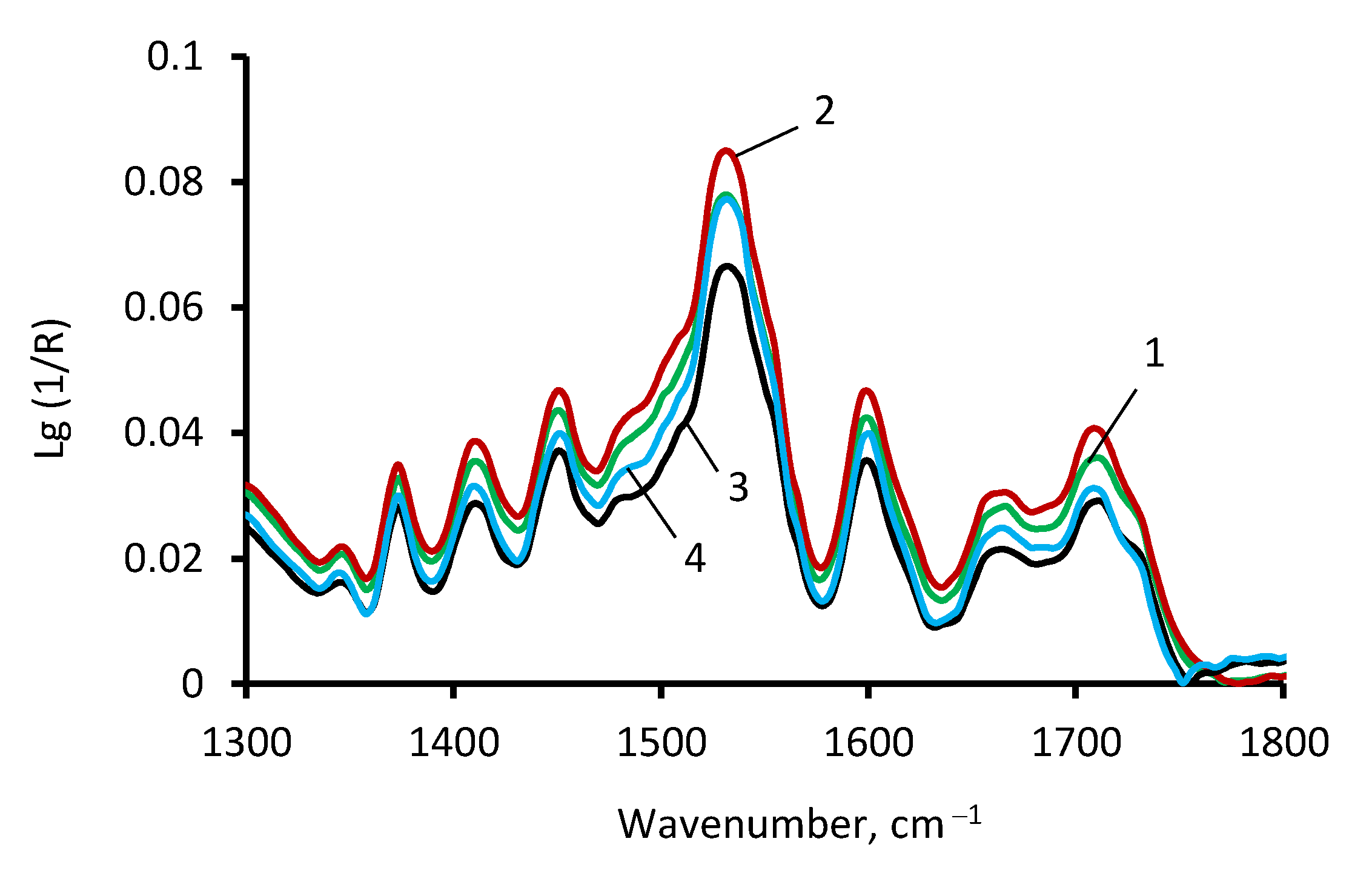
2.4.1. Fourier Transform Infrared Spectroscopy Analysis (FTIR)
2.4.2. Thermal Gravimetric Analysis (TGA)
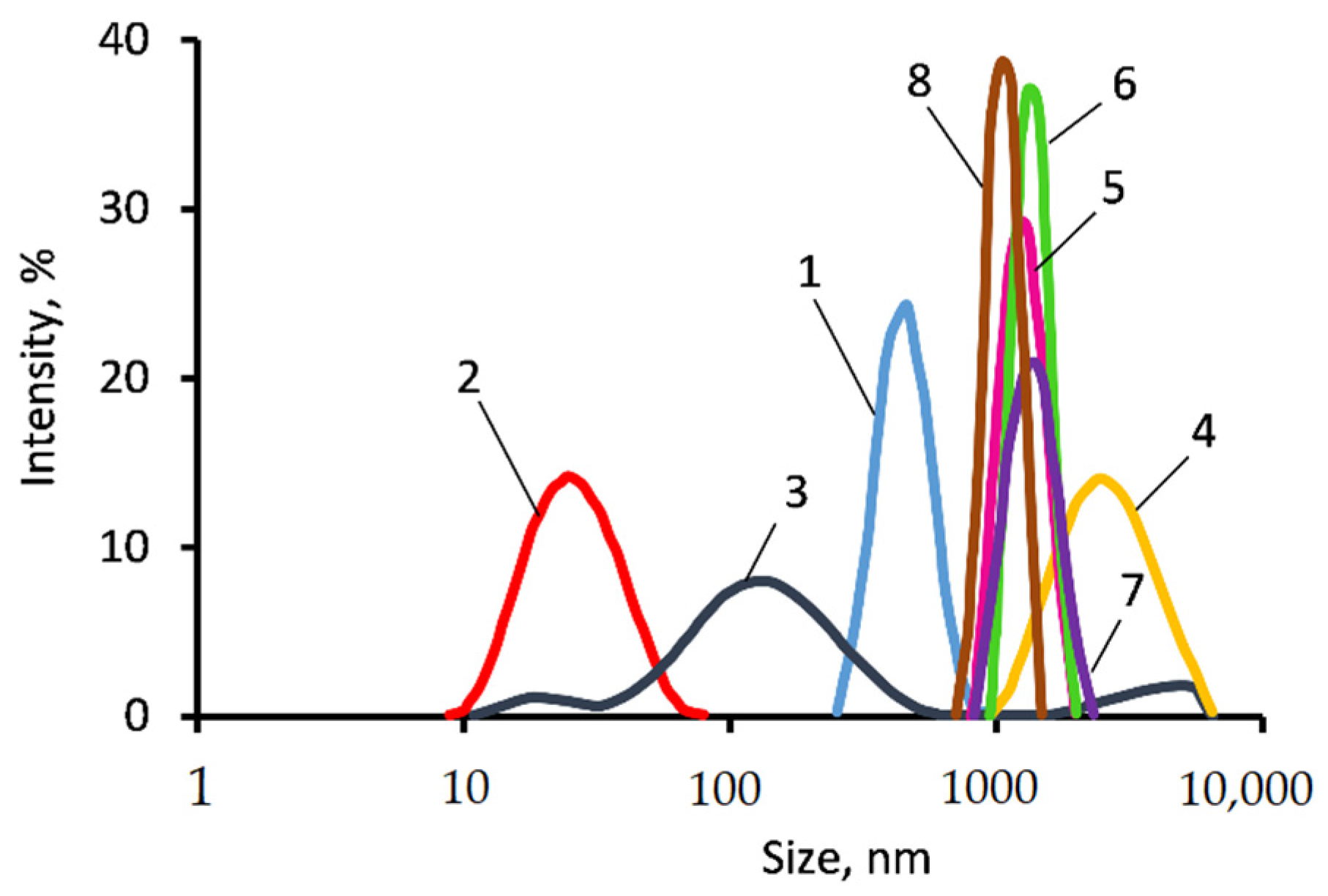
2.4.3. Light-Scattering (DLS)
2.4.4. Tensile Stress–Strain Measurements
2.4.5. Electron Spectroscopy
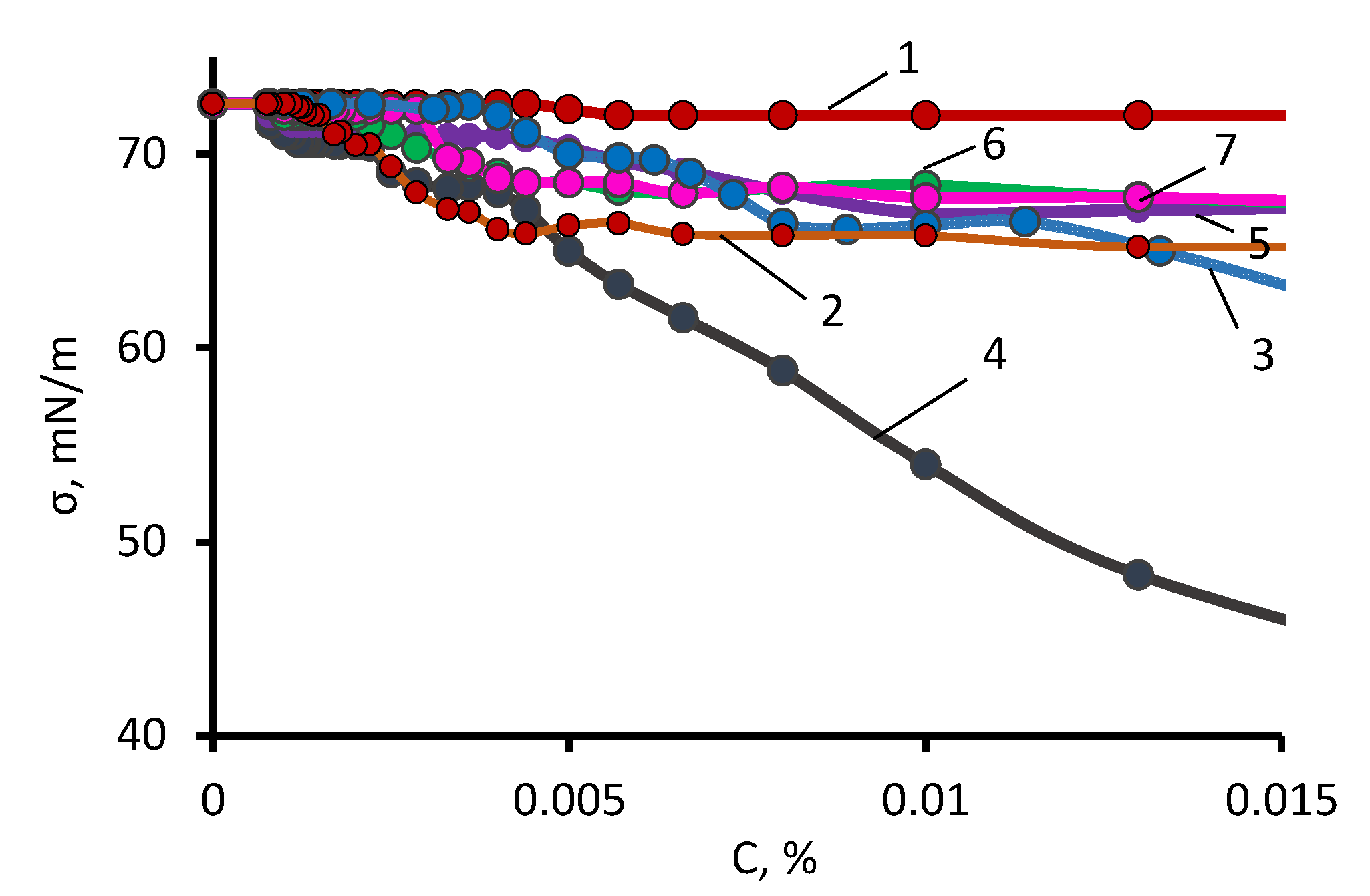
2.4.6. Measurements of the Surface Tension
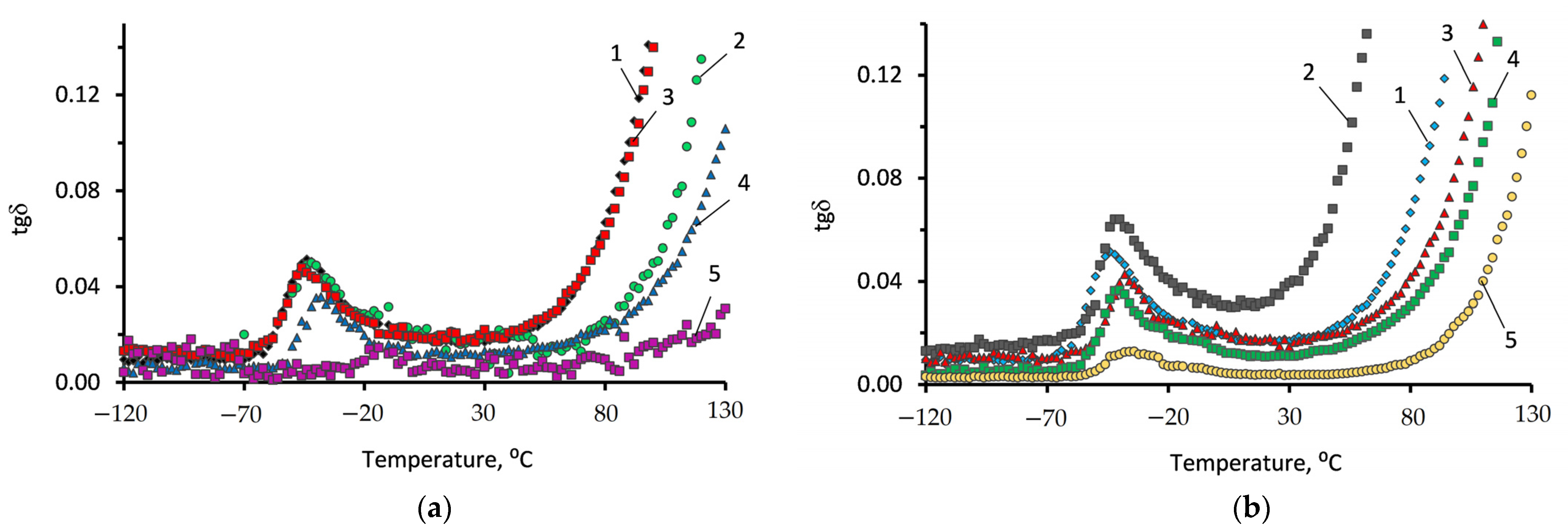
2.4.7. Measurement of Temperature Dependence of Dielectric Loss Tangent (DLT)
2.4.8. Permeability Measurements
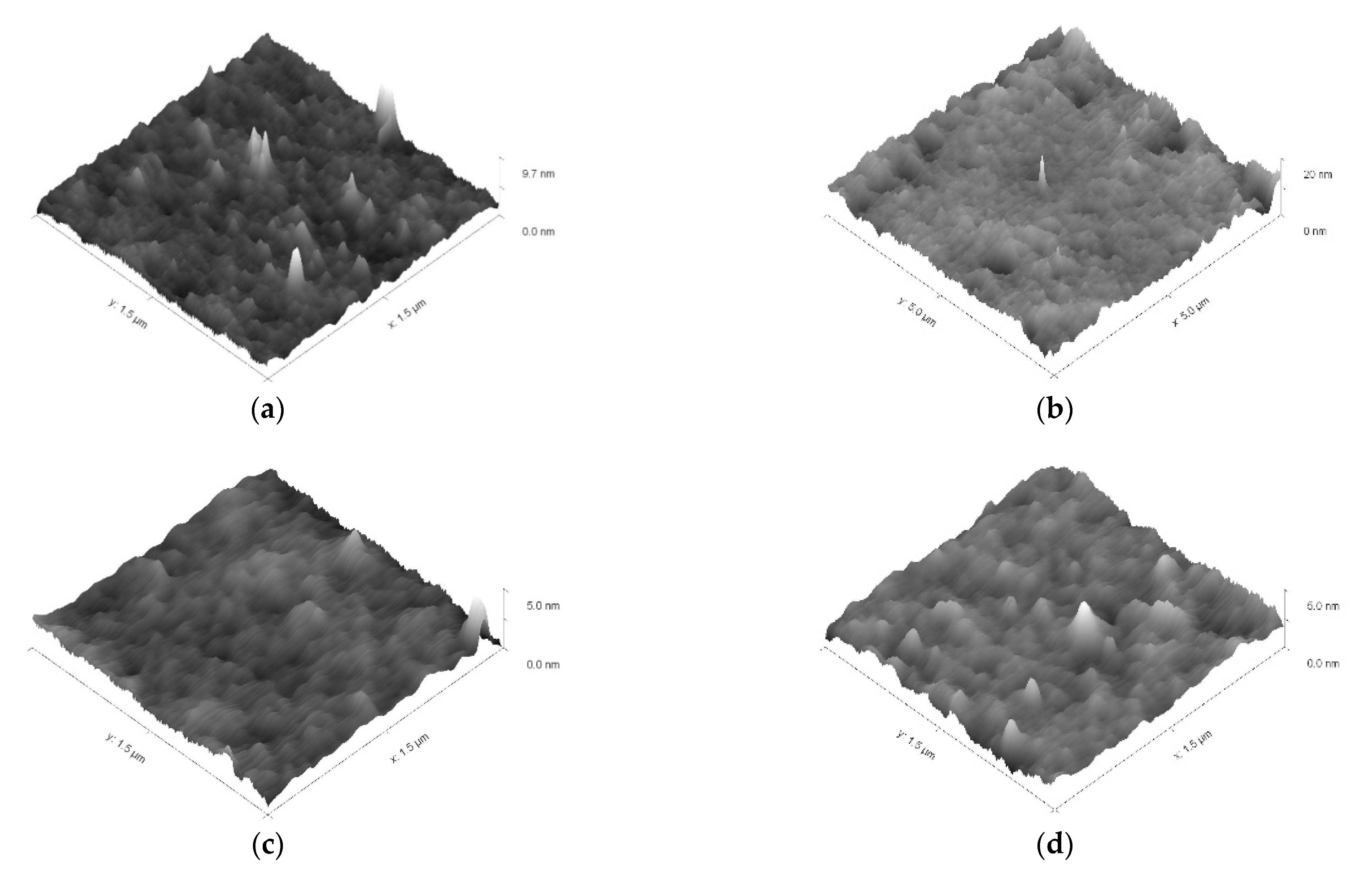
2.4.9. AFM Studies
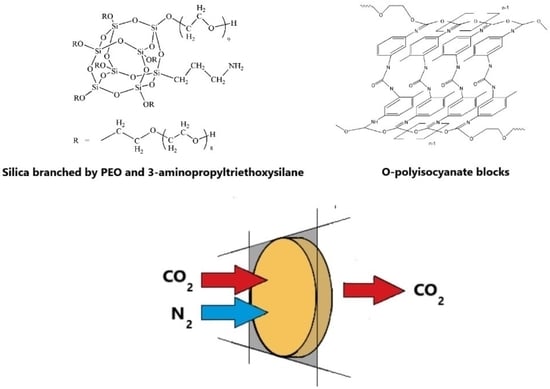
3. Results and Discussion
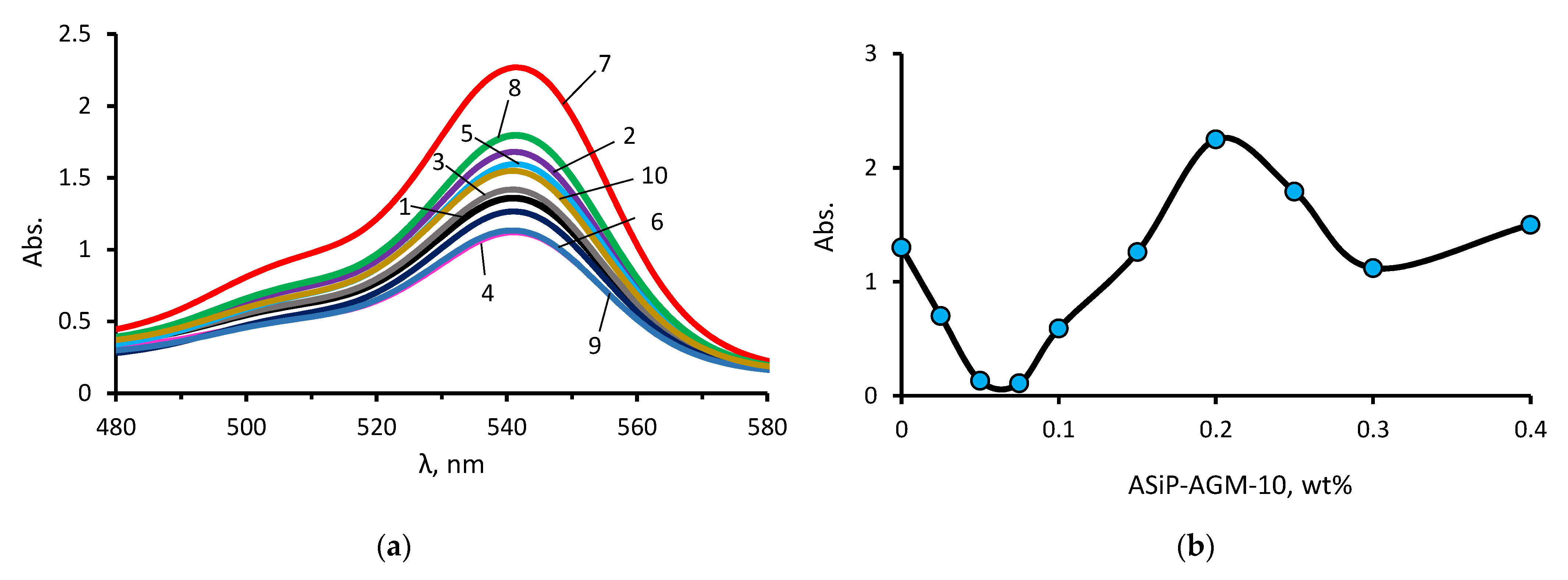
3.1. The ASiP–AGM Characterization
3.2. Investigation of MBC Modified by the ASiP–AGM
3.3. Mechanical Behavior of Modified Polymers
3.4. Investigation of the Sorption Activity of Modified Polymers
3.5. Gas Transport Properties of MBC Modified with the ASiP–AGM-10 and the ASiP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPEG | block copolymer of propylene and ethylene oxide |
| TDI | 2,4-tolylene diisocyanate |
| ASiP | silica branched by polyoxyethylene oxide and PDMS |
| ASiP–AGM-(10 ÷ 100) | silica branched by polyoxyethylene oxide and AGM-(10 ÷ 100 wt.% of TEOS total amount) |
| PEOASiP | silica branched by polyoxyethylene oxide |
| PEO | polyoxyethylene oxide |
| TEOS | tetraethoxysilane |
| DEG-K | potassium diethylene glycolate |
| AGM | 3-aminopropyltriethoxysilane |
| PDMS | polydimethylsiloxane (MW ≈ 30,000 g/mol) |
| TMA | thermomechanical analysis |
| TGA | thermogravimetric analysis |
| DLT | dielectric loss tangent |
| FTIR | Fourier transform infrared spectroscopy analysis |
| CMC | critical micelle concentration |
References
- Aritra, S.; Sasmal, R.; Das, A.; Venugopal, A.; Agasti, S.S.; George, S.J. Tricomponent Supramolecular Multiblock Copolymers with Tunable Composition via Sequential Seeded Growth. Angew. Chem. Int. Ed. 2021, 60, 18209–18216. [Google Scholar]
