High Cyclability Energy Storage Device with Optimized Hydroxyethyl Cellulose-Dextran-Based Polymer Electrolytes: Structural, Electrical and Electrochemical Investigations
Abstract
:1. Introduction
2. Experimental
2.1. Electrolyte Preparation
2.2. Electrolyte Characterization
2.3. EDLC Preparation
3. Result and Discussion
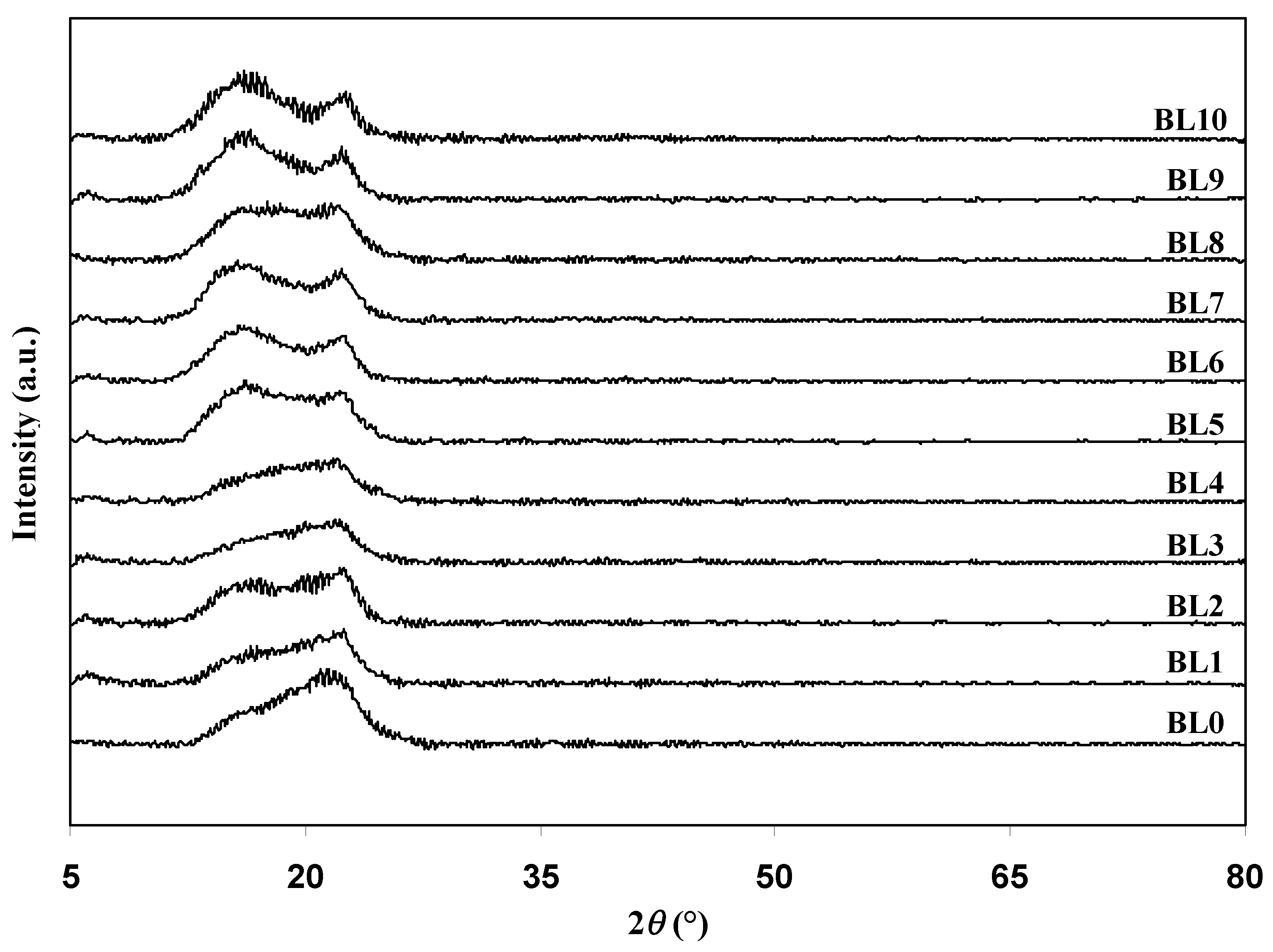
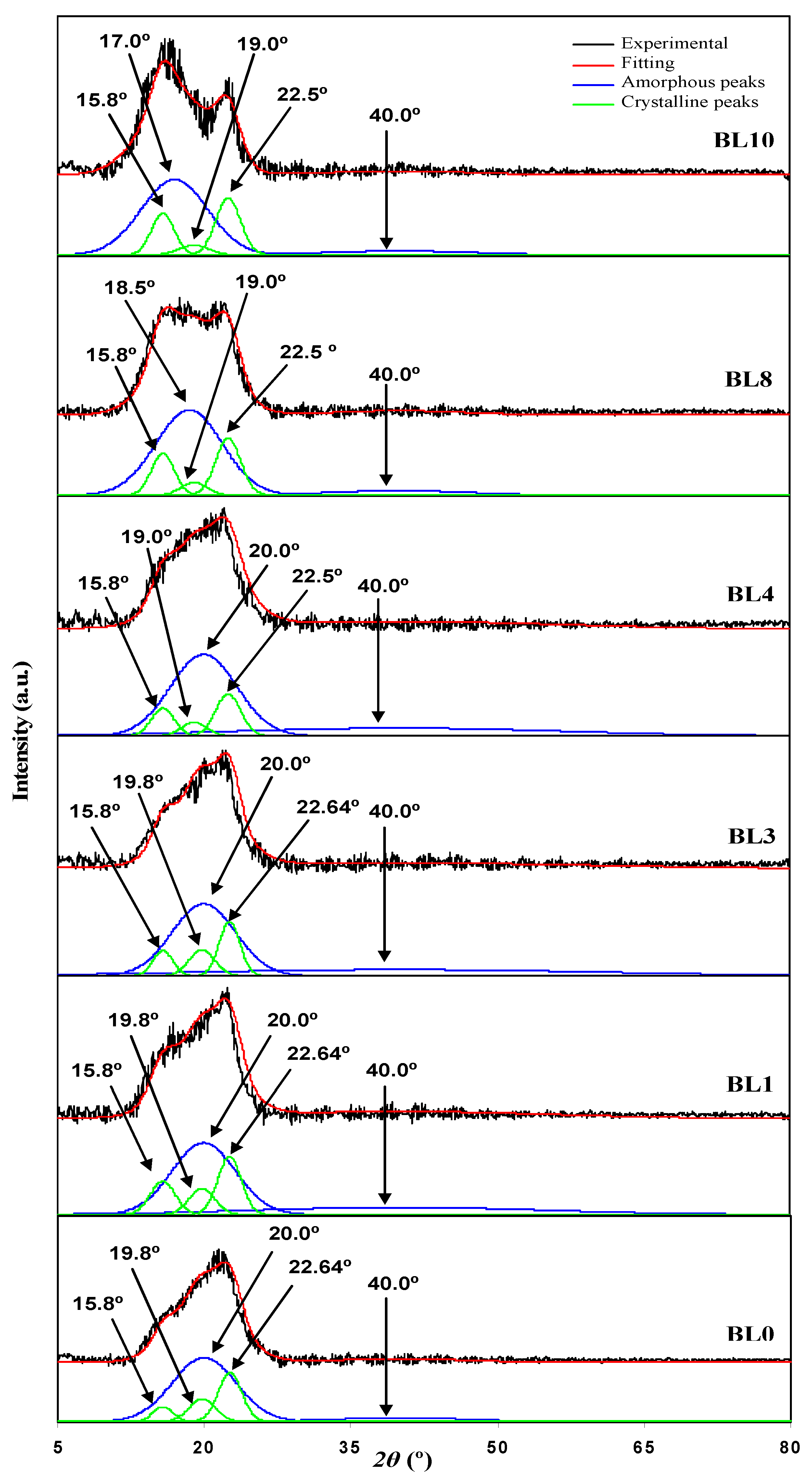
3.1. Ideal Composition for the Polymer Blend Host of the Electrodes Separator
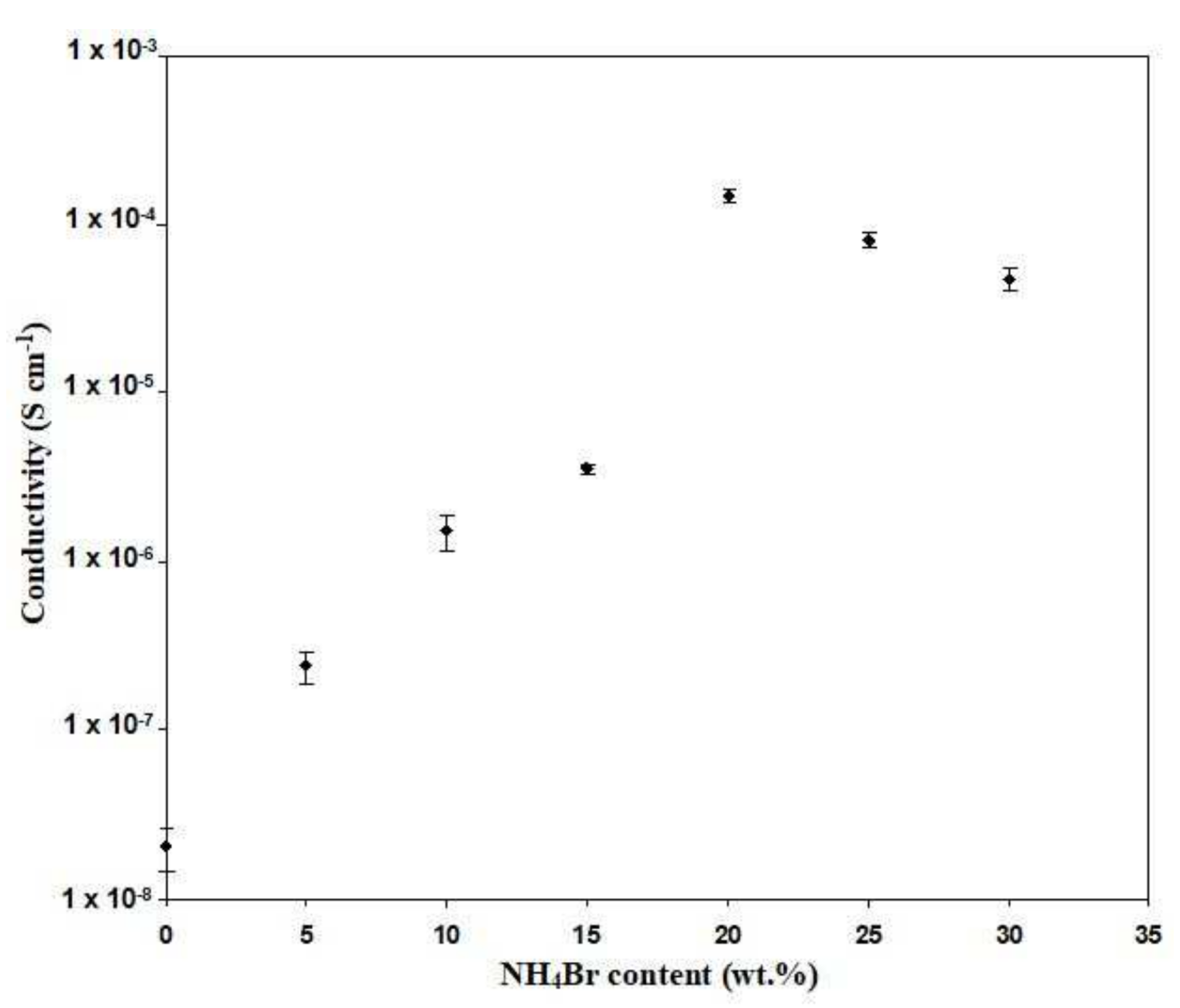
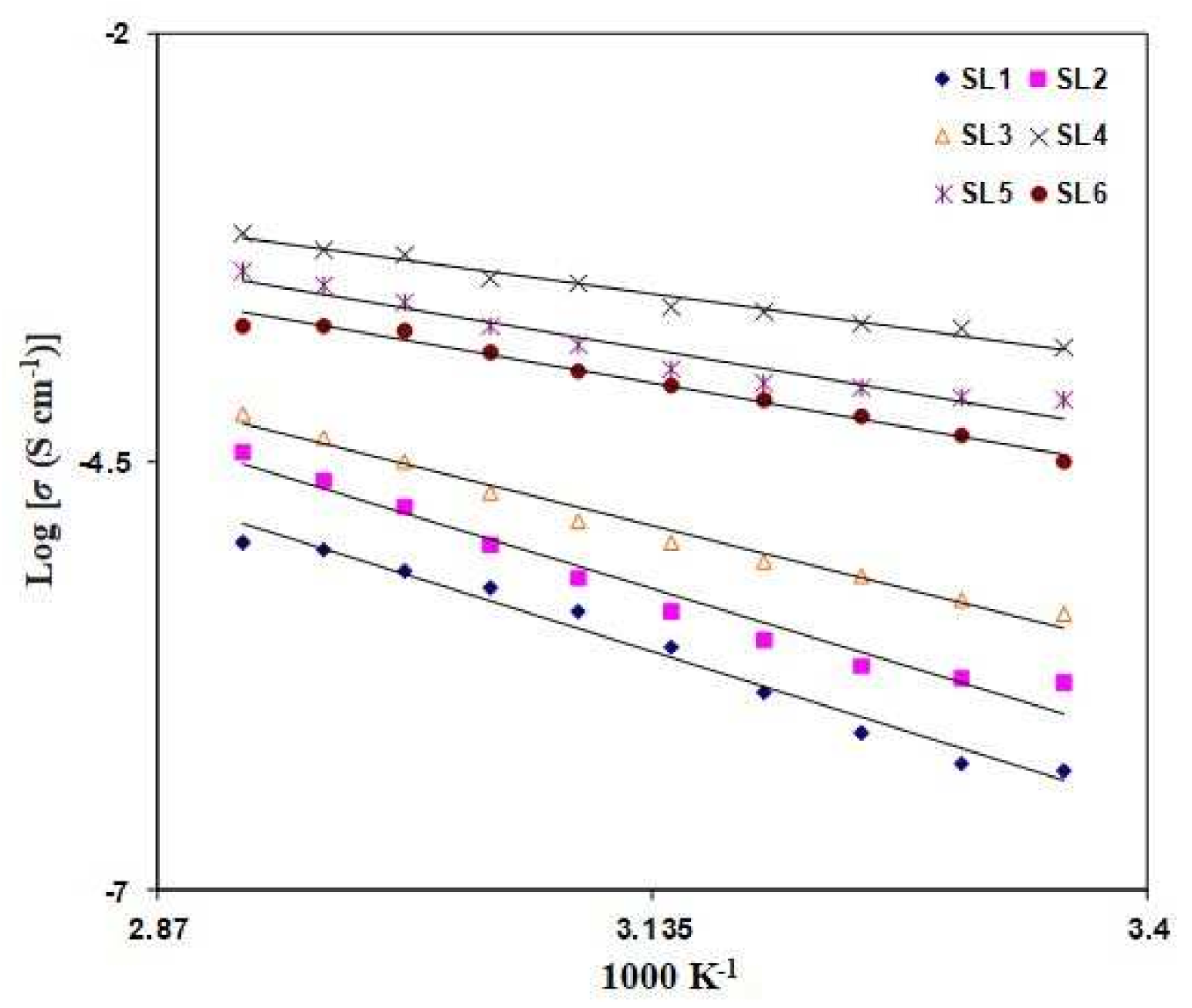
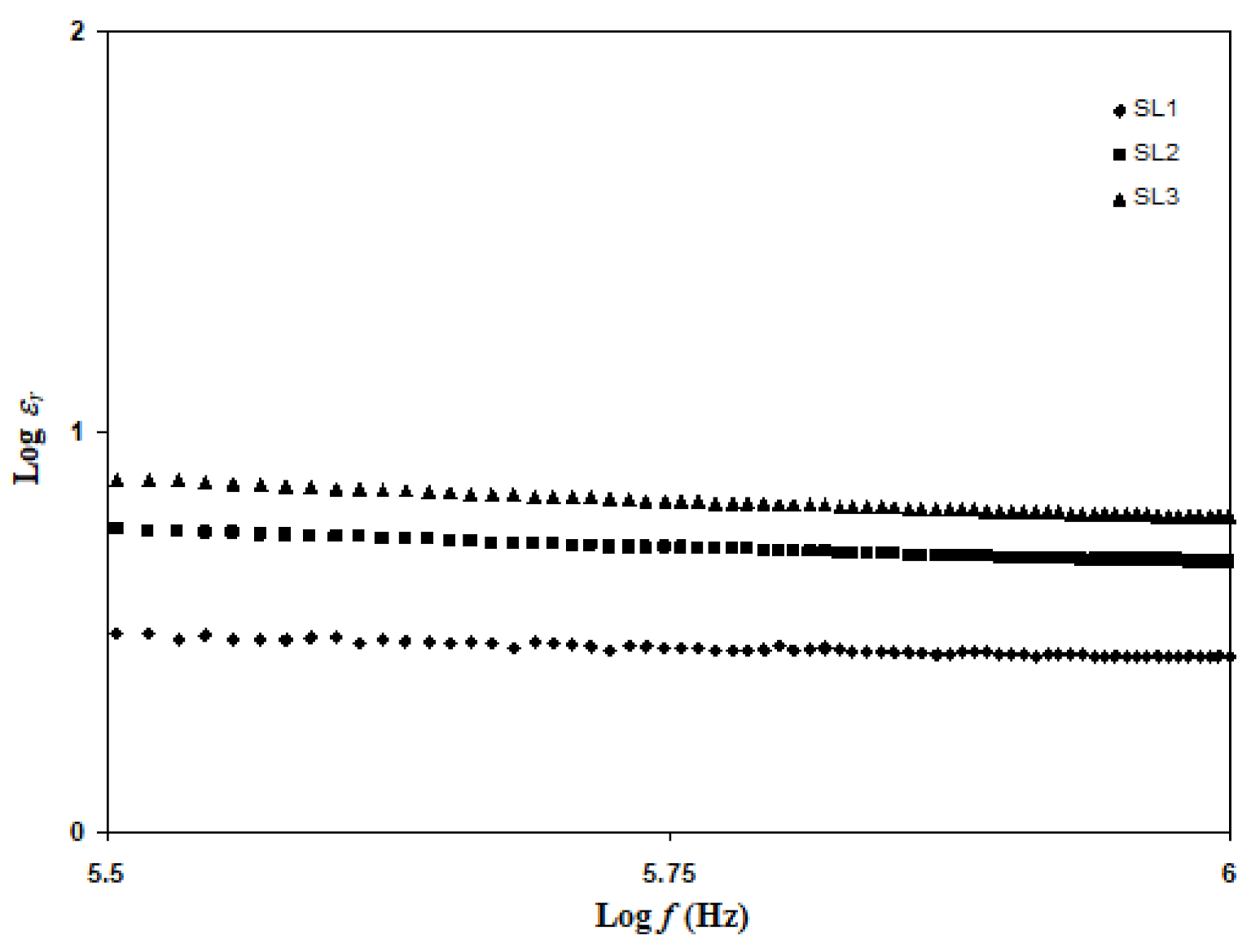
3.2. Conductivity Analysis
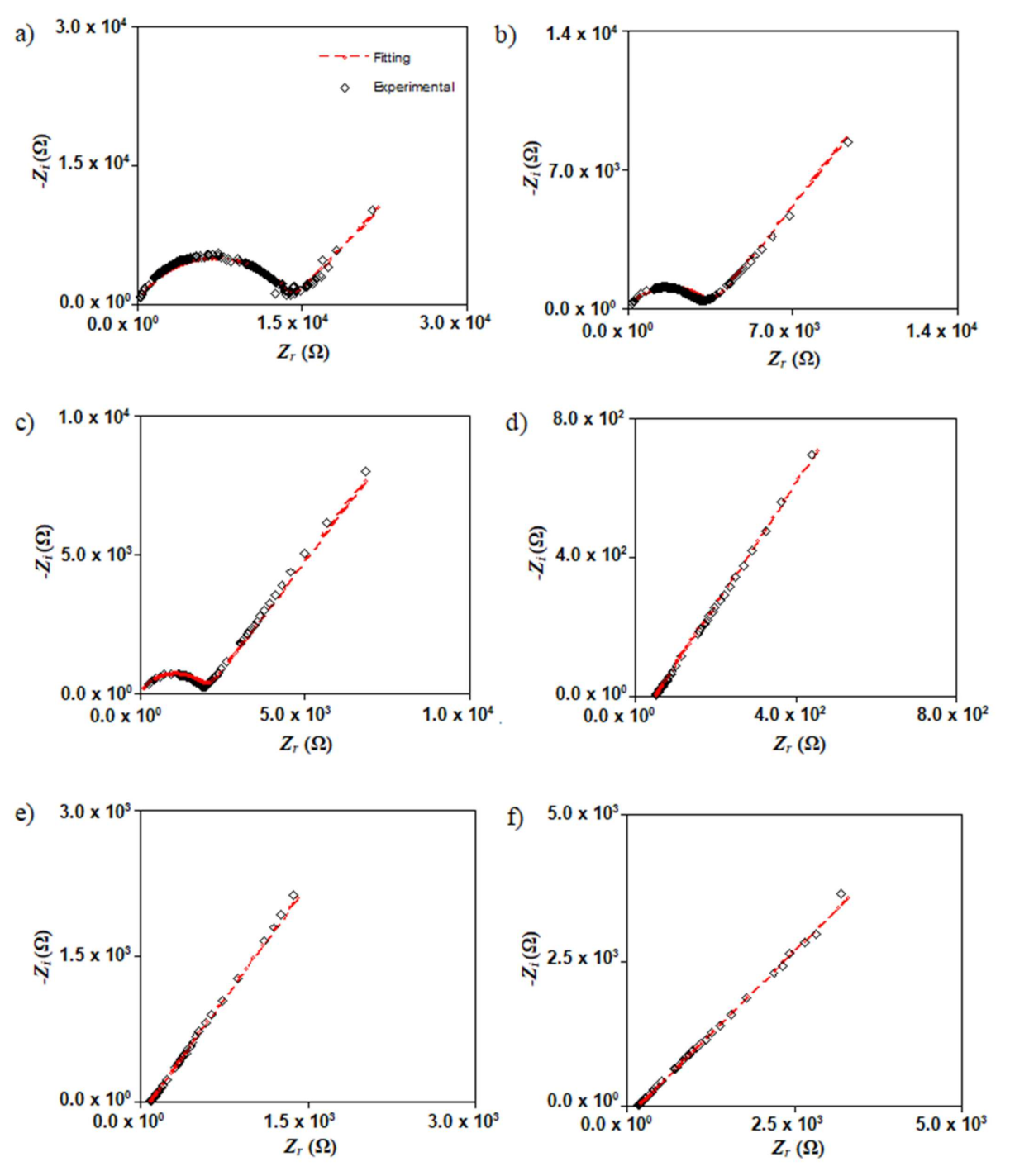
3.3. Impedance Analysis
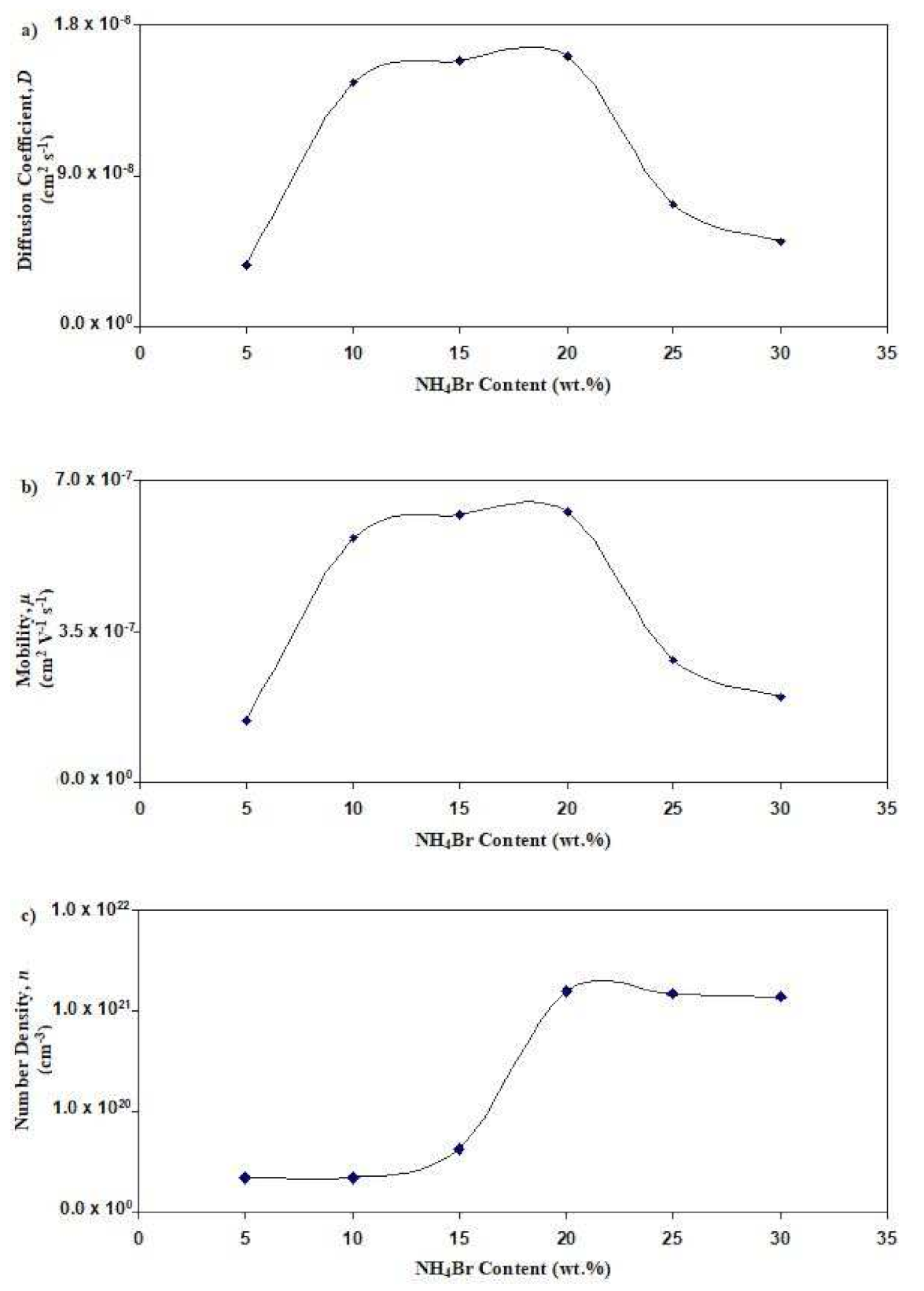
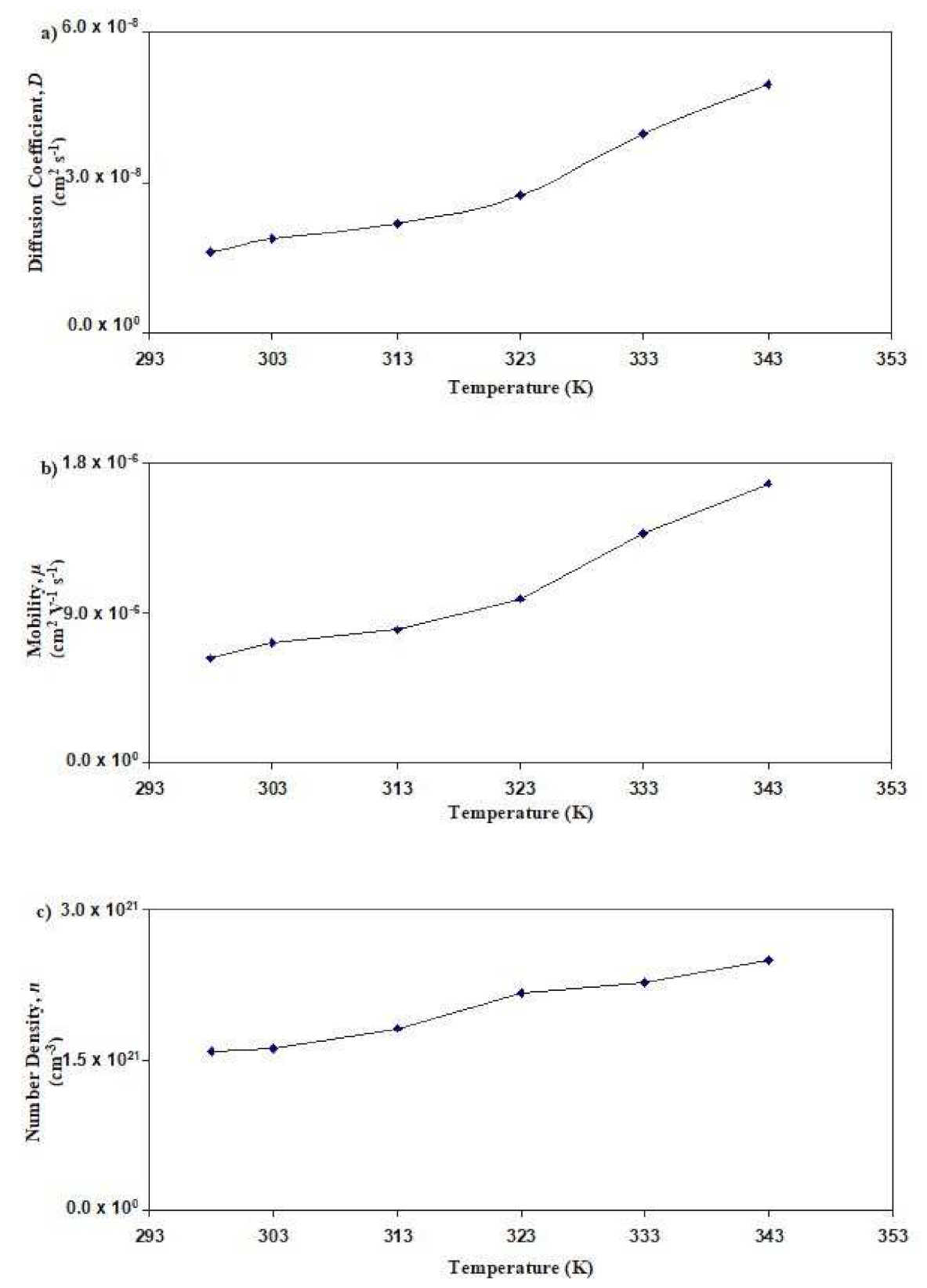
3.4. Determination of Transport Parameters
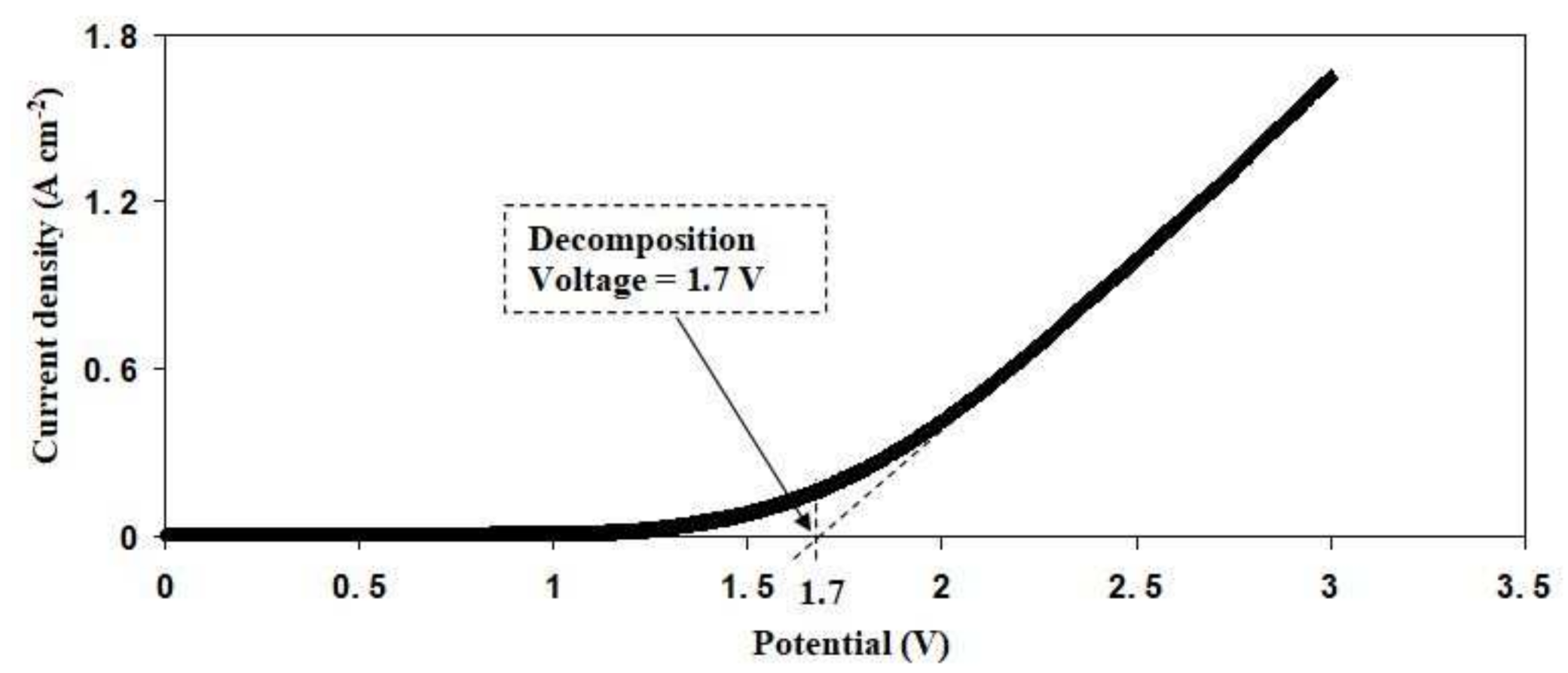
3.5. Linear Sweep Voltammetry (LSV)
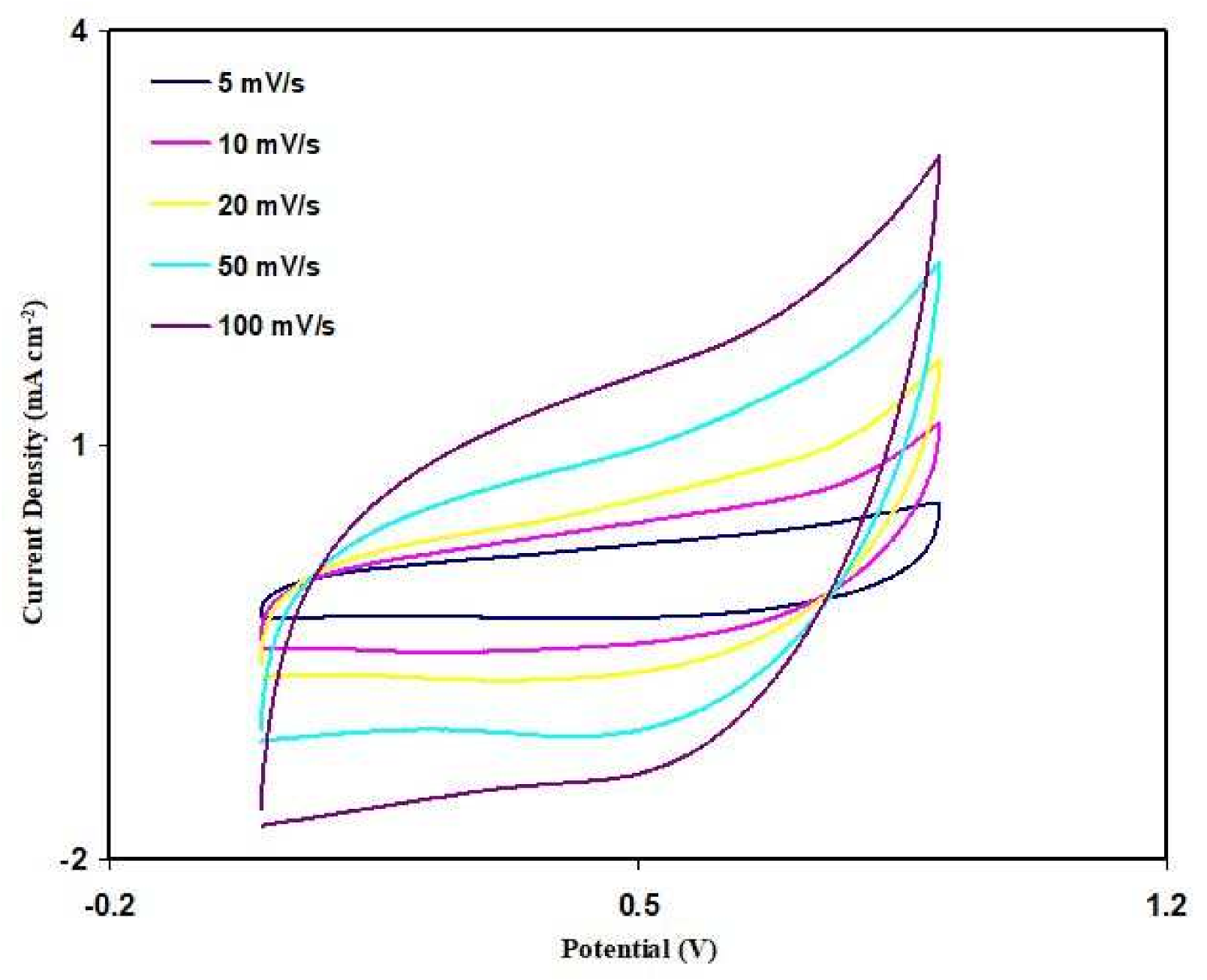
3.6. Cyclic Voltammetry (CV)
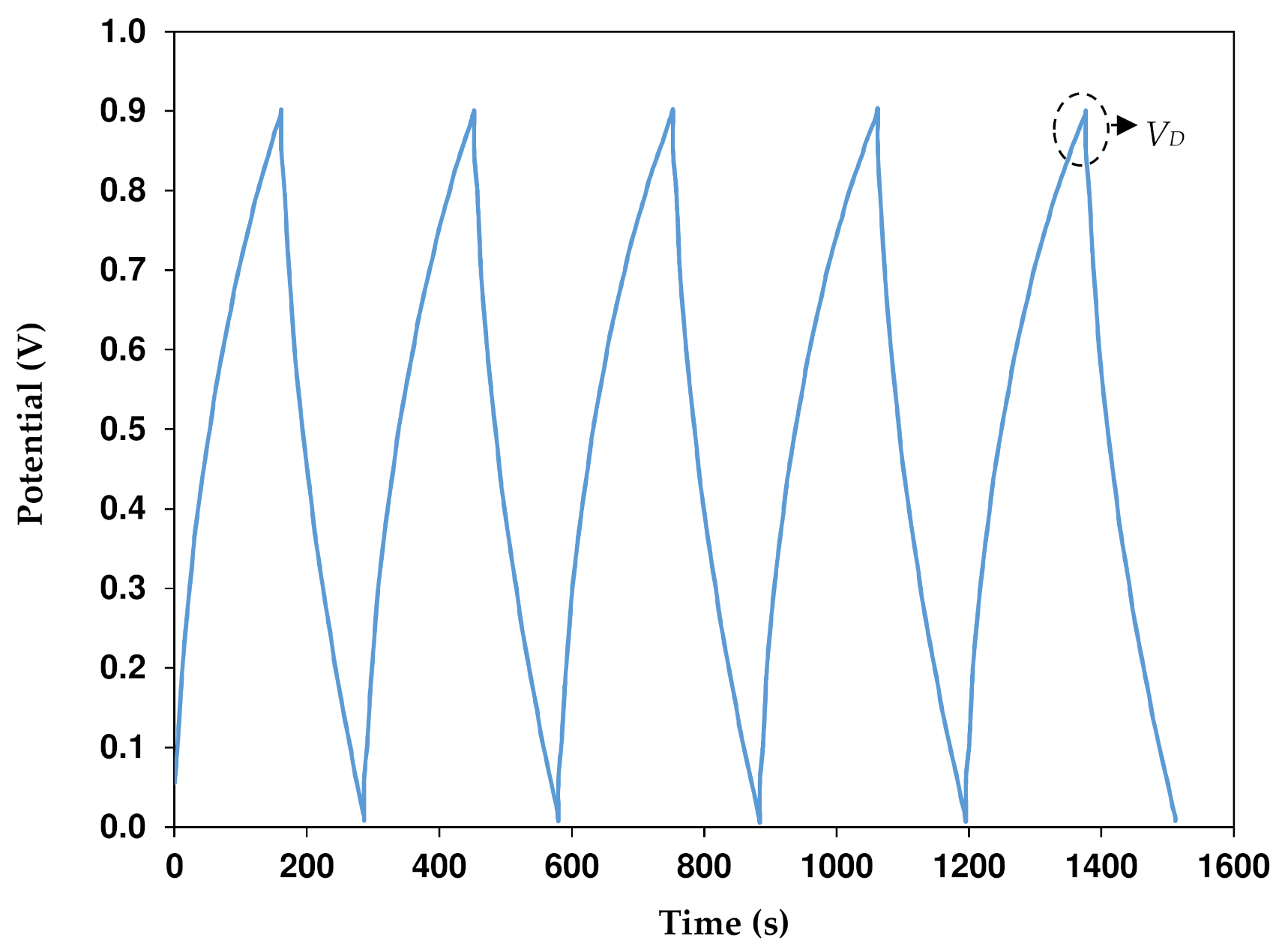
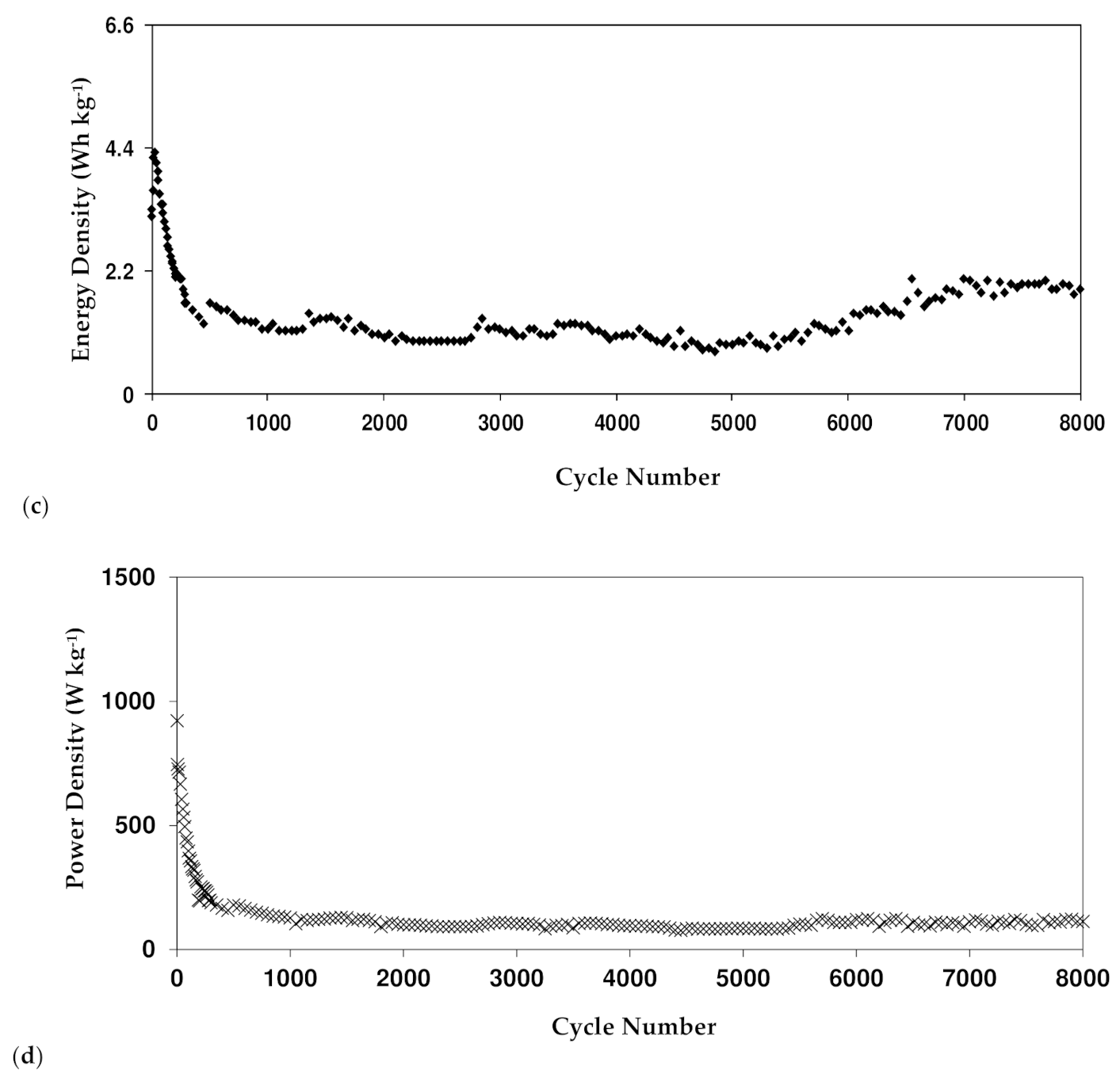
3.7. Dex-HEC-NH4Br-Based EDLC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, M.Z.; Rafiuddin, S. Structural, electrical conductivity and dielectric behavior of Na2SO4–LDT composite solid electrolyte. J. Adv. Res. 2016, 7, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Tomboc, G.M.; Kim, H. Derivation of both EDLC and pseudocapacitance characteristics based on synergistic mixture of NiCo2O4 and hollow carbon nanofiber: An efficient electrode towards high energy density supercapacitor. Electrochim. Acta 2019, 318, 392–404. [Google Scholar] [CrossRef]
- Amran, N.N.A.; Manan, N.S.A.; Kadir, M.F.Z. The effect of LiCF3SO3 on the complexation with potato starch-chitosan blend polymer electrolytes. Ionics 2016, 22, 1647–1658. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Polymer electrolytes for lithium ion batteries: A critical study. Ionics 2017, 23, 497–540. [Google Scholar] [CrossRef]
- Salleh, N.S.; Aziz, S.B.; Aspanut, Z.; Kadir, M.F.Z. Electrical impedance and conduction mechanism analysis of biopolymer electrolytes based on methyl cellulose doped with ammonium iodide. Ionics 2016, 22, 2157–2216. [Google Scholar] [CrossRef]
- Li, H.; Han, C.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Zhi, C. An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ. Sci. 2018, 11, 941–951. [Google Scholar] [CrossRef]
