Liquid and Solid Functional Bio-Based Coatings
Abstract
:1. Introduction
2. Bio-Based Coatings—Properties, Processing, Testing and Applications
2.1. Key Properties of Bio-Based Coatings
- Antimicrobial coatings produced with chitin nano-fibrils and/or chitosan can be useful for cellulose tissues (e.g., personal care), paper and cardboard (e.g., packaging for fresh products like pasta, tableware), woven and non-woven (e.g., sanitary, personal care), plastic substrates (e.g., bio-polyesters) for active packaging.
- Gas barrier improvements for multilayer food packaging (e.g., bio polyester-based), with sustainable end of life options could be achieved by protein-based coatings
- Water-repellent properties for paper cups, but also non-food packaging, could be imparted by including cutin, thanks to its hydro-repellence
2.2. Main Physico-Chemical Surface Treatments and Measurement Protocols
3. Innovative Coatings Based on Chitosan-Chitin, Proteins and Cutin
3.1. Innovation on Chitosan- and Chitin-Based Coatings
3.2. Innovation on Protein-Based Coatings
3.3. Innovation on Cutin-Based Coatings
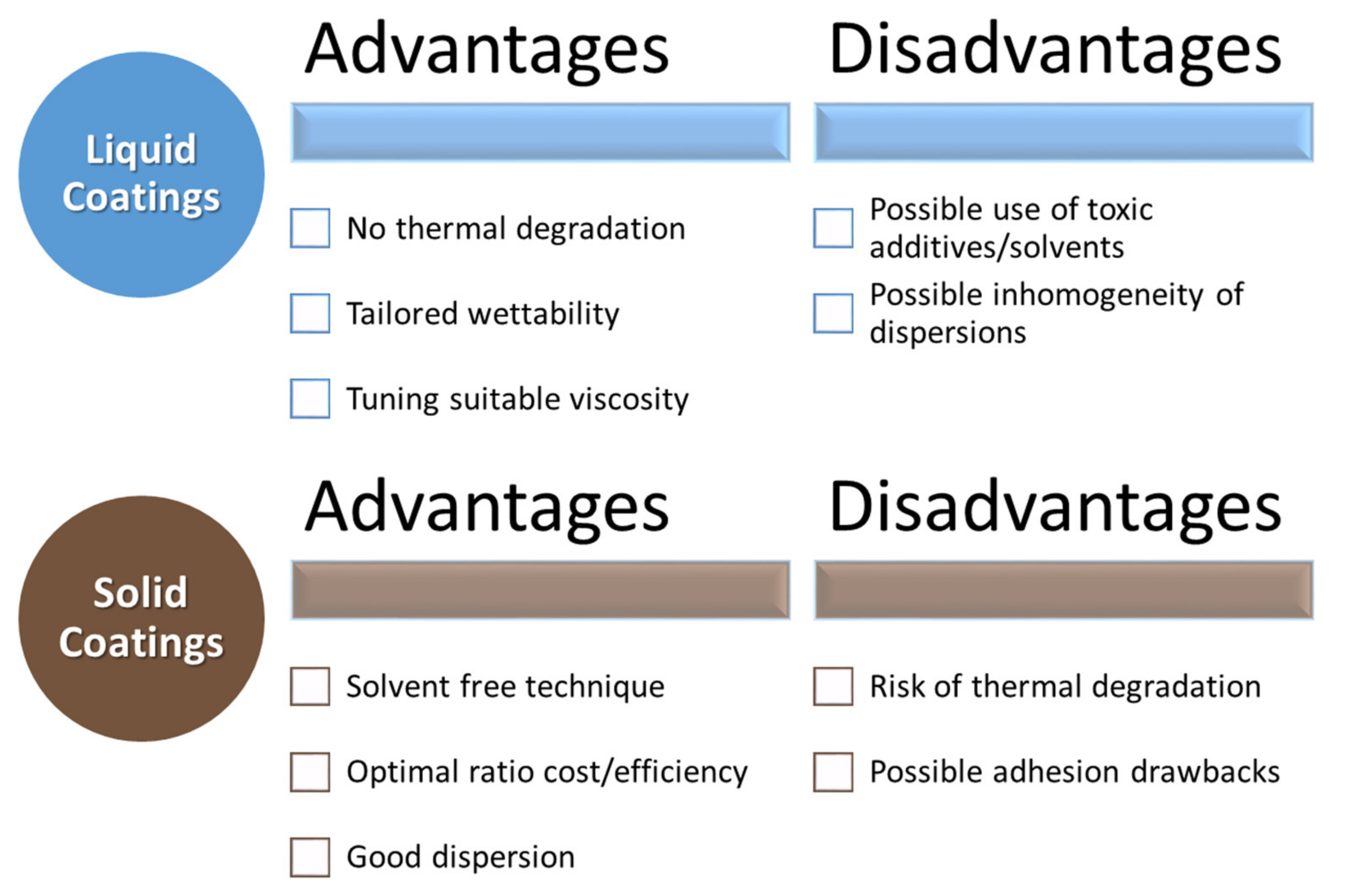
4. Liquid Bio-Based Coatings
5. Solid Functional Bio-Based Coatings
| Solid Coatings | ||
|---|---|---|
| Technique | Description | Meaningful applications with solid coatings |
| Co-extrusion | Co-extrusion is a process that allows the simultaneous extrusion of two or more materials along the same production line, resulting in a multilayer final product [188]. | [189] |
| Compression molding | A method based on the application of a pressure on a powder or another solid placed on a substrate in the lower plate of the press. The equipment is heated guaranteing a good adhesion between the layers [190]. | [191] |
| Fluidized bed dipping | A powder is transformed in an entirely consolidated film thanks to electrostatic forces [192]. | [193] |
| Electrostatic Spray | The coating method is characterized by the deposition of the solid coating through electrostatic atomization [194]. | [195] |
| Roll-to-Roll Coating | The coating or printing process is performed spreading a solid coating on a moving substrate, constitued above all by thin and flexible polymers, papers, ot textiles [196]. | [197] |
6. Future Perspectives for Liquid and Solid Bio-Based Coatings
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Fei, T.; Metzger, K.; Wang, T. Coating performance and rheological characteristics of novel soybean oil-based wax emulsions. Ind. Crops Prod. 2019, 140, 111654. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Jubete, E.; Liauw, C.M.; Allen, N.S. Water uptake and tensile properties of carboxylated styrene butadiene rubber based water born paints: Models for water uptake prediction. Prog. Org. Coat. 2007, 59, 126–133. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradable Polymers. In BT—Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 145–155. ISBN 978-3-642-29648-2. [Google Scholar]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Van Tuil, R.; Fowler, P.; Lawther, M.; Weber, C.J. Properties of biobased packaging materials. In Biobased Packaging Material for the Food Industry-Status and Perspectives; Royal Veterinary and Agricultural University; Woodhead Publishing: Copenhagen, Denmark, 2000; pp. 13–44. [Google Scholar]