- Hong, W.; Lin, J.; Tian, X.; Wang, L. Distinct Viscoelasticity of Hierar-chical Nanostructures Self-Assembled from Multiblock Copolymers. Macromolecules 2020, 53, 10955–10963. [Google Scholar] [CrossRef]
- Rikiyama, K.; Horiuchi, T.; Koga, N.; Sanada, Y.; Watanabe, K.; Aida, M.; Katsumoto, Y. Micellization of poly(ethylene oxide)-poly(propylene oxide) alternating multiblock copolymers in water. Polymer 2018, 156, 102–110. [Google Scholar] [CrossRef]
- Wieczorek, J.; Ulbricht, M. Amphiphilic poly(arylene ether sulfone) multiblock copolymers with quaternary ammonium groups for novel thin-film composite nanofiltration membranes. Polymer 2021, 217, 123446. [Google Scholar] [CrossRef]
- Vilesov, A.D.; Floudas, G.; Pakula, T.; Melenevskaya, E.Y.; Birshtein, T.M.; Lyatskaya, Y.V. Lamellar structure formation in the mixture of two cylinder-forming block copolymers. Macromol. Chem. Phys. 1994, 195, 2317–2326. [Google Scholar] [CrossRef]
- Xiao, L.; Li, J.; Li, W.; Li, W.; Huang, G. The synthesis of multiblock copolymer brush based on DSPAAC and CuAAC click reaction. J. Polym. Sci. 2021, 59, 100–107. [Google Scholar] [CrossRef]
- Entezami, A.A.; Abbasian, M. Recent advances in synthesis of new polymers by living free radical polymerization. Iran. Polym. J. 2006, 15, 583–611. [Google Scholar]
- Riess, G.; Bahadur, P.; Hurtrez, G. Encyclopedia of Polymer Science and Engineering, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1985; Volume 2, pp. 324–434. [Google Scholar]
- Brittain, W. A Review of Group-Transfer Polymerization. Rubber Chem. Technol. 1992, 65, 580–600. [Google Scholar] [CrossRef]
- Qiang, X.; Chakroun, R.; Janoszka, N.; Gröschel, A.H. Self-assembly of Multiblock Copolymers. Isr. J. Chem. 2019, 59, 945–958. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Dzhabbarov, I.M.; Gumerov, A.M.; Zaripov, I.I.; Davletbaev, R.S.; Atlaskin, A.A.; Sazanova, T.S.; Vorotyntsev, I.V. Amphiphilic Poly(dimethylsiloxane-ethylene-propylene oxide)-polyisocyanurate Cross-Linked Block Copolymers in a Membrane Gas Separation. Membranes 2021, 11, 94. [Google Scholar] [CrossRef]
