Wheat Biocomposite Extraction, Structure, Properties and Characterization: A Review
Abstract
:1. Introduction
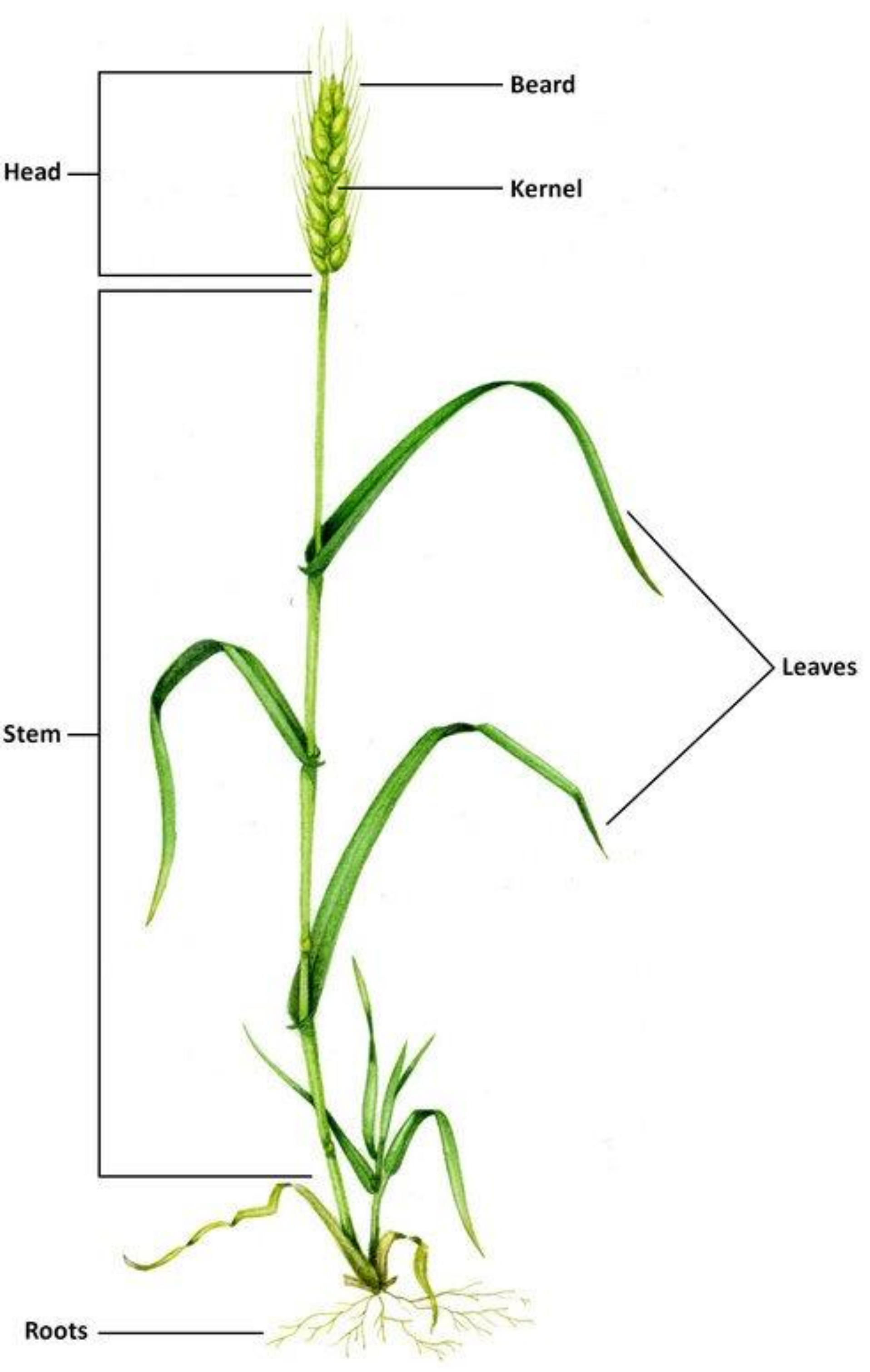
2. Wheat Plant
3. Film Preparation and Properties Characterization of Films Based Wheat Starch
3.1. Physical and Chemical Properties of Wheat Starch
| Type of Starch | ||||
|---|---|---|---|---|
| Parameter | Wheat Starch | Corn Starch | Rice Starch | Potato Starch |
| Amylose (%) | 16.0–31.5 | 20.0–28 | 20–28 | 25–31 |
| Amylopectin (%) | 68.5–75 | 75–83 | 65–85 | 76–83 |
| Ash (%) | 0.20–0.29 | 0.32–0.62 | 0.17–0.19 | 15.95–16.05 |
| Proteins (%) | 0.40–0.46 | 0.38–7.7 | 0.33–0.38 | 4.26–4.82 |
| Density (g/cm3) | 1.5 | 1.356–1.4029 | 1.282 | 0.763 |
| Moisture content (%) | 10.65–13.3 | 10.45–10.82 | 3.60 | 15.98 ± 0.36 |
3.2. Production of Films Based Wheat Starch
3.2.1. Wheat Starch Isolation
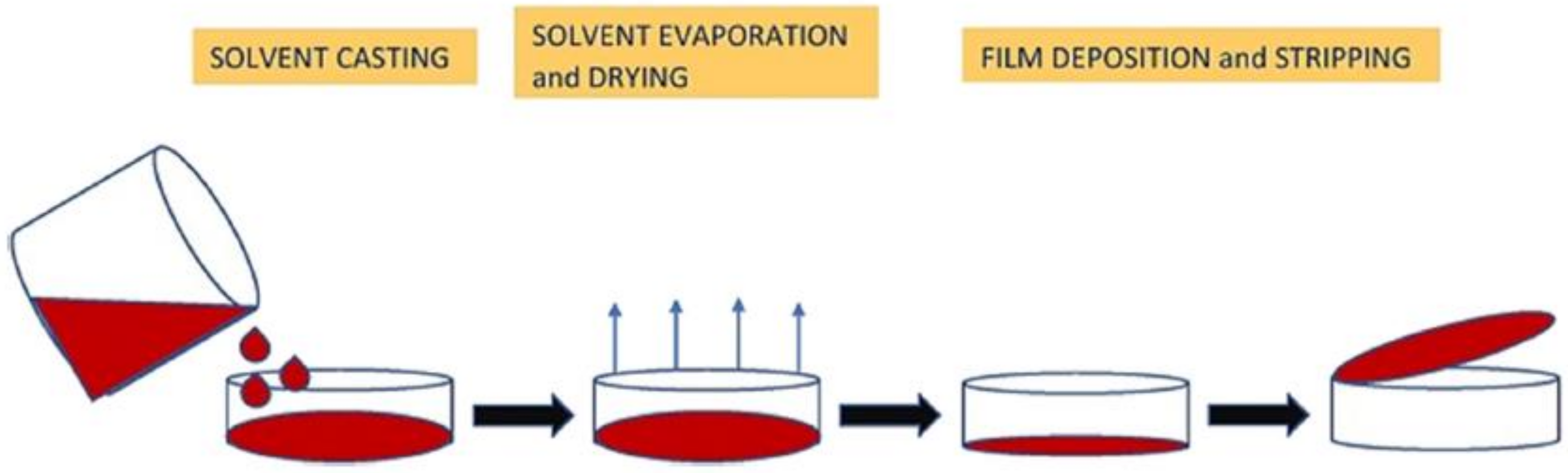
3.2.2. Wheat Starch Film Preparation
3.3. Properties Characteristics of Wheat Starch-Based Film
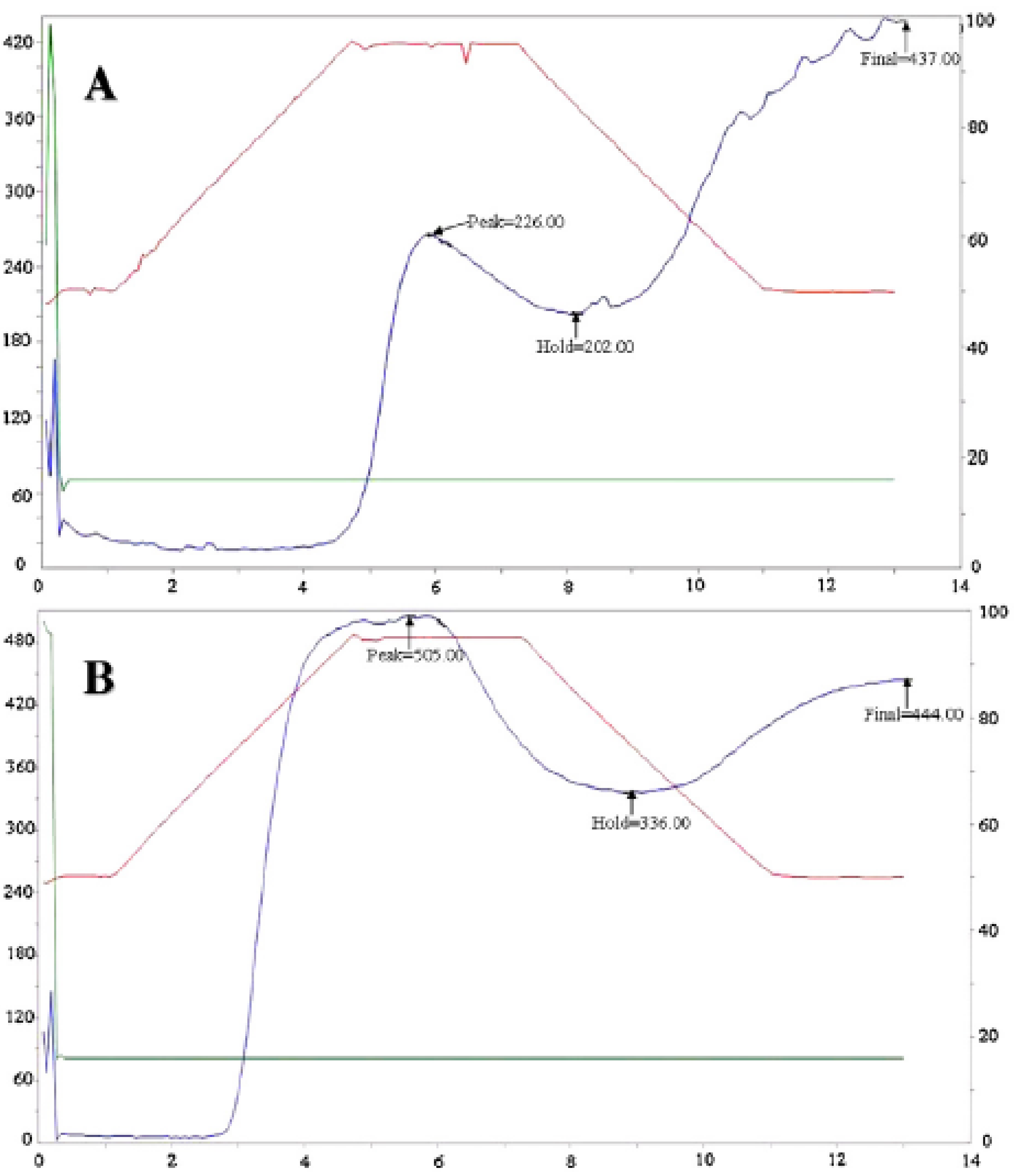
3.3.1. Pasting Properties
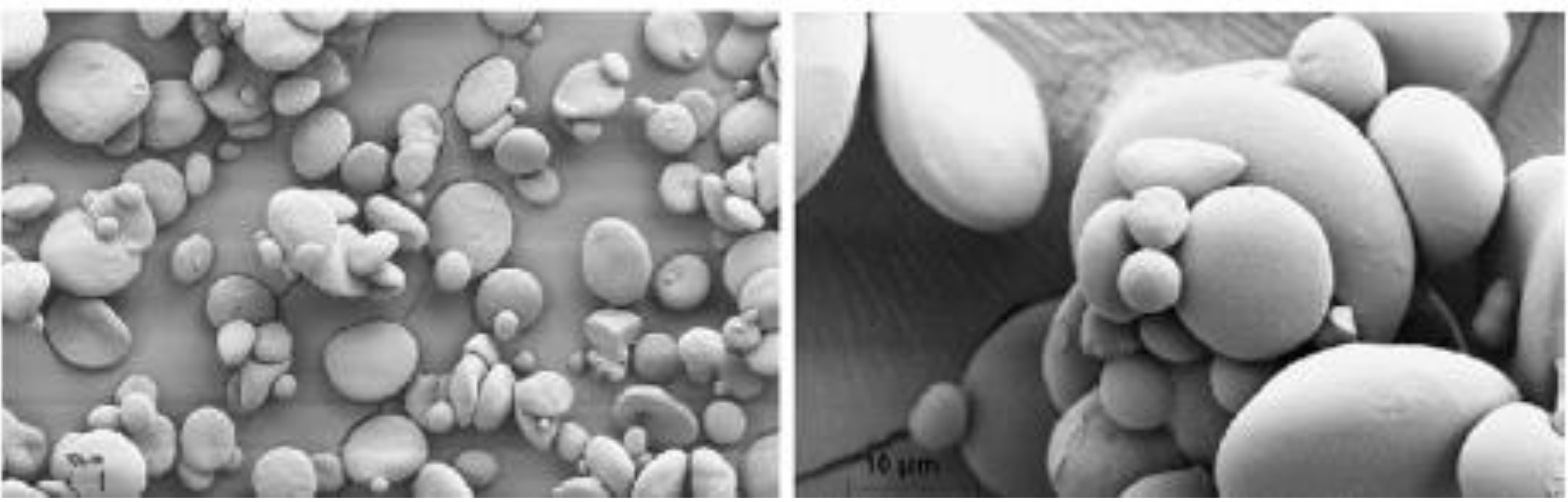
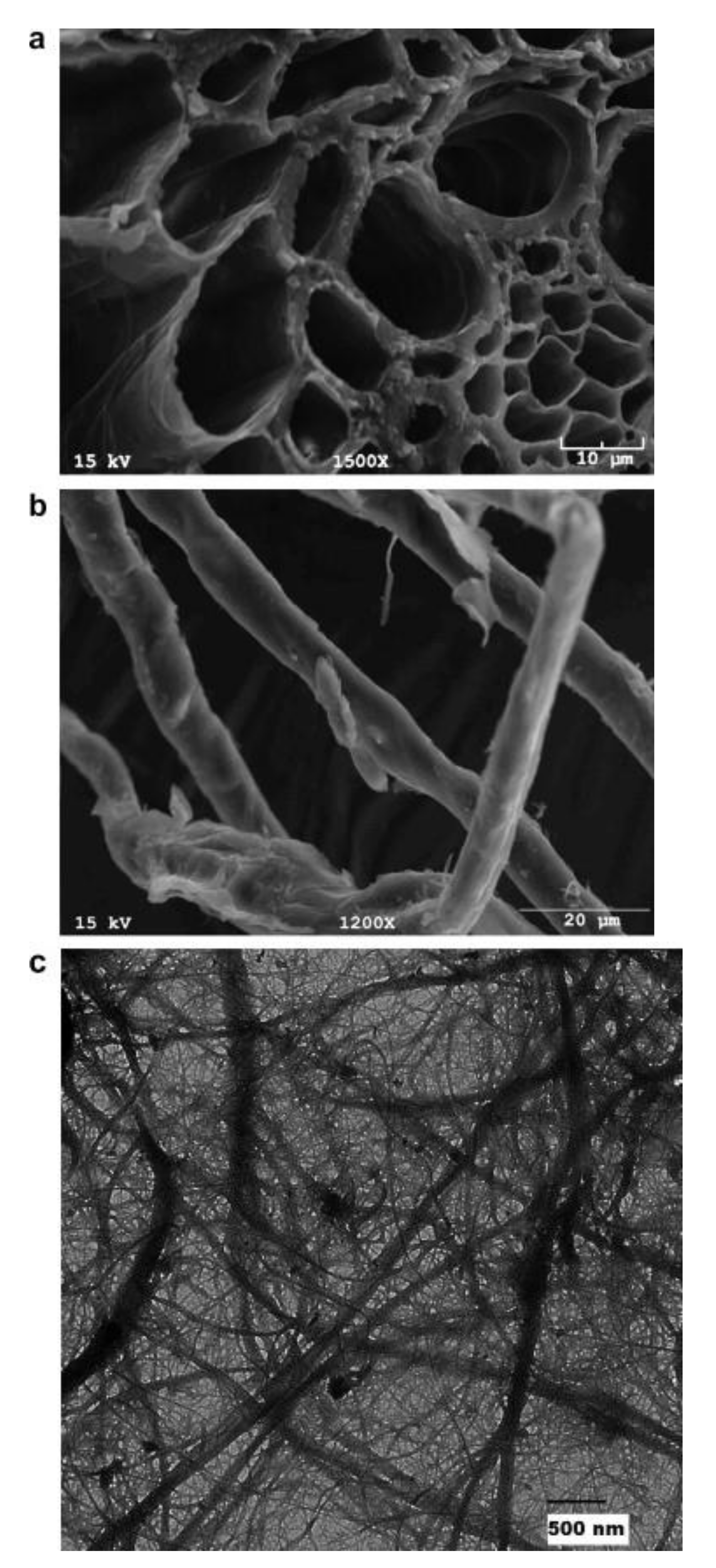
3.3.2. Morphological Properties
3.3.3. Film Transparency
3.3.4. Thermal Properties
3.3.5. Water Vapor Permeability (WVP)
3.3.6. Crystallinity
4. Wheat Gluten-Based Film; Preparation and Characterization
4.1. Production of Wheat Gluten-Based Film
4.1.1. Wet Method
4.1.2. Dry Method
4.2. Properties Characterization of Films Based Wheat Gluten
5. Wheat Fiber
| Type of Husk | |||
|---|---|---|---|
| Parameter | Wheat Husk | Corn Husk | Rice Husk |
| Density (g/cm3) | 0.75 | 1.49–1.18 | 0.1214 |
| Moisture content (%) | 6–6.05 | 7.6–8.7 | 9 |
| Cellulose (%) | 36–39.2 | 31.3–47 | 34.34–43.80 |
| Hemicellulose (%) | 18–26.4 | 34–43.91 | 19–25 |
| Protein (%) | 6 | 7 | 1.70–7.26 |
| Fats (%) | 5 | 17.2 | 0.38–2.98 |
| Lignin (%) | 6.8–16 | 1.5–14.3 | 16 |
| Type of Straw | |||
|---|---|---|---|
| Parameter | Wheat Straw | Corn Straw | Rice Straw |
| Density (g/cm3) | 0.3231–0.871 | 0.033–0.069 | 0.194 |
| Moisture content (%) | 8–60 | 25–30 | 6.58–18 |
| Cellulose (%) | 28.8–51.5 | 28–44 | 29.2–38 |
| Hemicellulose (%) | 10.5–39.1 | 36.05–36.83 | 12.0–29.3 |
| Protein (%) | 3–6.3 | 4–9 | 3–7 |
| Lignin (%) | 5.4–30 | 7–29 | 12–19.0 |
| Wheat Bran | |
|---|---|
| Parameter | Amount |
| Density (g/cm3) | 0.17–0.25 |
| Water holding capacity (g/g) | 3.39–6.49 |
| Water retention capacity (g/g) | 2.17–5.76 |
| Moisture content (%) | 8.2 |
| Cellulose (%) | 11.65–13.15 |
| Hemicellulose (%) | 49.7 |
| Starch (%) | 55.9–70.53 |
| Protein (%) | 15.8–16.88 |
| Lipid (%) | 3.8–4.13 |
| Lignin (%) | 5.3 |
6. Antioxidant Properties of Wheat Based Film
7. Antimicrobial Properties of Wheat Based Film
8. Wheat Biocomposite
8.1. Wheat Biocomposite Advantages and Applications
8.2. Wheat Biocomposite Fabrication
| Polymer | Filler | Fabrication Process | Filler Loading (%) | Optimum Tensile Modulus (MPa) | Optimum Yield Strength (MPa) | Ref. |
|---|---|---|---|---|---|---|
| Modified potato starch | Wheat straw nanofiber | Solution casting | 2–10 | 271 ± 27.4 | 7.71 ± 0.67 | [98] |
| Wheat gluten | Coconut coir | Mixing and compression molding | 10 | 2.29 ± 0.47 | 123.2 ± 34.7 | [144] |
| Natural rubber | Wheat bran | Mixing and compression molding | 10–50 phr | - | 22 | [245] |
| Wheat gluten | Wheat straw fibers | Mixing and compression molding | 0–11.1 | 18.4 ± 2.3 | 41.7 ± 3.4 | [154] |
| Polyethylene | Wheat Bran | Extrusion | 10–50 | 371 | 11.5 | [246] |
| Wheat gluten | Hydroxyethyl cellulose | Mixing and compression molding | 0–35 | 70 | 2.4 | [247] |
| Ecovio | Wheat husk | Mixing and compression molding | 13.5 | Flexural: 60 | Flexural: 0.75 | [248] |
| Wheat gluten | Chemlal olive pomace | Mixing and compression molding | 0–20 | 40 | 3.5 | [249] |
| Native Wheat | CNCs rice | Solution casting | 0.18 g | 34.86 ± 3.3 | 3.64 ± 0.18 | [236] |
| CNCs oat | 56.58 ± 9.06 | 5.07 ± 0.33 | ||||
| CNCs eucalyptus | 70.81 ± 8.22 | 4.32 ± 0.13 | ||||
| Phosphorylated Wheat | CNCs rice | 31.94 ± 1.38 | 3.78 ± 0.08 | |||
| CNCs oat | 24.37 ± 1.5 | 3.52 ± 0.14 | ||||
| CNCs eucalyptus | 30.12 ± 0.35 | 3.08 ± 0.02 | ||||
| PHBV | Wheat straw fibers | Extrusion and compression molding | 20 | 3100 ± 200 | 21 ± 2 | [235] |
| PLA | Wheat straw fibers | Extrusion and injection molding | 0–40 | 3450 | 61.2 | [234] |
| Polyester resin | Wheat straw strands | Mixing and compression molding | 25 | Flexural: 2427.2 | Flexural: 28.21 | [250] |