- Narodoslawsky, M.; Shazad, K.; Kollmann, R.; Schnitzer, H. LCA of PHA production–Identifying the ecological potential of bio-plastic. Chem. Biochem. Eng. Q. 2015, 29, 299–305. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Samyn, P. Bio-based coatings for paper applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef] [Green Version]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Cinelli, P.; Seggiani, M.; Coltelli, M.B.; Danti, S.; Righetti, M.C.; Gigante, V.; Sandroni, M.; Signori, F.; Lazzeri, A. Overview of Agro-Food Waste and By-Products Valorization for Polymer Synthesis and Modification for Bio-Composite Production. Proceedings 2021, 69, 22. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Gicquel, E.; Martin, C.; Yanez, J.G.; Bras, J. Cellulose nanocrystals as new bio-based coating layer for improving fiber-based mechanical and barrier properties. J. Mater. Sci. 2017, 52, 3048–3061. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Gigante, V.; Cinelli, P.; Lazzeri, A. Flexible Food Packaging Using Polymers from Biomass. In Bionanotechnology to Save the Environment; Morganti, P., Ed.; MDPI: Basel, Switzerland, 2019; pp. 272–298. ISBN 978-3-03842-693-6. [Google Scholar]
- Molinari, G.; Gigante, V.; Fiori, S.; Aliotta, L.; Lazzeri, A. Dispersion of Micro Fibrillated Cellulose (MFC) in Poly(lactic acid) (PLA) from Lab-Scale to Semi-Industrial Processing Using Biobased Plasticizers as Dispersing Aids. Chemistry 2021, 3, 896–915. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Panariello, L.; Gigante, V.; Coltelli, M.B.; Lazzeri, A. Sustainable Micro and Nano Additives for Controlling the Migration of a Biobased Plasticizer from PLA-Based Flexible Films. Polymers 2020, 12, 1366. [Google Scholar] [CrossRef]
- Gigante, V.; Coltelli, M.B.; Vannozzi, A.; Panariello, L.; Fusco, A.; Trombi, L.; Donnarumma, G.; Danti, S.; Lazzeri, A. Flat Die Extruded Biocompatible Poly(Lactic Acid) (PLA)/Poly(Butylene Succinate) (PBS) Based Films. Polymers 2019, 11, 1857. [Google Scholar] [CrossRef] [Green Version]
- Scaffaro, R.; Botta, L.; Lopresti, F.; Maio, A.; Sutera, F. Polysaccharide nanocrystals as fillers for PLA based nanocomposites. Cellulose 2017, 24, 447–478. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Ashter, S.A. Introduction to Bioplastics Engineering; Andrew, W., Ed.; William Andrew: London, UK, 2016; ISBN 0323394078. [Google Scholar]
- Shao, J.; Ünal, E. What do consumers value more in green purchasing? Assessing the sustainability practices from demand side of business. J. Clean. Prod. 2019, 209, 1473–1483. [Google Scholar] [CrossRef]
- Cunningham, M.F.; Campbell, J.D.; Fu, Z.; Bohling, J.; Leroux, J.G.; Mabee, W.; Robert, T. Future green chemistry and sustainability needs in polymeric coatings. Green Chem. 2019, 21, 4919–4926. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Chinnan, M.S. Role of packaging in quality preservation of frozen foods. In Quality in Frozen Food; Springer: New York, NY, USA, 1997; pp. 296–309. [Google Scholar]
- Pietrosanto, A.; Scarfato, P.; Di Maio, L.; Incarnato, L. Development of Eco-Sustainable PBAT-Based Blown Packaging Applications. Materials 2020, 13, 5395. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, K.; Alvarez, V.A.; Gutiérrez, T.J. Biopolymer Composite Materials with Antimicrobial Effects Applied to the Food Industry BT—Functional Biopolymers. In Handbook of Sustainable Polymers: Structure and Chemistry; Thakur, V.K., Thakur, M.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 57–96. ISBN 978-3-319-66417-0. [Google Scholar]