- Du, B.W.; Hu, S.Y.; Singh, R.; Tsai, T.T.; Lin, C.C.; Ko, F.U. Eco-friendly and biodegradable biopolymer chitosan/Y2O3 composite materials in flexible organic thin-film transistors. Materials 2017, 10, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azli, A.A.; Manan, N.S.A.; Kadir, M.F.Z. The development of Li+ conducting polymer electrolyte based on potato starch/graphene oxide blend. Ionics 2017, 23, 411–425. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Illias, H.A.; Shukur, M.F.; Kadir, M.F.Z. Characterization of starch-chitosan blend-based electrolyte doped with ammonium iodide for application in proton batteries. Ionics 2017, 23, 681–697. [Google Scholar] [CrossRef]
- Chong, M.Y.; Numan, A.; Liew, C.-W.; Ramesh, K.; Ramesh, S. Comparison of the performance of copper oxide and yttrium oxide nanoparticle based hydroxylethyl cellulose electrolytes for supercapacitors. J. Appl. Polym. Sci. 2017, 134, 44636. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Li, M.X.; Chang, Z.; Wang, Y.F.; Gao, J.; Zhu, Y.S.; Wu, Y.P.; Huang, W. A sandwich PVDF/HEC/PVDF gel polymer electrolyte for lithium ion battery. Electrochim. Acta 2017, 245, 752–759. [Google Scholar] [CrossRef]
- Li, M.X.; Wang, X.W.; Yang, Y.Q.; Chang, Z.; Wu, Y.P.; Holze, R. A dense cellulose-based membrane as a renewable host for gel polymer electrolyte of lithium ion batteries. J. Membr. Sci. 2015, 476, 112–118. [Google Scholar] [CrossRef]
- Selvanathan, V.; Halim, M.N.A.; Azzahari, A.D.; Rizwan, M.; Shahabudin, N.; Yahya, R. Effect of polar aprotic solvents on hydroxyethyl cellulose-based gel polymer electrolyte. Ionics 2018, 24, 1955–1964. [Google Scholar] [CrossRef]
- Tiwari, S.; Patil, R.; Bahadur, P. Polysaccharide based scaffolds for soft tissue engineering applications. Polymers 2019, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Sirisha, V.L.; Souza, J.; Kim, S.-K. Marine OMICS: Principles and Applications; Marine Foodomics; CRC Press: Boca Raton, FL, USA, 2016; pp. 643–682. [Google Scholar]
- Netsopa, S.; Niamsanit, S.; Sakloetsakun, D.; Milintawisamai, D. Characterization and rheological behavior of dextran from weissellaconfusa R003. Int. J. Polym. Sci. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Aziz, S.B.; Hadi, J.M.; Elham, E.M.; Abdulwahid, R.T.; Saeed, S.R.; Marf, A.S.; Karim, W.O.; Kadir, M.F.Z. The study of plasticized amorphous biopolymer blend electrolytes based on polyvinyl alcohol (PVA): Chitosan with high ion conductivity for energy storage electrical double-layer capacitors (EDLC) device application. Polymers 2020, 12, 1938. [Google Scholar] [CrossRef]
- Rajendran, S.; Mahendran, O. Experimental investigations on plasticized PMMA/PVA polymer blend electrolytes. Ionics 2001, 7, 463–468. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z. Electrical and transport properties of NH4Br-doped cornstarch-based solid biopolymer electrolyte. Ionics 2015, 21, 111–124. [Google Scholar] [CrossRef]