| Polypropylene | Wheat straw/Clay | Extrusion and injection molding | Wheat: 0–50Clay: 0–5 | Flexural: 2400 | - | [245] |
| Fabrication, filler loading and optimum mechanical properties of corn biocomposite | ||||||
| PLA | Corn Cob | Mixing and compression molding | 0–40 | 3.7 | 53 | [251] |
| Corn starch | Corn husk | Solution casting | 0–8 | 620 | 13 | [40] |
| Polypropylene (PP) | Corn stalk | Mixing and injection molding | 40 | 4.3 | 34.1 | [252] |
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Aisyah, H.A.; Rafiqah, S.A.; Sabaruddin, F.A.; Kamarudin, S.H.; Norrrahim, M.N.F.; Ilyas, R.A.; et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers 2021, 13, 646. [Google Scholar] [CrossRef]
- Alsubari, S.; Zuhri, M.Y.M.; Sapuan, S.M.; Ishak, M.R.; Ilyas, R.A.; Asyraf, M.R.M. Potential of natural fiber reinforced polymer composites in sandwich structures: A review on its mechanical properties. Polymers 2021, 13, 423. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Supian, A.B.M.; Sapuan, S.M.; Jawaid, M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syamsir, A. Crashworthiness Response of Filament Wound Kenaf/Glass Fibre-reinforced Epoxy Composite Tubes with Influence of Stacking Sequence under Intermediate-velocity Impact Load. Fibers Polym. 2021, 1–12. [Google Scholar] [CrossRef]
- Simon, F.; Loussert-Ajaka, I.; Damond, F.; Saragosti, S.; Barin, F.; Brun-Vézinet, F. A REVIEW ON NATURAL FIBERS. AIDS Res. Hum. Retrovir. 1996, 12, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Netravali, A.N. Green composites. I. physical properties of ramie fibers for environment-friendly green composites. Fibers Polym. 2006, 7, 372–379. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant fibre based bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications—a review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Liu, W.; Mohanty, A.K.; Askeland, P.; Drzal, L.T.; Misra, M. Influence of fiber surface treatment on properties of Indian grass fiber reinforced soy protein based biocomposites. Polymer 2004, 45, 7589–7596. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Gassan, J. Composites reinforced with cellulose based fibers. Prog. Polym. Sci. 1999, 24, 221–274. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Huzaifah, M.R.M.; Farid, M.A.A.; Shazleen, S.S.; Misenan, M.S.M.; Yasim-Anuar, T.A.T.; Naveen, J.; Nurazzi, N.M.; Rani, M.S.A.; Hakimi, M.I.; et al. Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts. Polymers 2021, 13, 2971. [Google Scholar] [CrossRef] [PubMed]
- Nurazzi, N.M.; Sabaruddin, F.A.; Harussani, M.M.; Kamarudin, S.H.; Rayung, M.; Asyraf, M.R.M.; Aisyah, H.A.; Norrrahim, M.N.F.; Ilyas, R.A.; Abdullah, N.; et al. Mechanical Performance and Applications of CNTs Reinforced Polymer Composites—A Review. Nanomaterials 2021, 11, 2186. [Google Scholar] [CrossRef]
- Chan, J.X.; Wong, J.F.; Petrů, M.; Hassan, A.; Nirmal, U.; Othman, N.; Ilyas, R.A. Effect of Nanofillers on Tribological Properties of Polymer Nanocomposites: A Review on Recent Development. Polymers 2021, 13, 2867. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, S.; Iyer, P.B.; Iyer, K.R.K. Influence of delignification and alkali treatment on the fine structure of coir fibres (Cocos Nucifera). J. Mater. Sci. 1996, 31, 721–726. [Google Scholar] [CrossRef]
- Gassan, J.; Bledzki, A.K. Alkali Treatment of Jute Fibers: Relationship Between. J. Appl. Polym. Sci. 1998, 71, 623–629. [Google Scholar] [CrossRef]
- Janker-Obermeier, I.; Sieber, V.; Faulstich, M.; Schieder, D. Solubilization of hemicellulose and lignin from wheat straw through microwave-assisted alkali treatment. Ind. Crops Prod. 2012, 39, 198–203. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Rafiqah, S.A.; Aisyah, H.A.; Nurazzi, N.M.; Norrrahim, M.N.F. Effect of hydrolysis time on the morphological, physical, chemical, and thermal behavior of sugar palm nanocrystalline cellulose ( Arenga pinnata (Wurmb.) Merr ). Text. Res. J. 2021, 91, 152–167. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019, 123, 379–388. [Google Scholar] [CrossRef]
- Ahmad Ilyas, R.; Mohd Sapuan, S.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Asrofi, M.; Siti Nur Atikah, M.; Muhammad Huzaifah, M.R.; Radzi, M.A.; et al. Sugar palm (Arenga pinnata (Wurmb.) Merr)cellulosic fibre hierarchy: A comprehensiveapproach from macro to nano scale. J. Mater. Res. Technol. 2019, 8, 2753–2766. [Google Scholar] [CrossRef]