- Panariello, L.; Coltelli, M.B.; Buchignani, M.; Lazzeri, A. Chitosan and nano-structured chitin for biobased anti-microbial treatments onto cellulose based materials. Eur. Polym. J. 2019, 113, 328–339. [Google Scholar] [CrossRef]
- Apicella, A.; Scarfato, P.; Di Maio, L.; Incarnato, L. Sustainable Active PET Films by Functionalization with Antimicrobial Bio-Coatings. Front. Mater. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Shah, M.Y.; Ahmad, S. Waterborne vegetable oil epoxy coatings: Preparation and characterization. Prog. Org. Coat. 2012, 75, 248–252. [Google Scholar] [CrossRef]
- Song, Z.; Xiao, H.; Li, Y. Effects of renewable materials coatings on oil resistant properties of paper. Nord. Pulp Pap. Res. J. 2015, 30, 344–349. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, S.; Yu, J.; Yang, J.; Xiong, L.; Sun, Q. Effects of chitin nano-whiskers on the antibacterial and physicochemical properties of maize starch films. Carbohydr. Polym. 2016, 147, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Panariello, L.; Vannozzi, A.; Morganti, P.; Coltelli, M.B.; Lazzeri, A. Biobased and Eco-Compatible Beauty Films Coated with Chitin. Cosmetics 2021, 8, 27. [Google Scholar] [CrossRef]
- Torlak, E.; Nizamlioğlu, M. Antimicrobial effectiveness of chitosan-essential oil coated plastic films against foodborne pathogens. J. Plast. Film Sheeting 2011, 27, 235–248. [Google Scholar] [CrossRef]
- Ye, M.; Neetoo, H.; Chen, H. Control of Listeria monocytogenes on ham steaks by antimicrobials incorporated into chitosan-coated plastic films. Food Microbiol. 2008, 25, 260–268. [Google Scholar] [CrossRef]
- Hult, E.L.; Ropponen, J.; Poppius-Levlin, K.; Ohra-Aho, T.; Tamminen, T. Enhancing the barrier properties of paper board by a novel lignin coating. Ind. Crops Prod. 2013, 50, 694–700. [Google Scholar] [CrossRef]
- Hult, E.L.; Koivu, K.; Asikkala, J.; Ropponen, J.; Wrigstedt, P.; Sipilä, J.; Poppius-Levlin, K. Esterified lignin coating as water vapor and oxygen barrier for fiber-based packaging. Holzforschung 2013, 67, 899–905. [Google Scholar] [CrossRef]
- Duan, H.; Shao, Z.; Zhao, M.; Zhou, Z. Preparation and properties of environmental-friendly coatings based on carboxymethyl cellulose nitrate ester & modified alkyd. Carbohydr. Polym. 2016, 137, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Obara, S.; Maruyama, N.; Nishiyama, Y.; Kokubo, H. Dry coating: An innovative enteric coating method using a cellulose derivative. Eur. J. Pharm. Biopharm. 1999, 47, 51–59. [Google Scholar] [CrossRef]
- Lin, S.; Krochta, J.M. Plasticizer effect on grease barrier and color properties of whey-protein coatings on paperboard. J. Food Sci. 2003, 68, 229–233. [Google Scholar] [CrossRef]
- Gällstedt, M.; Brottman, A.; Hedenqvist, M.S. Packaging-related properties of protein-and chitosan-coated paper. Packag. Technol. Sci. Int. J. 2005, 18, 161–170. [Google Scholar] [CrossRef]
- Dabral, M.; Francis, L.F.; Scriven, L.E. Drying process paths of ternary polymer solution coating. AIChE J. 2002, 48, 25–37. [Google Scholar] [CrossRef]