- Zaripov, I.I.; Davletbaeva, I.M.; Faizulina, Z.Z.; Davletbaev, R.S.; Gubaidullin, A.T.; Atlaskin, A.A.; Vorotyntsev, I.V. Synthesis and Characterization of Novel Nanoporous Gl-POSS-Branched Polymeric Gas Separation Membranes. Membranes 2020, 10, 110. [Google Scholar] [CrossRef]
- Baker, R.W. Membranes for Vapor/Gas Separation; Membrane Technology and Research, Inc.: Menlo Park, CA, USA, 2006. [Google Scholar]
- Alentiev, A.Y.; Yampolskii, Y.; Shantarovich, V.P.; Nemser, S.M.; Plate, N.A. High transport parameters and free volume of perfluorodioxole copolymers. J. Membr. Sci. 1997, 126, 123–132. [Google Scholar] [CrossRef]
- Patel, N.P.; Spontak, R.J. Mesoblends of polyether block copolymers with Poly(ethylene glycol). Macromolecules 2004, 37, 1394–1402. [Google Scholar] [CrossRef]
- Lee, H.S.; Roy, A.; Lane, O.; Dunn, S.; McGrath, J.E. Hydrophilic–hydrophobic multiblock copolymers based on poly(arylene ether sulfone) via low–temperature coupling reactions for proton exchange membrane fuel cells. Polymer 2008, 49, 715–723. [Google Scholar] [CrossRef]
- Scholes, C.A.; Chen, G.Q.; Lu, H.T.; Kentish, S.E. Crosslinked PEG and Pebax Membranes for Concurrent Permeation of Water and Carbon Dioxide. Membranes 2015, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pegoraro, M.; Zanderighi, L.; Penati, A.; Severini, F.; Bianchi, F.; Cao, N.; Sisto, R.; Valentini, C. Polyurethane membranes from polyether and polyester diols for gas fractionation. J. Appl. Polym. Sci. 1991, 43, 687–697. [Google Scholar] [CrossRef]
- Hassanajili, S.; Masoudi, E.; Karimi, G.; Khademi, M. Mixed matrix membranes based on polyetherurethane and polyesterurethane containing silica nanoparticles for separation of CO2/CH4 gases. Sep. Purif. Technol. 2013, 116, 1–12. [Google Scholar] [CrossRef]
- Khosravi, A.; Sadeghi, M. Separation performance of poly(urethane–urea) membranes in the separation of C2 and C3 hydrocarbons from methane. J. Membr. Sci. 2013, 434, 171–183. [Google Scholar] [CrossRef]