- Shukur, M.F.; Majid, N.A.; Ithnin, R.; Kadir, M.F.Z. Effect of plasticization on the conductivity and dielectric properties of starch-chitosan blend biopolymer electrolytes infused with NH4Br. Phys. Scr. 2013, 57, 014051–014057. [Google Scholar] [CrossRef]
- Nayak, R.; Dey, T.; Ghosh, P.; Bhattacharyya, A.R. Phosphoric acid doped poly(2,5-benzimidazole)-based proton exchange membrane for high temperature fuel cell application. J. Polym. Eng. Sci. 2016, 56, 20–25. [Google Scholar] [CrossRef]
- Pujiastuti, S.; Onggo, H. Effect of various concentration of sulfuric acid for Nafion membrane activation on the performance of fuel cell. AIP Conf. Proc. 2016, 1711, 060006. [Google Scholar]
- Hemalatha, R.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Moniha, V.; Boopathi, G.; Selvin, P.C. Preparation and characterization of proton-conducting polymer electrolyte based on PVA, amino acid proline, and NH4Cl and its applications to electrochemical devices. Ionics 2019, 25, 141–154. [Google Scholar] [CrossRef]
- Aziz, S.B.; Dannoun, E.M.A.; Hamsan, M.H.; Ghareeb, H.O.; Nofal, M.M.; Karim, W.O.; Asnawi, A.S.F.M.; Hadi, J.M.; Kadir, M.F.Z.A. A polymer blend electrolyte based on cs with enhanced ion transport and electrochemical properties for electrical double layer capacitor applications. Polymers 2021, 13, 930. [Google Scholar] [CrossRef]
- Vijaya, N.; Selvasekarapandian, S.; Hirankumar, G.; Karthikeyan, S.; Nithya, H.; Ramya, C.S.; Prabu, M. Structural, vibrational, thermal, and conductivity studies on proton-conducting polymer electrolyte based on poly (N-vinylpyrrolidone). Ionics 2012, 18, 91. [Google Scholar] [CrossRef]
- Hafiza, M.N.; Bashirah, A.N.A.; Bakar, N.Y.; Isa, M.I.N. Electrical properties of carboxyl methylcellulose/chitosan dual-blend green polymer doped with ammonium bromide. Int. J. Polym. Anal. Charact. 2014, 19, 151–158. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Aziz, S.B.; Kadir, M.F.Z. Dextran from Leuconostocmesenteroides-doped ammonium salt-based green polymer electrolyte. Bull. Mater. Sci. 2019, 42, 57. [Google Scholar] [CrossRef] [Green Version]
- Samsudin, A.S.; Isa, M.I.N. Structural and ionic transport study on CMC doped NH4Br: A new types of biopolymer electrolytes. J. Appl. Sci. 2012, 12, 174–179. [Google Scholar] [CrossRef]