- Xu, L.C.; Vadillo-Rodriguez, V.; Logan, B.E. Residence time, loading force, pH, and ionic strength affect adhesion forces between colloids and biopolymer-coated surfaces. Langmuir 2005, 21, 7491–7500. [Google Scholar] [CrossRef] [PubMed]
- Van der Wel, G.K.; Adan, O.C. Moisture in organic coatings—A review. Prog. Org. Coat. 1999, 37, 1–14. [Google Scholar] [CrossRef]
- Mazela, B.; Broda, M.; Perdoch, W.; Ross Gobakken, L.; Ratajczak, I.; Cofta, G.; Grześkowiak, W.; Komasa, A.; Przybył, A. Bio-friendly preservative systems for enhanced wood durability–1st periodic report on DURAWOOD project. In Proceedings of the 46th International Research Group on Wood Protection (IRG46),Viña del Mar, Chile, 10–14 May 2015; IRG Secretariat: Stockholm, Sweden, 2015; pp. 10–14. [Google Scholar]
- Bugnicourt, E.; Kehoe, T.; Latorre, M.; Serrano, C.; Philippe, S.; Schmid, M. Recent prospects in the inline monitoring of nanocomposites and nanocoatings by optical technologies. Nanomaterials 2016, 6, 150. [Google Scholar] [CrossRef] [Green Version]
- Merklein, T.M. High resolution measurement of multilayer structures. Appl. Opt. 1990, 29, 505–511. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Morishita, T.Y.; El-Tayeb, A.B.; Ison, A.J.; Zhang, Q. Comparison of antimicrobial susceptibility testing of Campylobacter spp. by the agar dilution and the agar disk diffusion methods. J. Clin. Microbiol. 2007, 45, 590. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Wang, L.; Shogren, R.L.; Carriere, C. Preparation and properties of thermoplastic starch-polyester laminate sheets by coextrusion. Polym. Eng. Sci. 2000, 40, 499–506. [Google Scholar] [CrossRef]
- Mittal, K.L. Advances in Contact Angle, Wettability and Adhesion; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 1119116996. [Google Scholar]
- Fernandes, S.C.; Freire, C.S.; Silvestre, A.J.; Desbrières, J.; Gandini, A.; Neto, C.P. Production of coated papers with improved properties by using a water-soluble chitosan derivative. Ind. Eng. Chem. Res. 2010, 49, 6432–6438. [Google Scholar] [CrossRef]
- Hershko, V.; Nussinovitch, A. The Behavior of Hydrocolloid Coatings on Vegetative Materials. Biotechnol. Prog. 1998, 14, 756–765. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2013, 33, 379–403. [Google Scholar] [CrossRef]
- Coltelli, M.; Aliotta, L.; Vannozzi, A.; Morganti, P.; Fusco, A.; Donnarumma, G.; Lazzeri, A. Properties and Skin Compatibility of Films Based on Poly (Lactic Acid) (PLA) Bionanocomposites Incorporating Chitin Nanofibrils (CN). J. Funct. Biomater. 2020, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Pottathara, Y.B.; Tiyyagura, H.R.; Ahmad, Z.; Thomas, S. Chapter 3—Chitin and chitosan composites for wearable electronics and energy storage devices. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–88. ISBN 978-0-12-817966-6. [Google Scholar]
- Eswara, P. Chitosan Market by Source, Application, Global Opportunity Analysis and Industry Forecast, 2020–2027; Allied Analytics LLP: Pune, India, 2017. [Google Scholar]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411. [Google Scholar]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Teleky, B.E.; Mitrea, L.; Călinoiu, L.F.; Martău, G.A.; Simon, E.; Varvara, R.A.; Vodnar, D.C. Active Packaging—Poly(Vinyl Alcohol) Films Enriched with Tomato By-Products Extract. Coatings 2020, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Tampucci, S.; Castagna, A.; Monti, D.; Manera, C.; Saccomanni, G.; Chetoni, P.; Zucchetti, E.; Barbagallo, M.; Fazio, L.; Santin, M.; et al. Tyrosol-Enriched Tomatoes by Diffusion across the Fruit Peel from a Chitosan Coating: A Proposal of Functional Food. Foods 2021, 10, 335. [Google Scholar] [CrossRef]