- Zhao, L.; Xia, W.; Tarverdi, K.; Song, J. Biocomposite boards from wheat straw without addition of bonding agent. Mater. Sci. Technol. 2014, 30, 603–610. [Google Scholar] [CrossRef]
- USDA. World agricultural production. Ekon. APK 2021. Available online: https://www.fas.usda.gov/data/world-agricultural-production (accessed on 14 August 2021).
- Dicharry, R.M.; Ye, P.; Saha, G.; Waxman, E.; Asandei, A.D.; Parnas, R.S. Wheat gluten-thiolated poly(vinyl alcohol) blends with improved mechanical properties. Biomacromolecules 2006, 7, 2837–2844. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Gällstedt, M.; Mattozzi, A.; Johansson, E.; Hedenqvist, M.S. Transport and Tensile Properties of Compression-Molded Wheat Gluten Films. Biomacromolecules 2004, 5, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Kunanopparat, T.; Menut, P.; Morel, M.-H.; Guilbert, S. Reinforcement of plasticized wheat gluten with natural fibers: From mechanical improvement to deplasticizing effect. Compos. Part A Appl. Sci. Manuf. 2008, 39, 777–785. [Google Scholar] [CrossRef]
- Zárate-Ramírez, L.S.; Martínez, I.; Romero, A.; Partal, P.; Guerrero, A. Wheat gluten-based materials plasticised with glycerol and water by thermoplastic mixing and thermomoulding. J. Sci. Food Agric. 2011, 91, 625–633. [Google Scholar] [CrossRef]
- Domenek, S.; Feuilloley, P.; Gratraud, J.; Morel, M.-H.; Guilbert, S. Biodegradability of wheat gluten based bioplastics. Chemosphere 2004, 54, 551–559. [Google Scholar] [CrossRef]
- Edwin, A.; Habeych, N. Development of Starch-Based Materials; Wageningen University: Wageningen, The Netherlands, 2009; ISBN 9789085854333. [Google Scholar]
- Woerdeman, D.L.; Veraverbeke, W.S.; Parnas, R.S.; Johnson, D.; Delcour, J.A.; Verpoest, I.; Plummer, C.J.G. Designing New Materials from Wheat Protein. Biomacromolecules 2004, 5, 1262–1269. [Google Scholar] [CrossRef]
- Lagrain, B.; Goderis, B.; Brijs, K.; Delcour, J.A. Molecular Basis of Processing Wheat Gluten toward Biobased Materials. Biomacromolecules 2010, 11, 533–541. [Google Scholar] [CrossRef]
- Jansens, K.J.A.; Vo, N.; Telen, L.; Brijs, K.; Lagrain, B.; Willem, A.; Vuure, V.; Van Acker, K.; Verpoest, I.; Van Puyvelde, P.; et al. Effect of molding conditions and moisture content on the mechanical properties of compression molded glassy, wheat gluten bioplastics. Ind. Crops Prod. 2013, 44, 480–487. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Nehra, M.; Siroha, A.K.; Petrů, M.; Ilyas, R.A.; Devi, U.; Devi, P. Development and Characterization of Physical Modified Pearl Millet Starch-Based Films. Foods 2021, 10, 1609. [Google Scholar] [CrossRef]
- Kumari, N.; Bangar, S.P.; Petrů, M.; Ilyas, R.A.; Singh, A.; Kumar, P. Development and Characterization of Fenugreek Protein-Based Edible Film. Foods 2021, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Isotton, F.S.; Bernardo, G.L.; Baldasso, C.; Rosa, L.M.; Zeni, M. The plasticizer effect on preparation and properties of etherified corn starchs films. Ind. Crops Prod. 2015, 76, 717–724. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Physical, thermal, morphological, and tensile properties of cornstarch-based films as affected by different plasticizers. Int. J. Food Prop. 2019, 22, 925–941. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Preparation and characterization of cornhusk/sugar palm fiber reinforced Cornstarch-based hybrid composites. J. Mater. Res. Technol. 2020, 9, 200–211. [Google Scholar] [CrossRef]
- Sanyang, M.; Sapuan, S.; Jawaid, M.; Ishak, M.; Sahari, J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymers 2015, 7, 1106. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Effect of various plasticizers and concentration on the physical, thermal, mechanical, and structural properties of cassava-starch-based films. Starch/Staerke 2017, 69, 1–11. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Noomhorm, A. Effect of Plasticizers on Mechanical and Barrier Properties of Rice Starch Film. Starch 2004, 56, 348–356. [Google Scholar] [CrossRef]
- Hong-rui, C.; Hai-tao, C.; Shuang, L.; Guo-qiang, D.; Ying, Z. ScienceDirect Effect of Plasticizers on Properties of Rice Straw Fiber Film. J. Northeast Agric. Univ. 2014, 21, 67–72. [Google Scholar] [CrossRef]
- Wheat | Production, Types, Nutrition, Uses, & Facts | Britannica. Available online: https://www.britannica.com/plant/wheat (accessed on 14 August 2021).