- Aziz, S.B.; Dannou, E.M.A.; Hamsan, M.H.; Abdulwahid, R.T.; Mishra, K.; Nofal, M.M.; Kadir, M.F.Z. Improving EDLC Device Performance Constructed from Plasticized Magnesium Ion Conducting Chitosan Based Polymer Electrolytes via Metal Complex Dispersion. Membranes 2021, 11, 289. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Mustafa, M.S.; Hamsan, M.H.; Abdulwahid, R.T.; Kadir, M.F.Z.; Ghareeb, H.O. Role of nano-capacitor on dielectric constant enhancement in PEO:NH4SCN:xCeO2 polymer nano-composites: Electrical and electrochemical properties. J. Mater. Res. Technol. 2020, 9, 9283–9294. [Google Scholar] [CrossRef]
- Marf, A.S.; Aziz, S.B.; Abdullah, R.M. Plasticized H+ ion-conducting PVA:CS-based polymer blend electrolytes for energy storage EDLC application. J. Mater. Sci. Mater. Electron. 2020, 31, 18554–18568. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Nofal, M.M.; Aziz, S.B.; Brza, M.A.; Dannou, E.M.A.; Murad, A.R.; Kadir, M.F.Z.; Muzakir, S.K. Plasticized Polymer Blend Electrolyte Based on Chitosan for Energy Storage Application: Structural, Circuit Modeling, Morphological and Electrochemical Properties. Polymer 2021, 13, 1233. [Google Scholar] [CrossRef]
- Hanif, M.P.M.; Jalilah, A.J.; Badrul, F.; Nuraqmar, S.M.S. The influence of graphite on conductivity, crystallinity and tensile properties of hydroxyethyl cellulose (hec)/graphite composite films. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 012015. [Google Scholar] [CrossRef]
- Li, R.; Zhang, H.; Hu, X.; Gan, W.; Li, Q. An efficiently sustainable dextran-based flocculant: Synthesis, characterization and flocculation. Chemosphere 2016, 159, 342–450. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Shukur, M.F.; Kadir, M.F.Z.; Yusof, Y.M. Ion conduction in chitosan-starch blend based polymer electrolyte with ammonium thiocyanate as charge provider. J. Polym. Res. 2020, 27, 149. [Google Scholar] [CrossRef]
- Ahmed, H.T.; Abdullah, O.G. Structural and ionic conductivity characterization of PEO: MC-NH4I proton-conducting polymer blend electrolytes based films. Results Phys. 2020, 16, 102861. [Google Scholar] [CrossRef]
- Pandi, D.V.; Selvasekarapandian, S.; Bhuvaneswari, R.; Premalatha, M.; Monisha, S.; Arunkumar, D.; Junichi, K. Development and characterization of proton conducting polymer electrolyte based on PVA, amino acid glycine and NH4SCN. Solid State Ion. 2016, 298, 15–22. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Rasheed, M.A. Incorporation of NH4NO3 into MC-PVA blend-based polymer to prepare proton-conducting polymer electrolyte films. Ionics 2018, 24, 777–785. [Google Scholar] [CrossRef]
- Sohaimy, M.I.H.; Isa, M.I.N. Ionic conductivity and conduction mechanism studies on cellulose based solid polymer electrolytes doped with ammonium carbonate. Polym. Bull. 2017, 74, 1371–1386. [Google Scholar] [CrossRef]