- Gnanasekaran, D.; Reddy, B.S. A facile synthesis of mixed soft-segmented poly(urethane-imide)-polyhedral oligomeric silsesquioxone hybrid nanocomposites and study of their structure-transport properties. Polym. Int. 2013, 63, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Chari, P.; Tseng, J.-K.; Zhang, G.; Cox, M.; Maia, J.M. Microconfinement effect on gas barrier and mechanical properties of multilayer rigid/soft thermoplastic polyurethane films. J. Appl. Polym. Sci. 2015, 132, 41849. [Google Scholar] [CrossRef]
- Wang, Y.; Gupta, M.; Schiraldi, D. Oxygen permeability in thermoplastic polyurethanes. J. Polym. Sci. B Polym. Phys. 2012, 50, 681–693. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, B.; Yang, Y.; Hu, C. Correlations between gas permeation and free-volume hole properties of polyurethane membranes. Eur. Polym. J. 2003, 39, 2345–2349. [Google Scholar] [CrossRef]
- Semsarzadeh, M.A.; Ghahramani, M. The effect of Poly(alkyl (meth)acrylate) segments on the thermodynamic properties, morphology and gas permeation properties of Poly(alkyl (meth)acrylate)-b-poly(dimethyl siloxane) triblock copolymer membranes. J. Membr. Sci. 2019, 594, 117400. [Google Scholar] [CrossRef]
- Li, H.; Freeman, B.D.; Ekiner, O.M. Gas permeation propertiesof poly(urethane–urea)s containing different polyethers. J. Membr. Sci. 2011, 369, 49–58. [Google Scholar] [CrossRef]
- Gisselfält, K.; Helgee, B. Effect of soft segment length and chain extender structure on phase separation and morphology in poly(urethaneurea)s. Macromol. Mater. Eng. 2003, 288, 265–271. [Google Scholar] [CrossRef]
- Mishra, A.; Maiti, P. Morphology of polyurethanes at various length scale: The influence of chain structure. J. Appl. Polym. Sci. 2011, 120, 3546–3555. [Google Scholar] [CrossRef]
- Gomes, D.; Peinemann, K.-V.; Nunes, S.P.; Kujawski, W.; Kozakiewicz, J. Gas transport properties of segmented poly(ether)siloxane urethane urea) membranes. J. Membr. Sci. 2006, 281, 747–753. [Google Scholar] [CrossRef]
- Mozaffari, V.; Sadeghi, M.; Fakhar, A.; Khanbabaei, G.; Ismail, A.F. Gas separation properties of polyurethane/poly(ether-block-amide) (PU/PEBA) blend membranes. Sep. Purif. Technol. 2017, 185, 202–214. [Google Scholar] [CrossRef]