- A Kernel of Wheat | National Festival of Breads. Available online: https://nationalfestivalofbreads.com/nutrition-education/a-kernel-of-wheat (accessed on 14 August 2021).
- The Parts of a Wheat Plant. Available online: https://sciencing.com/the-parts-of-a-wheat-plant-12211988.html (accessed on 14 August 2021).
- Wheat — Louisiana Ag in the Classroom, Reproduced with the permission of Louisiana Agriculture in the Classroom. Available online: https://aitcla.org/wheat (accessed on 14 August 2021).
- Zhang, W.; Gu, J.; Wang, Z.; Wei, C.; Yang, J.; Zhang, J. Comparison of Structural and Functional Properties of Wheat Starch under Different Soil Drought Conditions. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose–Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-Based Bionanocomposite Films Incorporated with CMC and Nanoclay. Starch/Staerke 2020, 72, 1–9. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Liu, H.; Yu, L.; Simon, G.; Zhang, X.; Dean, K.; Chen, L. Effect of annealing and pressure on microstructure of cornstarches with different amylose/amylopectin ratios. Carbohydr. Res. 2009, 344, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Zhu, T.; Jackson, D.S.; Wehling, R.L.; Geera, B. Comparison of amylose determination methods and the development of a dual wavelength iodine binding technique. Cereal Chem. 2008, 85, 51–58. [Google Scholar] [CrossRef]
- Avaro, M.R.A.; Pan, Z.; Yoshida, T.; Wada, Y. Two Alternative Methods to Predict Amylose Content of Rice Grain by Using Tristimulus CIE Lab Values and Developing a Specific Color Board Of Starch-Iodine Complex Solution. Plant Prod. Sci. 2011, 14, 164–168. [Google Scholar] [CrossRef]
- Jian, Y.; Xiaorong, Y.; Zhaoci, W.; Xiarong, Y.; Zhaoci, W. Research on method for determination of amylose content in rice. Proc. 7th Int. Work. Conf. Stored-Product Prot. 1998, 2, 1710–1714. [Google Scholar]
- Boonpo, S.; Kungwankunakorn, S. Study on Amylose Iodine Complex from Cassava Starch by Colorimetric Method. J. Adv. Agric. Technol. 2017, 4, 345–349. [Google Scholar] [CrossRef]
- Landers, P.S.; Gbur, E.E.; Sharp, R.N. Comparison of Two Models to Predict Amylose Concentration in Rice Flours as Determined by Spectrophotometric Assay. Cereal Chem. 1991, 68, 545–548. [Google Scholar]
- Cauvain, S.P. Bread Making: Improving Quality; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9781855735538. [Google Scholar]
- Chen, X.; He, X.; Fu, X.; Huang, Q. In vitro digestion and physicochemical properties of wheat starch/flour modified by heat-moisture treatment. J. Cereal Sci. 2015, 63, 109–115. [Google Scholar] [CrossRef]
- Chen, G.X.; Zhou, J.W.; Liu, Y.L.; Lu, X.B.; Han, C.X.; Zhang, W.Y.; Xu, Y.H.; Yan, Y.M. Biosynthesis and Regulation of Wheat Amylose and Amylopectin from Proteomic and Phosphoproteomic Characterization of Granule-binding Proteins. Sci. Rep. 2016, 6, 33111. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Yadav, M.P.; Liu, Y.; Chen, H.; Tatsumi, E.; Yin, L. Effects of corn fiber gum with different molecular weights on the gelatinization behaviors of corn and wheat starch. Food Hydrocoll. 2016, 53, 180–186. [Google Scholar] [CrossRef]
- De Pilli, T.; Legrand, J.; Derossi, A.; Severini, C. Effect of proteins on the formation of starch-lipid complexes during extrusion cooking of wheat flour with the addition of oleic acid. Int. J. Food Sci. Technol. 2015, 50, 515–521. [Google Scholar] [CrossRef]
- Wang, S.; Luo, H.; Zhang, J.; Zhang, Y.; He, Z.; Wang, S. Alkali-induced changes in functional properties and in vitro digestibility of wheat starch: The role of surface proteins and lipids. J. Agric. Food Chem. 2014, 62, 3636–3643. [Google Scholar] [CrossRef] [PubMed]
- Dengate, H.N.; Baruch, D.W.; Meredith, P. The Density of Wheat Starch Granules: A Tracer Dilution Procedure for Determining the Density of an Immiscible Dispersed Phase. Starch-Stärke 1978, 30, 80–84. [Google Scholar] [CrossRef]
- Jang, J.K.; Pyun, Y.R. Effect of moisture content on the melting of wheat starch. Starch/Staerke 1996, 48, 48–51. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Zakaria, N.H.; Muhammad, N.; Abdullah, M.M.A.B. Potential of Starch Nanocomposites for Biomedical Applications. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012087. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.H.; Chang, Y.H. Effect of tara gum addition on steady and dynamic shear rheological properties of rice starch isolated from the Korean rice variety “Boramchan”. Prev. Nutr. Food Sci. 2018, 23, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Puncha-arnon, S.; Uttapap, D. Rice starch vs. rice flour: Differences in their properties when modified by heat-moisture treatment. Carbohydr. Polym. 2013, 91, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tharise, N.; Julianti, E.; Nurminah, M. Evaluation of physico-chemical and functional properties of composite flour from cassava, rice, potato, soybean and xanthan gum as alternative of wheat flour. Int. Food Res. J. 2014, 21, 1641–1649. [Google Scholar]
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Gifuni, I.; Olivieri, G.; Krauss, I.R.; D’Errico, G.; Pollio, A.; Marzocchella, A. Microalgae as new sources of starch: Isolation and characterization of microalgal starch granules. Chem. Eng. Trans. 2017, 57, 1423–1428. [Google Scholar] [CrossRef]
- Zhang, Z.; Saleh, A.S.M.; Wu, H.; Gou, M.; Liu, Y.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Li, W. Effect of Starch Isolation Method on Structural and Physicochemical Properties of Acorn Kernel Starch. Starch/Staerke 2020, 72, 1900122. [Google Scholar] [CrossRef]
- Ali, A.; Wani, T.A.; Wani, I.A.; Masoodi, F.A. Comparative study of the physico-chemical properties of rice and corn starches grown in Indian temperate climate. J. Saudi Soc. Agric. Sci. 2016, 15, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of glycerol on physical and mechanical properties of wheat starch edible films. J. Texture Stud. 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Xu, C.; Zhou, X. Morphological features and physicochemical properties of waxy wheat starch. Int. J. Biol. Macromol. 2013, 62, 304–309. [Google Scholar] [CrossRef]
- Zelaziński, T.; Ekielski, A.; Tulska, E.; Vladut, V.; Durczak, K. Wood dust application for improvement of selected properties of thermoplastic starch. INMATEH-Agric. Eng. 2019, 58, 37–44. [Google Scholar] [CrossRef]
- Fahma, F.; Sunarti, T.C.; Indriyani, S.M.; Lisdayana, N. Thermoplastic Cassava Starch-PVA Composite Films with Cellulose Nanofibers from Oil Palm Empty Fruit Bunches as Reinforcement Agent. Int. J. Polym. Sci. 2017, 2017, 2745721. [Google Scholar] [CrossRef] [Green Version]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Liu, H.; Corke, H.; Ramsden, L. Functional properties and enzymatic digestibility of cationic and cross-linked cationic ae, wx, and normal maize starch. J. Agric. Food Chem. 1999, 47, 2523–2528. [Google Scholar] [CrossRef]
- Jane, J.-L.; Kasemsuwan, T.; Leas, S.; Zobel, H.; Robyt, J.F. Anthology of starch granule morphology by scanning electron microscopy. Starch-Stärke 1994, 46, 121. [Google Scholar] [CrossRef]
- Kim, H.S.; Huber, K.C. Physicochemical properties and amylopectin fine structures of A- and B-type granules of waxy and normal soft wheat starch. J. Cereal Sci. 2010, 51, 256–264. [Google Scholar] [CrossRef]
- Kim, H.S.; Huber, K.C. Channels within soft wheat starch A- and B-type granules. J. Cereal Sci. 2008, 48, 159–172. [Google Scholar] [CrossRef]
- Properties and Biodegradation Nature of Thermoplastic Starch. Available online: https://books.google.com.hk/books?hl=zh-TW&lr=&id=e8qgDwAAQBAJ&oi=fnd&pg=PA57&dq=Properties+and+Biodegradation+Nature+of+Thermoplastic+Starch&ots=zPn6_EwbF6&sig=qfWSktlyiYCQanAQG5YwYYZgjjg&redir_esc=y#v=onepage&q=Properties%20and%20Biodegradation%20Nature%20of%20Thermoplastic%20Starch&f=false (accessed on 15 August 2021).