- Mazuki, N.; Majeed, A.P.P.A.; Samsudin, A.S. Study on electrochemical properties of CMC-PVA doped NH4Br based solid polymer electrolytes system as application for EDLC. J. Polym. Res. 2020, 27, 135. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Khairul, W.M.; Isa, M.I.N. Characterization on potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J. Non-Cryst. Solids 2012, 358, 1104–1112. [Google Scholar] [CrossRef]
- Kartha, S.A. A Preparation of B2O3-Li2O-MO (M=Pb, Zn) Glass Thin Films and Study of Thin Properties. Ph.D. Thesis, Mahatma Gandhi University, Kerala, India, 2013. [Google Scholar]
- Shukur, M.F. Characterization of Ion Conducting Solid Bio-Polymer Electrolytes Based on Starch-Chitosan Blend and Application in Electrochemical Devices. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2015. [Google Scholar]
- Yulianti, E.; Deswita, D.; Sudaryanto, S.; Mashadi, S. Study of solid polymer electrolyte based on biodegradable polymer polycaprolactone. Mal. J. Fund. Appl. Sci. 2019, 15, 467–471. [Google Scholar]
- Yao, P.; Yu, P.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on polymer-based composite electrolytes for lithium batteries. Front. Chem. 2019, 7, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, X.; Gu, N.; Cheng, Z.; Yang, X.; Wang, E.; Dong, S. Impedance study of (PEO)10LiClO4-Al2O3 composite polymer electrolyte with blocking electrodes. Electrochim. Acta 2001, 46, 1829–1836. [Google Scholar] [CrossRef]
- Kumar, M.S.; Rao, M.C. Effect of Al2O3 on structural and dielectric properties of PVP-CH3COONa based solid polymer electrolyte films for energy storage devices. Heliyon 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuhaimi, N.E.A.; Teo, L.P.; Woo, H.J.; Majid, S.R.; Arof, A.K. Electrical double-layer capacitors with plasticized polymer electrolyte based on methyl cellulose. Polym. Bull. 2012, 69, 807–826. [Google Scholar] [CrossRef]
- Arof, A.K.; Amirudin, S.; Yusof, S.Z.; Noor, I.M. A method based on impedance spectroscopy to determine transport properties of polymer electrolytes. Phys. Chem. 2014, 16, 1856–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadzallah, I.A.; Noor, I.M.; Careem, M.A.; Arof, A.K. Investigation of transport properties of chitosan-based electrolytes utilizing impedance spectroscopy. Ionics 2016, 22, 1635–1645. [Google Scholar] [CrossRef]