- Stratigaki, M.; Choudalakis, G.; Gotsis, A.D. Gas transport properties in waterborne polymer nanocomposite coatings containing organomodified clays. J. Coatings Technol. Res. 2014, 11, 899–911. [Google Scholar] [CrossRef]
- Talakesh, M.; Sadeghi, M.; Chenar, M.; Khosravi, A. Gas separation properties of poly(ethyleneglycol)/poly(tetramethyleneglycol) based polyurethane membranes. J. Membr. Sci. 2012, 415, 469–477. [Google Scholar] [CrossRef]
- Ganß, M.; Staudinger, U.; Satapathy, B.; Leuteritz, A.; Weidisch, R. Mechanism of strengthening and toughening of a nanostructured styrene-butadiene based block copolymer by oligostyrene-modified montmorillonites. Polymer 2021, 213, 123328. [Google Scholar] [CrossRef]
- Li, L.; Ge, W.; Zhao, B.; Adeel, M.; Mei, H.; Zheng, S. Polyhydroxyurethane thermosets from novolac epoxide: Synthesis and its nanostructured blends with poly(trifluoroethylacrylate)-block-poly(N-vinylpyrrolidone) diblock copolymer. Polymer 2021, 213, 123314. [Google Scholar] [CrossRef]
- Yang, A.; Zou, L.; Zha, S.; Zhou, P.; Wu, P. Nano-cavitation structure toughness mechanism and optical properties of amphiphilic acrylate block copolymer modified epoxy system. J. Polym. Res. 2021, 28, 55. [Google Scholar] [CrossRef]
- Zhang, D.; Seong, J.G.; Lee, W.H.; Ando, S.; Wan, Y.; Lee, Y.M.; Zhuang, Y. Effects of sulfonate incorporation and structural isomerism on physical and gas transport properties of soluble sulfonated polyimides. Polymer 2020, 191, 122263. [Google Scholar] [CrossRef]
- Smaihi, M.; Schrotter, J.-C.; Lesimple, C.; Prevost, I.; Guizard, C. Gas separation properties of hybrid imide–siloxane copolymers with various silica contents. J. Membr. Sci. 1999, 161, 157–170. [Google Scholar] [CrossRef]