- Oromiehie, A.R.; Taherzadeh, T.; Rabiee, A. Physical and Thermal Mechanical Properties of Corn Starch/LDPE Composites. J. Appl. Polym. Sci. 2013, 127, 1128–1134. [Google Scholar] [CrossRef]
- Sondari, D.; Falah, F.; Suryaningrum, R.; Sari, F.P.; Sari, F.P.; Septefani, A.A.; Septefani, A.A.; Restu, W.K.; Restu, W.K.; Sampora, Y.; et al. Biofilm Based on Modified Sago Starch: Preparation and Characterization. Reaktor 2019, 19, 125–130. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of starch composition and molecular weight on physicochemical properties of biodegradable films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Li, Y.; Liang, W.; Liu, L.; Li, S.; Xue, J.; Guo, D. Comparison of gelatinization method, starch concentration, and plasticizer on physical properties of high-amylose starch films. J. Food Process Eng. 2017, 41, e12645. [Google Scholar] [CrossRef]
- Luchese, C.; Garrido, T.; Spada, J.; Tessaro, I.; De la Caba, K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2017, 106, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhang, J.; Cheng, F. Effects of starches from different botanical sources and modification methods on physicochemical properties of starch-based edible films. Int. J. Biol. Macromol. 2019, 132, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Song, X.; Chen, F.; Shen, Z. Physical and structural characterization of edible bilayer films made with zein and corn-wheat starch. J. Saudi Soc. Agric. Sci. 2019, 18, 324–331. [Google Scholar] [CrossRef]
- Mali, S.; Karam, L.B.; Ramos, L.P.; Grossmann, M.V.E. Relationships among the composition and physicochemical properties of starches with the characteristics of their films. J. Agric. Food Chem. 2004, 52, 7720–7725. [Google Scholar] [CrossRef] [PubMed]
- Karak, N. 10—Vegetable oil-based polymer composites. In Vegetable Oil-Based Polymers: Properties, Processing and Applications; Woodhead Publishing: Cambridge, UK, 2012; pp. 247–270. ISBN 978-0-85709-710-1. [Google Scholar]
- Sui, Z.; Yao, T.; Zhao, Y.; Ye, X.; Kong, X.; Ai, L. Effects of heat-moisture treatment reaction conditions on the physicochemical and structural properties of maize starch: Moisture and length of heating. Food Chem. 2015, 173, 1125–1132. [Google Scholar] [CrossRef]
- Krieger, K.M.; Duvick, S.A.; Pollak, L.M.; White, P.J. Thermal properties of corn starch extracted with different blending methods: Microblender and homogenizer. Cereal Chem. 1997, 74, 553–555. [Google Scholar] [CrossRef] [Green Version]
- Alemdar, A.; Sain, M. Biocomposites from wheat straw nanofibers: Morphology, thermal and mechanical properties. Compos. Sci. Technol. 2008, 68, 557–565. [Google Scholar] [CrossRef]
- Zeng, J.; Gao, H.; Li, G. Functional properties of wheat starch with different particle size distribution. J. Sci. Food Agric. 2014, 94, 57–62. [Google Scholar] [CrossRef]
- Taylor, J.R.N. Chapter 1—Sorghum and Millets: Taxonomy, History, Distribution, and Production. In Sorghum and Millets: Chemistry, Technology, and Nutritional Attributes, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; AACC International Press: Duxford, UK, 2019; pp. 1–21. ISBN 978-0-12-811527-5. [Google Scholar]
- Nascimento, T.A.; Calado, V.; Carvalho, C.W.P. Development and characterization of flexible film based on starch and passion fruit mesocarp flour with nanoparticles. Food Res. Int. 2012, 49, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Wang, X.; Cheng, M. Preparation and characterization of potato starch film with various size of Nano-SiO2. Polymers 2018, 10, 1172. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Sun, C.; Xiong, L. Mechanical, barrier and morphological properties of pea starch and peanut protein isolate blend films. Carbohydr. Polym. 2013, 98, 630–637. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Patricia Miranda, S.; Garnica, O.; Lara-Sagahon, V.; Cárdenas, G. Water Vapor Permeability and Mechanical Properties of Chitosan Composite Films. J. Chil. Chem. Soc. 2004, 49, 173–178. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Castro Vidaurre, E.F.; Armada, M.; Gottifredi, J.C. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Guo, X.; Lu, Y.; Cui, H.; Jia, X.; Bai, H.; Ma, Y. Factors Affecting the Physical Properties of Edible Composite Film Prepared from Zein and Wheat Gluten. Molecules 2012, 17, 3794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Kester, J.J.; Fennema, O.R. Edible films and coatings: A review. Food Technol. 1986, 40, 47–59. [Google Scholar]
- Henrique, C.M.; Teófilo, R.F.; Sabino, L.; Ferreira, M.M.C.; Cereda, M.P. Classification of Cassava Starch Films by Physicochemical Properties and Water Vapor Permeability Quantification by FTIR and PLS. J. Food Sci. 2007, 72, E184–E189. [Google Scholar] [CrossRef]
- Bénière, F.; Bocquet, J.L.; Brébec, G.; Limoge, Y. Diffusion in Solids. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Ed.; Elsevier: Oxford, UK, 2001; pp. 2159–2170. ISBN 978-0-08-043152-9. [Google Scholar]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-Ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.J.; Donald, A.M. Gelatinisation of starch: A combined SAXS/WAXS/DSC and SANS study. Carbohydr. Res. 1998, 308, 133–147. [Google Scholar] [CrossRef]
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced material applications of starch and its derivatives. Eur. Polym. J. 2018, 108, 570–581. [Google Scholar] [CrossRef]
- Ruiz, E.; Srikaeo, K.; la Revilla, L.S. Effects of Heat Moisture Treatment on Physicochemical Properties and Starch Digestibility of Rice Flours Differing in Amylose Content. Food Appl. Biosci. J. 2018, 6, 140–153. [Google Scholar]
- Rindlav-Westling, Å.; Stading, M.; Gatenholm, P. Crystallinity and morphology in films of starch, amylose and amylopectin blends. Biomacromolecules 2002, 3, 84–91. [Google Scholar] [CrossRef]
- Pouplin, M.; Redl, A.; Gontard, N. Glass transition of wheat gluten plasticized with water, glycerol, or sorbitol. J. Agric. Food Chem. 1999, 47, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Patni, N.; Yadava, P.; Agarwal, A.; Maroo, V. Study on Wheat Gluten Biopolymer: A Novel Way to Eradicate Plastic Waste. Indian J. Appl. Res. 2011, 3, 253–255. [Google Scholar] [CrossRef]
- ROY, S.B.; SHIT, D.S.C.; SEN GUPTA, D.R.A.; SHUKLA, D.P.R. A Review on Bio-Composites: Fabrication, Properties and Applications. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 03, 16814–16824. [Google Scholar] [CrossRef]
- Vo Hong, N.; Van Puyvelde, P.; Van Vuure, A.W.; Verpoest, I. Preparation of biocomposites based on gluten resin and unidirectional flax fibers. In Proceedings of the 15th European Conference on Composite Materials (ECCM 2012), Venice, Italy, 24–28 June 2012. [Google Scholar]
- Hou, T.H.; Su, C.H.; Liu, W.L. Parameters optimization of a nano-particle wet milling process using the Taguchi method, response surface method and genetic algorithm. Powder Technol. 2007, 173, 153–162. [Google Scholar] [CrossRef]
- Hong, N.V.; Pyka, G.; Wevers, M.; Goderis, B.; Van Puyvelde, P.; Verpoest, I.; Van Vuure, A.W. Processing rigid wheat gluten biocomposites for high mechanical performance. Compos. Part A 2015, 79, 74–81. [Google Scholar] [CrossRef]
- Kim, J.T.; Netravali, A.N. Mechanical, thermal, and interfacial properties of green composites with ramie fiber and soy resins. J. Agric. Food Chem. 2010, 58, 5400–5407. [Google Scholar] [CrossRef] [PubMed]
- Zelaziński, T.; Słoma, J.; Skudlarski, J.; Ekielski, A. The rape pomace and microcrystalline cellulose composites made by press processing. Sustainability 2020, 12, 1311. [Google Scholar] [CrossRef] [Green Version]
- Hemsri, S.; Thongpin, C.; Supatti, N.; Manomai, P.; Socharoentham, A. Bio-based Blends of Wheat Gluten and Maleated Natural Rubber: Morphology, Mechanical Properties and Water Absorption. Energy Procedia 2016, 89, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Cassava/sugar palm fiber reinforced cassava starch hybrid composites: Physical, thermal and structural properties. Int. J. Biol. Macromol. 2017, 101, 75–83. [Google Scholar] [CrossRef]
- Lee, J.; Cousineau, A. Production and Characterization of Wheat Gluten Films. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2012. [Google Scholar]
- Mojumdar, S.C.; Moresoli, C.; Simon, L.C.; Legge, R.L. Edible wheat gluten (WG) protein films. J. Therm. Anal. Calorim. J. Therm. Anal. Calorim. 2011, 104, 929–936. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, L.; Zhang, B.; He, M.; Shi, W.; Yang, S.; Cui, Y.; Yin, G. Preparation and characterization of edible dialdehyde carboxymethyl cellulose crosslinked feather keratin films for food packaging. Polymers 2020, 12, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, P.; Reitz, L.; Horan, C.; Parnas, R. Manufacture and biodegradation of wheat gluten/basalt composite material. J. Polym. Environ. 2006, 14, 1–7. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Biocomposites developed using water-plasticized wheat gluten as matrix and jute fibers as reinforcement. Polym. Int. 2011, 60, 711–716. [Google Scholar] [CrossRef] [Green Version]