- Ahmad, N.H.; Isa, M.I.N. Proton conducting solid polymer electrolytes based carboxymethyl cellulose doped ammonium chloride: Ionic conductivity and transport studies. Int. J. Plast. Technol. 2015, 19, 47–55. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Aziz, S.B.; Yusof, Y.M.; Kadir, M.F.Z. Influence of NH4Br as an ionic source on the structural/electrical properties of dextran-based biopolymer electrolytes and EDLC application. Bull. Mater. Sci. 2020, 43, 30. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Saeed, S.R.; Brza, M.A.; Abdulwahid, R.T.; Hamsan, M.H.; Abdullah, R.M.; Kadir, M.F.Z.; Muzakir, S.K. Investigation of ion transport parameters and electrochemical performance of plasticized biocompatible chitosan-based proton conducting polymer composite electrolytes. Membranes 2020, 10, 363. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Mishra, K.; Hamsan, M.H.; Karim, W.O.; Abdullah, R.M.; Kadir, M.F.Z.; Abdulwahid, R.T. Fabrication of high performance energy storage EDLC device from proton conducting methylcellulose: Dextran polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 1137–1150. [Google Scholar] [CrossRef]
- Noor, N.A.M.; Isa, M.I.N. Investigation on transport and thermal studies of solid polymer electrolyte based on carboxymethyl cellulose doped ammonium thiocyanate for potential application in electrochemical devices. Int. J. Hydrog. Energy 2019, 44, 8298–8306. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S. Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydr. Polym. 2015, 124, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Singh, M.B. Theory of the electrochemical impedance of mesostructured electrodes embedded with heterogeneous micropores. J. Phys. Chem. C 2017, 121, 7164–7174. [Google Scholar] [CrossRef]
- Shukur, M.F.; Hamsan, M.H.; Kadir, M.F.Z. Investigation of plasticized ionic conductor based on chitosan and ammonium bromide for EDLC application. Mater. Today Proc. 2019, 17, 490–498. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Dannoun, E.M.A.; Hamsan, M.H.; Hadi, J.M.; Kadir, M.F.Z.; Abdulwahid, R.T. The Study of Electrical and Electrochemical Properties of Magnesium Ion Conducting CS: PVA Based Polymer Blend Electrolytes: Role of Lattice Energy of Magnesium Salts on EDLC Performance. Molecules 2020, 25, 4503. [Google Scholar] [CrossRef]
- Teoh, K.H.; Liew, C.W.; Ramesh, S. Electric double layer capacitor based on activated carbon electrode and biodegradable composite polymer electrolyte. Ionics 2014, 20, 251–258. [Google Scholar]
- Teoh, K.H.; Lim, C.S.; Liew, C.W.; Ramesh, S. Electric double-layer capacitors with corn starch-based biopolymer electrolytes incorporating silica as filler. Ionics 2015, 21, 2061–2068. [Google Scholar] [CrossRef]