- Hu, C.-C.; Cheng, P.-H.; Chou, S.-C.; Lai, C.-L.; Huang, S.-H.; Tsai, H.-A.; Lee, K.-R. Separation behavior of amorphous amino-modified silica nanoparticle/polyimide mixed matrix membranes for gas separation. J. Membr. Sci. 2019, 595, 117542. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.M. Gas permeation properties of poly(amide-6-b-ethylene oxide)–silica hybrid membranes. J. Membr. Sci. 2001, 193, 209–225. [Google Scholar] [CrossRef]
- Sadeghi, M.; Khanbabaei, G.; Dehaghani, A.H.S.; Sadeghi, M.; Aravand, M.A.; Akbarzade, M.; Khattia, S. Gas permeation properties of ethylene vinyl acetate–silica nanocomposite membranes. J. Membr. Sci. 2008, 322, 423–428. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.J.; Pinnau, I.; Guiver, M.D. Polysulfone/silica nanoparticle mixed-matrix membranes for gas separation. J. Membr. Sci. 2008, 314, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Janke, A.; Formanek, P.; Fery, A.; Stamm, M.; Tripathi, B.P. High permeation and antifouling polysulfone ultrafiltration membranes with in situ synthesized silica nanoparticles. Mater. Today Commun. 2019, 22, 100784. [Google Scholar] [CrossRef]
- Davletbaev, R.S.; Zaripov, I.I.; Faizulina, Z.Z.; Davletbaeva, I.M.; Domrachova, D.S.; Gumerov, A.M. Synthesis and characterization of amphiphilic branched silica derivatives associated with oligomeric medium. RSC Adv. 2019, 9, 21233–21242. [Google Scholar] [CrossRef] [Green Version]
- Davletbaeva, I.M.; Mazil’nikov, A.I.; Zaripov, I.I.; Davletbaev, R.S.; Gumerov, A.M.; Parfenov, V.V. Synthesis of Block Copolymers Based on a Macroinitiator and 2,4-Toluene Diisocyanate. Polym. Sci. Ser. B 2018, 60, 51–57. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Finkelshtein, E. Membrane Materials for Gas and Vapor Separation: Synthesis and Application of Silicon-Containing Polymers; Wiley: Chichester, UK, 2017. [Google Scholar]
- Changa, S.; Matsumoto, T.; Matsumoto, H.; Unno, M. Synthesis and characterization of heptacyclic laddersiloxanes and ladder polysilsesquioxane. Appl. Organometal. Chem. 2010, 24, 241–246. [Google Scholar] [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, T. Ladder siloxanes—Silsesquioxanes with defined ladder structure. Russ. Chem. Rev. 2013, 82, 289–302. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons Ltd.: Chichester, England, 2004. [Google Scholar]