- Muneer, F.; Johansson, E.; Hedenqvist, M.S.; Gällstedt, M.; Newson, W.R. Preparation, properties, protein cross-linking and biodegradability of plasticizer-solvent free hemp fibre reinforced wheat gluten, glutenin, and gliadin composites. BioResources 2014, 9, 5246–5261. [Google Scholar] [CrossRef] [Green Version]
- Kunanopparat, T.; Menut, P.; Morel, M.H.; Guilbert, S. Plasticized wheat gluten reinforcement with natural fibers: Effect of thermal treatment on the fiber/matrix adhesion. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1787–1792. [Google Scholar] [CrossRef]
- Hemsri, S.; Grieco, K.; Asandei, A.D.; Parnas, R.S. Wheat gluten composites reinforced with coconut fiber. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1160–1168. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Thuy Mai, T.T.; Nguyen, N.B.; Dang, T.D.; Phung Le, M.L.; Dang, T.T. A novel method for preparing microfibrillated cellulose from bamboo fibers. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 015016. [Google Scholar] [CrossRef]
- Tan, M.Y.; Nicholas Kuan, H.T.; Lee, M.C. Characterization of Alkaline Treatment and Fiber Content on the Physical, Thermal, and Mechanical Properties of Ground Coffee Waste/Oxobiodegradable HDPE Biocomposites. Int. J. Polym. Sci. 2017, 2017, 6258151. [Google Scholar] [CrossRef] [Green Version]
- Harwalkar, C.Y.M. Thermal analysis of food carbohydrates FCA. Elsevier Applied Science: London, UK, 1990; pp. 168–222. [Google Scholar]
- Rouilly, A.; Rigal, L. Agro-materials: A bibliographic review. J. Macromol. Sci.-Polym. Rev. 2002, 42, 441–479. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zheng, Q. Improved tensile strength of glycerol-plasticized gluten bioplastic containing hydrophobic liquids. Bioresour. Technol. 2008, 99, 7665–7671. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Dicharry, R.; Waxman, E.; Parnas, R.S.; Asandei, A.D. Imaging and thermal studies of wheat gluten/poly(vinyl alcohol) and wheat gluten/thiolated poly(vinyl alcohol) blends. Biomacromolecules 2008, 9, 568–573. [Google Scholar] [CrossRef]
- Mangavel, C.; Rossignol, N.; Perronnet, A.; Barbot, J.; Popineau, Y.; Guéguen, J. Properties and microstructure of thermo-pressed wheat gluten films: A comparison with cast films. Biomacromolecules 2004, 5, 1596–1601. [Google Scholar] [CrossRef] [Green Version]
- Kayserilioǧlu, B.Ş.; Bakir, U.; Yilmaz, L.; Akkaş, N. Drying temperature and relative humidity effects on wheat gluten film properties. J. Agric. Food Chem. 2003, 51, 964–968. [Google Scholar] [CrossRef]
- Muensri, P.; Kunanopparat, T.; Menut, P.; Siriwattanayotin, S. Composites: Part A Effect of lignin removal on the properties of coconut coir fiber / wheat gluten biocomposite. Compos. Part A 2011, 42, 173–179. [Google Scholar] [CrossRef]
- Chen, L.; Reddy, N.; Wu, X.; Yang, Y. Thermoplastic films from wheat proteins. Ind. Crops Prod. 2012, 35, 70–76. [Google Scholar] [CrossRef]
- Zubeldía, F.; Ansorena, M.R.; Marcovich, N.E. Wheat gluten films obtained by compression molding. Polym. Test. 2015, 43, 68–77. [Google Scholar] [CrossRef]
- Gianibelli, M.C.; Larroque, O.R.; MacRitchie, F.; Wrigley, C.W. Biochemical, genetic, and molecular characterization of wheat glutenin and its component subunits. Cereal Chem. 2001, 78, 635–646. [Google Scholar] [CrossRef]
- “RightFiber Wheat Fiber | The Ingredient House.”. Available online: https://theingredienthouse.com/product/wheat-fiber/ (accessed on 27 October 2020).
- Huang, J.; Yu, C. Determination of cellulose, hemicellulose and lignin content using near-infrared spectroscopy in flax fiber. Text. Res. J. 2019, 89, 4875–4883. [Google Scholar] [CrossRef]
- The Canadian Society for Bioengineering Qualitative and Quantitative Analysis of Lignocellulosic Biomass using Infrared Spectroscopy. Available online: https://library.csbe-scgab.ca/docs/meetings/2009/CSBE09307.pdf (accessed on 14 August 2021).
- Wolfrum, E.J.; Lorenz, A.J.; deLeon, N. Correlating detergent fiber analysis and dietary fiber analysis data for corn stover collected by NIRS. Cellulose 2009, 16, 577–585. [Google Scholar] [CrossRef]
- Hindrichsen, I.K.; Kreuzer, M.; Madsen, J.; Bach Knudsen, K.E. Fiber and lignin analysis in concentrate, forage, and feces: Detergent versus enzymatic-chemical method. J. Dairy Sci. 2006, 89, 2168–2176. [Google Scholar] [CrossRef]
- Sain, M.; Panthapulakkal, S. Bioprocess preparation of wheat straw fibers and their characterization. Ind. Crops Prod. 2006, 23, 1–8. [Google Scholar] [CrossRef]
- Monta, B.; Ghizzi, G.; Silva, D.; Gastaldi, E.; Torres-chávez, P.; Gontard, N.; Angellier-coussy, H. Biocomposites from wheat proteins and fibers: Structure/mechanical properties relationships. Ind. Crops Prod. 2013, 43, 545–555. [Google Scholar] [CrossRef]
- Zou, Y.; Huda, S.; Yang, Y. Lightweight composites from long wheat straw and polypropylene web. Bioresour. Technol. 2010, 101, 2026–2033. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, R.; Misra, M.; Erickson, L.; Mohanty, A. Compostability and biodegradation study of PLA-wheat straw and PLA-soy straw based green composites in simulated composting bioreactor. Bioresour. Technol. 2010, 101, 8489–8491. [Google Scholar] [CrossRef]
- Le Digabel, F.; Boquillon, N.; Dole, P.; Monties, B.; Averous, L. Properties of thermoplastic composites based on wheat-straw lignocellulosic fillers. J. Appl. Polym. Sci. 2004, 93, 428–436. [Google Scholar] [CrossRef]
- Mengeloglu, F.; Karakus, K. Thermal degradation, mechanical properties and morphology of wheat straw flour filled recycled thermoplastic composites. Sensors 2008, 8, 500. [Google Scholar] [CrossRef] [Green Version]
- Karr, G.S.; Cheng, E.; Sun, X.S. Physical properties of strawboard as affected by processing parameters. Ind. Crops Prod. 2000, 12, 19–24. [Google Scholar] [CrossRef]
- Zhao, L. Novel Bio-Composites Based on Whole Utilisation of Wheat Straw. Ph.D. Thesis, Brunel University, London, UK, 2013. [Google Scholar]
- A Biocomposite Made with Wheat Bran | JEC Group. Available online: http://www.jeccomposites.com/knowledge/international-composites-news/biocomposite-made-wheat-bran (accessed on 28 October 2020).
- Fama, L.; Bittante, A.M.B.Q.; Sobral, P.J.A.; Goyanes, S.; Gerschenson, L.N. Garlic powder and wheat bran as fillers: Their effect on the physicochemical properties of edible biocomposites. Mater. Sci. Eng. C 2010, 30, 853–859. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Wu, H.J.; Wu, M.; Huang, Z.G.; Zhang, M. Effect of Wheat Bran Fiber on the Behaviors of Maize Starch Based Films. Starch/Staerke 2020, 72, 1900319. [Google Scholar] [CrossRef]
- Oishi, Y.; Nakaya, M.; Matsui, E.; Hotta, A. Structural and mechanical properties of cellulose composites made of isolated cellulose nanofibers and poly(vinyl alcohol). Compos. Part A Appl. Sci. Manuf. 2015, 73, 72–79. [Google Scholar] [CrossRef]
- Wei, X.; Wei, W.; Cui, Y.H.; Lu, T.J.; Jiang, M.; Zhou, Z.W.; Wang, Y. All-cellulose composites with ultra-high mechanical properties prepared through using straw cellulose fiber. RSC Adv. 2016, 6, 93428–93435. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, chemical and surface properties of wheat husk, rye husk and soft wood and their polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Jagwani, D.; Joshi, P. Deportation of Toxic Phenol From Aqueous System by Wheat Husk. Int. J. Plant Anim. Environ. Sci. 2014, 4, 58–64. [Google Scholar]
- Mendes, C.A.D.C.; Adnet, F.A.D.O.; Leite, M.C.A.M.; Furtado, C.R.G.; Sousa, A.M.F. De Chemical, physical, mechanical, thermal and morphological characterization of corn husk residue. Cellul. Chem. Technol. 2014, 49, 727–735. [Google Scholar]
- Pandecha, K.; Pongtornkulpanich, A.; Sukchai, S. Thermal properties of corn husk fiber as insulation for flat plate solar collector. J. Renew. Energy Smart Grid Technol. 2015, 10, 27–36. [Google Scholar]
- Norashikin, M.Z.; Ibrahim, M.Z. The potential of natural waste (corn husk) for production of environmental friendly biodegradable film for seedling. World Acad. Sci. Eng. Technol. 2009, 58, 176–180. [Google Scholar] [CrossRef]
- Table 1 | Physical and Acoustical Properties of Corn Husk Fiber Panels. Available online: https://www.hindawi.com/journals/aav/2016/5971814/tab1/ (accessed on 8 June 2020).