- Arof, A.K.; Kufian, M.Z.; Syukur, M.F.; Aziz, M.F.; Abdelrahman, A.E.; Majid, S.R. Electrical double layer capacitor using poly(methyl methacrylate)–C4BO8Li gel polymer electrolyte and carbonaceous material from shells of matakucing (Dimocarpuslongan) fruit. Electrochim. Acta 2012, 74, 39–45. [Google Scholar] [CrossRef]
- Chong, M.Y.; Numan, A.; Liew, C.W.; Ng, H.; Ramesh, K.; Ramesh, S. Enhancing the performance of green solid-state electric double-layer capacitor incorporated with fumed silica nanoparticles. J. Phys. Chem. Solids 2018, 117, 194–203. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Brza, M.A.; Kadir, M.F.Z.; Muzakir, S.K.; Abdulwahid, R.T. Effect of glycerol on EDLC characteristics of chitosan:methylcellulose polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 8355–8366. [Google Scholar] [CrossRef]
- Lim, C.S.; Teoh, K.H.; Liew, C.W.; Ramesh, S. Capacitive behavior studies on electrical double layer capacitor using poly (vinyl alcohol)-lithium perchlorate based polymer electrolyte incorporated with TiO2. Mater. Chem. Phys. 2014, 143, 661–667. [Google Scholar] [CrossRef]
- Farah, N.; Ng, H.M.; Numan, A.; Liew, C.W.; Latip, N.A.A.; Ramesh, K.; Ramesh, S. Solid polymer electrolytes based on poly(vinyl alcohol) incorporated with sodium salt and ionic liquid for electrical double layer capacitor. Mater. Sci. Eng. B 2019, 251, 114468. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Kadir, M.F.Z. NH4NO3 as charge carrier contributor in glycerolized potato starch-methyl cellulose blend-based polymer electrolyte and the application in electrochemical double-layer capacitor. Ionics 2017, 23, 3429–3453. [Google Scholar] [CrossRef]



| Dextran:HEC Composition (wt%) | Designation |
|---|---|
| 0:100 | BL0 |
| 10:90 | BL1 |
| 20:80 | BL2 |
| 30:70 | BL3 |
| 40:60 | BL4 |
| 50:50 | BL5 |
| 60:40 | BL6 |
| 70:30 | BL7 |
| 80:20 | BL8 |
| 90:10 | BL9 |
| 100:0 | BL10 |
| Dextran:HEC:NH4Br Composition (wt%) | Designation |
|---|---|
| 40:60:0 | BL4 |
| 38:57:5 | SL1 |
| 36:54:10 | SL2 |
| 34:51:15 | SL3 |
| 32:48:20 | SL4 |
| 30:45:25 | SL5 |
| 28:42:30 | SL6 |
| Designation | Degree of Crystallinity (χc) |
|---|---|
| BL0 | 30.53 |
| BL1 | 29.97 |
| BL2 | 28.57 |
| BL3 | 24.44 |
| BL4 | 20.17 |
| BL5 | 24.59 |
| BL6 | 28.52 |
| BL7 | 29.02 |
| BL8 | 29.93 |
| BL9 | 30.88 |
| BL10 | 32.06 |
| Electrolyte | Eac(eV) |
|---|---|
| SL1 | 0.68 |
| SL2 | 0.65 |
| SL3 | 0.54 |
| SL4 | 0.26 |
| SL5 | 0.38 |
| SL6 | 0.61 |
| Electrolyte | k1 (F−1) | k2 (F−1) | C1 (F) | C2 (F) |
|---|---|---|---|---|
| SL1 | 8.00 × 108 | 3.70 × 105 | 1.25 × 10−9 | 2.70 × 10−6 |
| SL2 | 1.40 × 108 | 3.68 × 105 | 7.14 × 10−9 | 2.72 × 10−6 |