- Alentiev, A.Y.; Belov, N.A.; Chirkov, S.V.; Yampolskii, Y.P. Gas diffusion characteristics as criteria of nonequilibrium state of amorphous glassy polymers. J. Membr. Sci. 2018, 547, 99–109. [Google Scholar] [CrossRef]






| Modifier | ASiP–AGM-10 | ASiP | ||||||
|---|---|---|---|---|---|---|---|---|
| C, % | 0.0 | 0.05 | 0.10 | 0.20 | 0.30 | 0.10 | 0.20 | 0.30 |
| Gas | P, Barrer * | |||||||
| He | 13 ± 0.7 | 12 ± 0.6 | 12 ± 0.6 | 14 ± 0.7 | 12 ± 0.6 | 11 ± 0.6 | 11 ± 0.6 | 12 ± 0.6 |
| H2 | 17 ± 0.9 | 19 ± 1 | 19 ± 1 | 22 ± 1 | 18 ± 0.9 | 15 ± 0.8 | 16 ± 0.8 | 17 ± 0.9 |
| O2 | 9.0 ± 0.5 | 9.8 ± 0.5 | 8.4 ± 0.4 | 10 ± 0.5 | 8.3 ± 0.4 | 8.5 ± 0.4 | 7.8 ± 0.4 | 7.8 ± 0.4 |
| N2 | 3.2 ± 0.2 | 3.4 ± 0.2 | 2.2 ± 0.1 | 4.0 ± 0.2 | 3.2 ± 0.2 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.9 ± 0.1 |
| CO2 | 82 ± 4 | 85 ± 4 | 85 ± 4 | 110 ± 6 | 84 ± 4 | 86 ± 4 | 78 ± 4 | 86 ± 4 |
| CH4 | 9.9 ± 0.5 | 10 ± 0.5 | 10 ± 0.5 | 13 ± 0.7 | 10 ± 0.5 | 11 ± 0.6 | 9.0 ± 0.5 | 0.6 |
| Modifier | ASiP–AGM-10 | ASiP | ||||||
|---|---|---|---|---|---|---|---|---|
| C, % | 0.0 | 0.05 | 0.10 | 0.20 | 0.30 | 0.10 | 0.20 | 0.30 |
| Gas pairs | α = Pi/Pj | |||||||
| O2/N2 | 2.8 ± 0.3 | 2.9 ± 0.3 | 3.8 ± 0.4 | 2.5 ± 0.3 | 2.6 ± 0.3 | 3.1 ± 0.3 | 2.9 ± 0.3 | 2.7 ± 0.3 |
| CO2/N2 | 26 ± 3 | 25 ± 3 | 39 ± 4 | 28 ± 3 | 26 ± 3 | 32 ± 3 | 29 ± 3 | 30 ± 3 |
| He/N2 | 4.1 ± 0.4 | 3.5 ± 0.4 | 5.5 ± 0.6 | 3.5 ± 0.4 | 3.8 ± 0.4 | 4.1 ± 0.4 | 4.1 ± 0.4 | 4.1 ± 0.4 |
| H2/N2 | 5.3 ± 0.5 | 5.4 ± 0.5 | 8.6 ± 0.9 | 5.5 ± 0.6 | 5.6 ± 0.6 | 8.6 ± 0.9 | 5.5 ± 0.6 | 5.6 ± 0.6 |
| CO2/CH4 | 8.2 ± 0.8 | 8.5 ± 0.9 | 8.5 ± 0.9 | 8.5 ± 0.9 | 8.4 ± 0.8 | 7.8 ± 0.8 | 8.7 ± 0.9 | 7.8 ± 0.8 |
| He/CH4 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| H2/CH4 | 1.7 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.2 | 1.4 ± 0.1 | 1.8 ± 0.2 | 1.5 ± 0.2 |
| Modifier | ASiP–AGM-10 | ASiP | ||||||
|---|---|---|---|---|---|---|---|---|
| C, % | 0.0 | 0.05 | 0.10 | 0.20 | 0.30 | 0.10 | 0.20 | 0.30 |