- Nordin, R.; Ismail, H. Properties of Rice Husk Powder/Natural Rubber Composite. Solid State Sci. Technol. 2007, 15, 83–91. [Google Scholar]
- Faisal Bukhori, M.S.D. Effect of Rice Husk Waste and Rice Husk Ash Composition as Filler in Plastic Bottle Drink Waste Composites on Water Absorption Properties. Int. J. Sci. Res. 2015, 4, 2146–2148. [Google Scholar]
- Gummert, M.; Van Hung, N.; Chivenge, P.; Douthwaite, B. Sustainable Rice Straw Management. 2020. Springer International Publishing: Midtown Manhattan, NY, USA, 2020; pp. 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, M.; Panwar, D.; Kaira, G.S. Bioprocesses for Enzyme Production Using Agro-Industrial Wastes: Technical Challenges and Commercialization Potential; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128026120. [Google Scholar]
- Galanakis, C.M. Food Waste Recovery: Processing Technologies and Industrial Techniques; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128004197. [Google Scholar]
- Lam, P.S.; Sokhansanj, S.; Bi, X.; Mani, S.; Lim, C.J.; Womac, A.R.; Hoque, M.; Peng, J.; Jayashankar, T.; Nalmi, L.J.; et al. Physical characterization of wet and dry wheat straw and switchgrass—Bulk and specific density. In Proceedings of the 2007 ASAE Annual Meeting, Minneapolis, MN, USA, 17–20 June 2007. [Google Scholar] [CrossRef]
- Jiang, D.; An, P.; Cui, S.; Sun, S.; Zhang, J.; Tuo, T. Effect of Modification Methods of Wheat Straw Fibers on Water Absorbency and Mechanical Properties of Wheat Straw Fiber Cement-Based Composites. Adv. Mater. Sci. Eng. 2020, 2020, 5031025. [Google Scholar] [CrossRef] [Green Version]
- Bouasker, M.; Belayachi, N.; Hoxha, D.; Al-Mukhtar, M. Physical characterization of natural straw fibers as aggregates for construction materials applications. Materials 2014, 7, 3034. [Google Scholar] [CrossRef] [Green Version]
- Passoth, V.; Sandgren, M. Biofuel production from straw hydrolysates: Current achievements and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5105–5116. [Google Scholar] [CrossRef] [Green Version]
- Rehman, N.; de Miranda, M.I.G.; Rosa, S.M.L.; Pimentel, D.M.; Nachtigall, S.M.B.; Bica, C.I.D. Cellulose and Nanocellulose from Maize Straw: An Insight on the Crystal Properties. J. Polym. Environ. 2014, 22, 252–259. [Google Scholar] [CrossRef]
- Li, Y.; Yan, F.; Li, T.; Zhou, Y.; Jiang, H.; Qian, M.; Xu, Q. High-solid anaerobic digestion of corn straw for methane production and pretreatment of bio-briquette. Bioresour. Technol. 2018, 250, 741–749. [Google Scholar] [CrossRef]
- Appendix XI: Bulk Density, Pelletability and Particle Size. Available online: http://www.fao.org/3/S4314E/s4314e0q.htm (accessed on 28 October 2020).
- Onipe, O.O.; Beswa, D.; Jideani, A.I.O. Effect of size reduction on colour, hydration and rheological properties of wheat bran. Food Sci. Technol. 2017, 37, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Mayo, B. The proteolytic system of lactic acid bacteria. Microbiologia 1993, 9, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Song, J.; Li, Y.; Li, P.; Wang, H. Robust and cost-saving static solid cultivation method for lipid production using the chlamydospores of Phanerochaete chrysosporium. Biotechnol. Biofuels 2019, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Matavire, T.O. Bran to produce entrapment materials for the controlled by Master of Engineering (Chemical Engineering). 2018. Available online: https://www.semanticscholar.org/paper/Extraction-and-modification-of-hemicellulose-from_Matavire/09d110349e4453a83d864a6c73183a5b7e2c9556?sort=is-influential&pdf=true (accessed on 8 June 2020).
- Nagarajan, S.; Nagarajan, R.; Kumar, J.; Salemme, A.; Togna, A.R.; Saso, L.; Bruno, F. Antioxidant activity of synthetic polymers of phenolic compounds. Polymers 2020, 12, 1646. [Google Scholar] [CrossRef]
- Eça, K.S.; Sartori, T.; Menegalli, F.C. Films and edible coatings containing antioxidants—A review. Braz. J. Food Technol. 2014, 17, 98–112. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, M.A.; Shaffei, K.A.; Abdelwahab, N. Evaluation of some conducting polymers as novel antioxidants for rubber vulcanizates. Int. J. Polym. Sci. 2014, 2014, 893542. [Google Scholar] [CrossRef]
- Jacob, J.; Thomas, S.; Loganathan, S.; Valapa, R.B. Chapter 10—Antioxidant incorporated biopolymer composites for active packaging. In Processing and Development of Polysaccharide-Based Biopolymers for Packaging Applications; Zhang, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 239–260. ISBN 978-0-12-818795-1. [Google Scholar]
- Cheremisinoff, N.P. Condensed Encyclopedia of polymer Engineering Terms. Choice Rev. Online 2001, 39, 1288. [Google Scholar]
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch-chitosan films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Yilmaz-Turan, S.; Jiménez-Quero, A.; Menzel, C.; de Carvalho, D.M.; Lindström, M.E.; Sevastyanova, O.; Moriana, R.; Vilaplana, F. Bio-based films from wheat bran feruloylated arabinoxylan: Effect of extraction technique, acetylation and feruloylation. Carbohydr. Polym. 2020, 250, 116916. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Antimicrobial polymer-based materials for food packaging applications. Polymers 2020, 12, 731. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-K.; Bae, D.H. Antimicrobial properties of wheat gluten-chitosan composite film in intermediate-moisture food systems. Food Sci. Biotechnol. 2006, 15, 133–137. [Google Scholar]
- Güçbilmez, Ç.M.; Yemenicioǧlu, A.; Arslanoǧlu, A. Antimicrobial and antioxidant activity of edible zein films incorporated with lysozyme, albumin proteins and disodium EDTA. Food Res. Int. 2007, 40, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Pintado, C.; Ferreira, S.; Sousa, I. Properties of Whey Protein-Based Films Containing Organic Acids and Nisin To Control Listeria monocytogenes. J. Food Prot. 2009, 72, 1891–1896. [Google Scholar] [CrossRef] [Green Version]
- Türe, H.; Eroglu, E.; Soyer, F.; Özen, B. Antifungal activity of biopolymers containing natamycin and rosemary extract against Aspergillus niger and Penicillium roquefortii. Int. J. Food Sci. Technol. 2008, 43, 2026–2032. [Google Scholar] [CrossRef] [Green Version]
- Pranoto, Y.; Rakshit, S.K.; Salokhe, V.M. Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT-Food Sci. Technol. 2005, 38, 859–865. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Sivarooban, T.; Hettiarachchy, N.S.; Johnson, M.G. Physical and antimicrobial properties of grape seed extract, nisin, and EDTA incorporated soy protein edible films. Food Res. Int. 2008, 41, 781–785. [Google Scholar] [CrossRef]
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J. Food Sci. Technol. 2018, 55, 1953–1959. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A. Application of encapsulated essential oils as antimicrobial agents in food packaging. Curr. Opin. Food Sci. 2017, 14, 78–84. [Google Scholar] [CrossRef]
- Kan, Y.; Chen, T.; Wu, Y.; Wu, J.; Wu, J. Antioxidant activity of polysaccharide extracted from Ganoderma lucidum using response surface methodology. Int. J. Biol. Macromol. 2015, 72, 151–157. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; El Gendy, A.E.N.G.S.; Sendra, E.; Fernández-López, J.; El Razik, K.A.A.; Omer, E.A.; Pérez-Alvarezj, J.A. Chemical composition and antioxidant and anti-Listeria activities of essential oils obtained from some Egyptian plants. J. Agric. Food Chem. 2010, 58, 9063–9070. [Google Scholar] [CrossRef] [PubMed]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Nazrin, A.; Sherwani, S.F.K.; Khalina, A. Antimicrobial activities of starch-based biopolymers and biocomposites incorporated with plant essential oils: A review. Polymers 2020, 12, 2403. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the physical properties, antioxidant and antimicrobial activity of ternary potato starch-furcellaran-gelatin films incorporated with lavender essential oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef]
- Türe, H.; Gällstedt, M.; Hedenqvist, M.S. Antimicrobial compression-moulded wheat gluten films containing potassium sorbate. Food Res. Int. 2012, 45, 109–115. [Google Scholar] [CrossRef]
- Khairuddin, N.; Muhamad, I.I.; Abd Rahman, W.A.W.; Siddique, B.M. Physicochemical and thermal characterization of hydroxyethyl cellulose—Wheat starch based films incorporated thymol intended for active packaging. Sains Malays. 2020, 49, 323–333. [Google Scholar] [CrossRef]
- Rozilah, A.; Jaafar, C.N.A.; Sapuan, S.M.; Zainol, I.; Ilyas, R.A. The Effects of Silver Nanoparticles Compositions on the Mechanical, Physiochemical, Antibacterial, and Morphology Properties of Sugar Palm Starch Biocomposites for Antibacterial Coating. Polymers 2020, 12, 2605. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atiqah, A.; Atikah, M.S.N.; Syafri, E.; Asrofi, M.; et al. Thermal, Biodegradability and Water Barrier Properties of Bio-Nanocomposites Based on Plasticised Sugar Palm Starch and Nanofibrillated Celluloses from Sugar Palm Fibres. J. Biobased Mater. Bioenergy 2020, 14, 234–248. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Development and characterization of sugar palm nanocrystalline cellulose reinforced sugar palm starch bionanocomposites. Carbohydr. Polym. 2018, 202, 186–202. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atikah, M.S.N.; Mohd Nurazzi, N.; Atiqah, A.; Ansari, M.N.M.; et al. Effect of sugar palm nanofibrillated celluloseconcentrations on morphological, mechanical andphysical properties of biodegradable films basedon agro-waste sugar palm (Arenga pinnata(Wurmb.) Merr) starch. J. Mater. Res. Technol. 2019, 8, 4819–4830. [Google Scholar] [CrossRef]