| SL3 | 1.20 × 108 | 3.65 × 105 | 8.33 × 10−9 | 2.74 × 10−6 |
| SL4 | - | 3.80 × 104 | - | 2.63 × 10−5 |
| SL5 | - | 9.50 × 104 | - | 1.05 × 10−5 |
| SL6 | - | 1.02 × 105 | - | 9.80 × 10−6 |
| Temperature (K) | k (F−1) | C (F) |
|---|---|---|
| 298 | 3.80 × 104 | 2.63 × 10−5 |
| 303 | 3.55 × 104 | 2.82 × 10−5 |
| 313 | 2.92 × 104 | 3.42 × 10−5 |
| 323 | 2.10 × 104 | 4.76 × 10−5 |
| 333 | 1.45 × 104 | 6.90 × 10−5 |
| 343 | 1.10 × 104 | 9.09 × 10−5 |
| Scan Rates (mV s−1) | Capacitance (F g−1) |
|---|---|
| 100 | 9.51 |
| 50 | 13.41 |
| 20 | 20.84 |
| 10 | 29.70 |
| 5 | 36.69 |
| Polymer Electrolyte | Current Density | CCD (F/g) | Cycles | Reference |
|---|---|---|---|---|
| Dex-NH4Br | 0.063 mA/cm2 | 2.05 | 100 | [52] |
| HEC-MgTf2-EMIMTf-SiO2 | 0.400 A/g | 25.00 | 1600 | [63] |
| CS-MC-NH4I-Gly | 0.400 mA/cm2 | 9.70 | 100 | [64] |
| PVA-Dex-NH4I | 0.500 mA/cm2 | 4.20 | 100 | [53] |
| PVA-LiClO4-TiO2 | - | 12.50 | 1000 | [65] |
| PVA-Naft-BmImBr | 0.200 A/g | 16.32 | 1000 | [66] |
| PS-MC-NH4NO3-Gly | 0.200 mA/cm2 | 31.00 | 1000 | [67] |
| Dex-HEC-NH4Br | 0.250 mA/cm2 | 31.70 | 8000 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azha, M.A.S.; Dannoun, E.M.A.; Aziz, S.B.; Kadir, M.F.Z.; Zaki, Z.I.; El-Bahy, Z.M.; Sulaiman, M.; Nofal, M.M. High Cyclability Energy Storage Device with Optimized Hydroxyethyl Cellulose-Dextran-Based Polymer Electrolytes: Structural, Electrical and Electrochemical Investigations. Polymers 2021, 13, 3602. https://doi.org/10.3390/polym13203602
Azha MAS, Dannoun EMA, Aziz SB, Kadir MFZ, Zaki ZI, El-Bahy ZM, Sulaiman M, Nofal MM. High Cyclability Energy Storage Device with Optimized Hydroxyethyl Cellulose-Dextran-Based Polymer Electrolytes: Structural, Electrical and Electrochemical Investigations. Polymers. 2021; 13(20):3602. https://doi.org/10.3390/polym13203602
Chicago/Turabian StyleAzha, Muhammad A. S., Elham M. A. Dannoun, Shujahadeen B. Aziz, Mohd F. Z. Kadir, Zaki Ismail Zaki, Zeinhom M. El-Bahy, Mazdida Sulaiman, and Muaffaq M. Nofal. 2021. "High Cyclability Energy Storage Device with Optimized Hydroxyethyl Cellulose-Dextran-Based Polymer Electrolytes: Structural, Electrical and Electrochemical Investigations" Polymers 13, no. 20: 3602. https://doi.org/10.3390/polym13203602
APA StyleAzha, M. A. S., Dannoun, E. M. A., Aziz, S. B., Kadir, M. F. Z., Zaki, Z. I., El-Bahy, Z. M., Sulaiman, M., & Nofal, M. M. (2021). High Cyclability Energy Storage Device with Optimized Hydroxyethyl Cellulose-Dextran-Based Polymer Electrolytes: Structural, Electrical and Electrochemical Investigations. Polymers, 13(20), 3602. https://doi.org/10.3390/polym13203602