| Gas | D·108, cm2/s | |||||||
| O2 | 94 ± 9 | 96 ± 10 | 85 ± 9 | 110 ± 10 | 85 ± 9 | 110 ± 10 | 90 ± 9 | 95 ± 10 |
| N2 | 67 ± 7 | 79 ± 8 | 66 ± 7 | 100 ± 10 | 74 ± 7 | 64 ± 6 | 57 ± 6 | 79 ± 8 |
| CO2 | 44 ± 4 | 45 ± 5 | 45 ± 5 | 66 ± 7 | 47 ± 5 | 48 ± 5 | 48 ± 5 | 47 ± 5 |
| CH4 | 39 ± 4 | 41 ± 4 | 41 ± 4 | 58 ± 4 | 42 ± 4 | 45 ± 5 | 45 ± 5 | 47 ± 5 |
| Modifier | ASiP–AGM-10 | ASiP | ||||||
|---|---|---|---|---|---|---|---|---|
| C, % | 0.0 | 0.05 | 0.10 | 0.20 | 0.30 | 0.10 | 0.20 | 0.30 |
| Gas | S·103, cm3(STP)/(cm3·cmHg) | |||||||
| O2 | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.1 | 1.0 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| N2 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| CO2 | 19 ± 3 | 19 ± 3 | 19 ± 3 | 17 ± 3 | 18 ± 3 | 18 ± 3 | 16 ± 2 | 18 ± 3 |
| CH4 | 2.5 ± 0.4 | 2.4 ± 0.4 | 2.4 ± 0.4 | 2.2 ± 0.3 | 2.4 ± 0.4 | 2.4 ± 0.4 | 2.0 ± 0.3 | 2.3 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davletbaeva, I.M.; Alentiev, A.Y.; Faizulina, Z.Z.; Zaripov, I.I.; Nikiforov, R.Y.; Parfenov, V.V.; Arkhipov, A.V. Organosilica-Modified Multiblock Copolymers for Membrane Gas Separation. Polymers 2021, 13, 3579. https://doi.org/10.3390/polym13203579
Davletbaeva IM, Alentiev AY, Faizulina ZZ, Zaripov II, Nikiforov RY, Parfenov VV, Arkhipov AV. Organosilica-Modified Multiblock Copolymers for Membrane Gas Separation. Polymers. 2021; 13(20):3579. https://doi.org/10.3390/polym13203579
Chicago/Turabian StyleDavletbaeva, Ilsiya M., Alexander Yu. Alentiev, Zulfiya Z. Faizulina, Ilnaz I. Zaripov, Roman Yu. Nikiforov, Victor V. Parfenov, and Alexander V. Arkhipov. 2021. "Organosilica-Modified Multiblock Copolymers for Membrane Gas Separation" Polymers 13, no. 20: 3579. https://doi.org/10.3390/polym13203579
APA StyleDavletbaeva, I. M., Alentiev, A. Y., Faizulina, Z. Z., Zaripov, I. I., Nikiforov, R. Y., Parfenov, V. V., & Arkhipov, A. V. (2021). Organosilica-Modified Multiblock Copolymers for Membrane Gas Separation. Polymers, 13(20), 3579. https://doi.org/10.3390/polym13203579