- Syafri, E.; Sudirman; Mashadi; Yulianti, E.; Deswita; Asrofi, M.; Abral, H.; Sapuan, S.M.; Ilyas, R.A.; Fudholi, A. Effect of sonication time on the thermal stability, moisture absorption, and biodegradation of water hyacinth (Eichhornia crassipes) nanocellulose-filled bengkuang (Pachyrhizus erosus) starch biocomposites. J. Mater. Res. Technol. 2019, 8, 6223–6231. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Pratama, A.B.; Fajri, N.; Sapuan, S.M.; Ilyas, R.A. Transparent and antimicrobial cellulose film from ginger nanofiber. Food Hydrocoll. 2020, 98, 105266. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sapuan, S.M.; Ilyas, R.A. Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym. Test. 2020, 81, 106186. [Google Scholar] [CrossRef]
- Kumar, T.S.M.; Chandrasekar, M.; Senthilkumar, K.; Ilyas, R.A.; Sapuan, S.M.; Hariram, N.; Rajulu, A.V.; Rajini, N.; Siengchin, S. Characterization, Thermal and Antimicrobial Properties of Hybrid Cellulose Nanocomposite Films with in-Situ Generated Copper Nanoparticles in Tamarindus indica Nut Powder. J. Polym. Environ. 2021, 29, 1134–1142. [Google Scholar] [CrossRef]
- Asrofi, M.; Sapuan, S.M.; Ilyas, R.A.; Ramesh, M. Characteristic of composite bioplastics from tapioca starch and sugarcane bagasse fiber: Effect of time duration of ultrasonication (Bath-Type). Mater. Today Proc. 2021, 46, 1626–1630. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A.; Sherwani, S.F.K.; Yusuf, J.; Ilyas, R.A. Recent developments in sustainable arrowroot (Maranta arundinacea Linn) starch biopolymers, fibres, biopolymer composites and their potential industrial applications: A review. J. Mater. Res. Technol. 2021, 13, 1191–1219. [Google Scholar] [CrossRef]
- Jumaidin, R.; Diah, N.A.; Ilyas, R.A.; Alamjuri, R.H.; Yusof, F.A.M. Processing and Characterisation of Banana Leaf Fibre Reinforced Thermoplastic Cassava Starch Composites. Polymers 2021, 13, 1420. [Google Scholar] [CrossRef]
- Aisyah, H.A.; Paridah, M.T.; Sapuan, S.M.; Ilyas, R.A.; Khalina, A.; Nurazzi, N.M.; Lee, S.H.; Lee, C.H. A comprehensive review on advanced sustainable woven natural fibre polymer composites. Polymers 2021, 13, 471. [Google Scholar] [CrossRef]
- Azammi, A.M.N.; Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Atikah, M.S.N.; Asrofi, M.; Atiqah, A. Characterization studies of biopolymeric matrix and cellulose fibres based composites related to functionalized fibre-matrix interface. In Interfaces in Particle and Fibre Reinforced Composites—From Macro to Nano Scales; Woodhead Publishing: London, UK, 2019; pp. 1–68. ISBN 9780081026656. [Google Scholar]
- Domenek, S.; Brendel, L.; Morel, M.-H.; Guilbert, S. Influence of Degree of Protein Aggregation on Mass Transport Through Wheat Gluten Membranes and Their Digestibility—An In Vitro Study. Cereal Chem. J. 2004, 81, 423–428. [Google Scholar] [CrossRef]
- Guillaume, C.; Pinte, J.; Gontard, N.; Gastaldi, E. Wheat gluten-coated papers for bio-based food packaging: Structure, surface and transfer properties. Food Res. Int. 2010, 43, 1395–1401. [Google Scholar] [CrossRef]
- Gontard, N.; Thibault, R.; Cuq, B.; Guilbert, S. Influence of Relative Humidity and Film Composition on Oxygen and Carbon Dioxide Permeabilities of Edible Films. J. Agric. Food Chem. 1996, 44, 1064–1069. [Google Scholar] [CrossRef]
- Chalier, P.; Peyches-Bach, A.; Gastaldi, E.; Gontard, N. Effect of Concentration and Relative Humidity on the Transfer of Alkan-2-ones through Paper Coated with Wheat Gluten. J. Agric. Food Chem. 2007, 55, 867–875. [Google Scholar] [CrossRef]
- Angellier-Coussy, H.; Torres-Giner, S.; Morel, M.-H.; Gontard, N.; Gastaldi, E. Functional properties of thermoformed wheat gluten/montmorillonite materials with respect to formulation and processing conditions. J. Appl. Polym. Sci. 2008, 107, 487–496. [Google Scholar] [CrossRef]
- Sun, S.; Song, Y.; Zheng, Q. Thermo-molded wheat gluten plastics plasticized with glycerol: Effect of molding temperature. Food Hydrocoll. 2008, 22, 1006–1013. [Google Scholar] [CrossRef]
- Yang, S.; Bai, S.; Wang, Q. Sustainable packaging biocomposites from polylactic acid and wheat straw: Enhanced physical performance by solid state shear milling process. Compos. Sci. Technol. 2018, 158, 34–42. [Google Scholar] [CrossRef]
- Berthet, M.-A.; Gontard, N.; Angellier-Coussy, H. Impact of fibre moisture content on the structure/mechanical properties relationships of PHBV/wheat straw fibres biocomposites. Compos. Sci. Technol. 2015, 117, 386–391. [Google Scholar] [CrossRef]
- Bruni, G.P.; Oliveira, J.P.; Fonseca, L.M.; Silva, F.T.; Dias, A.R.G.; da Rosa Zavareze, E. Biocomposite Films Based on Phosphorylated Wheat Starch and Cellulose Nanocrystals from Rice, Oat, and Eucalyptus Husks. Starch-Stärke 2020, 72, 1900051. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Khalina, A.; Sapuan, S.M.; Ilyas, R.A. Mechanical properties of sugar palm yarn / woven glass fiber reinforced unsaturated polyester composites: Effect of fiber loadings and alkaline treatment. Polimery 2019, 64, 12–22. [Google Scholar] [CrossRef]
- Suriani, M.J.; Radzi, F.S.M.; Ilyas, R.A.; Petrů, M.; Sapuan, S.M.; Ruzaidi, C.M. Flammability, Tensile, and Morphological Properties of Oil Palm Empty Fruit Bunches Fiber/Pet Yarn-Reinforced Epoxy Fire Retardant Hybrid Polymer Composites. Polymers 2021, 13, 1282. [Google Scholar] [CrossRef]
- Suriani, M.J.; Rapi, H.Z.; Ilyas, R.A.; Petrů, M.; Sapuan, S.M. Delamination and Manufacturing Defects in Natural Fiber-Reinforced Hybrid Composite: A Review. Polymers 2021, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Suriani, M.J.; Sapuan, S.M.; Ruzaidi, C.M.; Nair, D.S.; Ilyas, R.A. Flammability, morphological and mechanical properties of sugar palm fiber/polyester yarn-reinforced epoxy hybrid biocomposites with magnesium hydroxide flame retardant filler. Text. Res. J. 2021, 004051752110086. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Fatimah Athiyah, S.; Shazleen, S.S.; Rafiqah, S.A.; Harussani, M.M.; Kamarudin, S.H.; Razman, M.R.; Rahmah, M.; Zainudin, E.S.; et al. A Review on Mechanical Performance of Hybrid Natural Fiber Polymer Composites for Structural Applications. Polymers 2021, 13, 2170. [Google Scholar] [CrossRef] [PubMed]
- Moazzen, N.; Khanmohammadi, M.; Bagheri Garmarudi, A.; Kazemipour, M.; Ansari Dogaheh, M. Optimization and infrared spectrometric evaluation of the mechanical properties of PLA-based biocomposites. J. Macromol. Sci. Part A Pure Appl. Chem. 2019, 56, 17–25. [Google Scholar] [CrossRef]
- Mat Zain, N.F. Preparation and Characterization of Cellulose and Nanocellulose From Pomelo (Citrus grandis) Albedo. J. Nutr. Food Sci. 2014, 5, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.R.; Sardashti, A.P.; Simon, L.C. Preparation and characterization of polypropylene–wheat straw–clay composites. Compos. Sci. Technol. 2010, 70, 1674–1680. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Piszczyk, Ł.; Saeb, M.R.; Colom, X. Processing and structure–property relationships of natural rubber/wheat bran biocomposites. Cellulose 2016, 23, 3157–3175. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Influence of the Design Solutions of Extruder Screw Mixing Tip on Selected Properties of Wheat Bran-Polyethylene Biocomposite. Polymers 2019, 11, 2120. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zheng, Q.; Liu, C. Green biocomposites from wheat gluten and hydroxyethyl cellulose: Processing and properties. Ind. Crops Prod. 2008, 28, 56–62. [Google Scholar] [CrossRef]
- Muthuraj, R.; Lacoste, C.; Lacroix, P.; Bergeret, A. Sustainable thermal insulation biocomposites from rice husk, wheat husk, wood fibers and textile waste fibers: Elaboration and performances evaluation. Ind. Crops Prod. 2019, 135, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Boudria, A.; Hammoui, Y.; Adjeroud, N.; Djerrada, N.; Madani, K. Effect of filler load and high-energy ball milling process on properties of plasticized wheat gluten/olive pomace biocomposite. Adv. Powder Technol. 2018, 29, 1230–1238. [Google Scholar] [CrossRef]
- Mahmood, H.; Mehmood, S.; Shakeel, A.; Iqbal, T.; Kazmi, M.A.; Khurram, A.R.; Moniruzzaman, M. Glycerol Assisted Pretreatment of Lignocellulose Wheat Straw Materials as a Promising Approach for Fabrication of Sustainable Fibrous Filler for Biocomposites. Polymers 2021, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.S.; Husseinsyah, S. Polylactic acid/corn cob eco-composites: Effect of new organic coupling agent. J. Thermoplast. Compos. Mater. 2014, 27, 1667–1678. [Google Scholar] [CrossRef]
- Rodriguez, M.; Rodriguez, A.; R, J.B.; Vilaseca, F.; Girones, J.; Mutje, P. Determination of corn stalk fibers’ strength through modeling of the mechanical properties of its composites. BioResources 2010, 5, 2535–2546. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.A.B.A.; Omran, A.A.B.; Hasan, Z.; Ilyas, R.A.; Sapuan, S.M. Wheat Biocomposite Extraction, Structure, Properties and Characterization: A Review. Polymers 2021, 13, 3624. https://doi.org/10.3390/polym13213624
Mohammed AABA, Omran AAB, Hasan Z, Ilyas RA, Sapuan SM. Wheat Biocomposite Extraction, Structure, Properties and Characterization: A Review. Polymers. 2021; 13(21):3624. https://doi.org/10.3390/polym13213624
Chicago/Turabian StyleMohammed, Abdulrahman A. B. A., Abdoulhdi A. Borhana Omran, Zaimah Hasan, R. A. Ilyas, and S. M. Sapuan. 2021. "Wheat Biocomposite Extraction, Structure, Properties and Characterization: A Review" Polymers 13, no. 21: 3624. https://doi.org/10.3390/polym13213624
APA StyleMohammed, A. A. B. A., Omran, A. A. B., Hasan, Z., Ilyas, R. A., & Sapuan, S. M. (2021). Wheat Biocomposite Extraction, Structure, Properties and Characterization: A Review. Polymers, 13(21), 3624. https://doi.org/10.3390/polym13213624