- Toan, N. Van Production of chitin and chitosan from partially autolyzed shrimp shell materials. Open Biomater. J. 2009, 1, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Huang, Y.; Lu, A.; Zhang, L. Recent advances in chitin based materials constructed via physical methods. Prog. Polym. Sci. 2018, 82, 1–33. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of Chitin Nanofibers with a Uniform Width as α-Chitin from Crab Shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.L.; Hao, L.T.; Kong, H.; Park, J.M.; Jung, S.H.; Cha, H.G.; Lee, J.Y.; Kim, H.; Hwang, S.Y.; et al. A ball milling-based one-step transformation of chitin biomass to organo-dispersible strong nanofibers passing highly time and energy consuming processes. Int. J. Biol. Macromol. 2019, 125, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, Q.; She, X.; Xia, Y.; Liu, Y.; Li, J.; Yang, D. Fabrication and characterisation of α-chitin nanofibers and highly transparent chitin films by pulsed ultrasonication. Carbohydr. Polym. 2013, 98, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nogi, M.; Yoshioka, M.; Morimoto, M.; Yano, H.; Saimoto, H. Fibrillation of dried chitin into 10–20 nm nanofibers by a simple grinding method under acidic conditions. Carbohydr. Polym. 2010, 81, 134–139. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Aklog, Y.F.; Nagae, T.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Preparation of chitin nanofibers by surface esterification of chitin with maleic anhydride and mechanical treatment. Carbohydr. Polym. 2016, 153, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S. Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef]
- Häckl, K.; Kunz, W. Some aspects of green solvents. C. R. Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Gigante, V.; Panariello, L.; Morganti, P.; Cinelli, P.; Danti, S.; Lazzeri, A. Chitin nanofibrils in renewable materials for packaging and personal care applications. Adv. Mater. Lett. 2018, 10, 425–430. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin Nanofibrils in Poly(Lactic Acid) (PLA) Nanocomposites: Dispersion and Thermo-Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [Green Version]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.B.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Danti, S.; Coltelli, M.B. Chitin and lignin to produce biocompatible tissues. Res. Clin. Dermatol. 2018, 1, 5–11. [Google Scholar] [CrossRef]
- Morganti, P.; Coltelli, M.B. A new carrier for advanced cosmeceuticals. Cosmetics 2019, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Danti, S.; Anand, S.; Azimi, B.; Milazzo, M.; Fusco, A.; Ricci, C.; Zavagna, L.; Linari, S.; Donnarumma, G.; Lazzeri, A.; et al. Chitin Nanofibril Application in Tympanic Membrane Scaffolds to Modulate Inflammatory and Immune Response. Pharmaceutics 2021, 13, 1440. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Schmid, M.; Nerney, O.M.; Wildner, J.; Smykala, L.; Lazzeri, A.; Cinelli, P. Processing and validation of whey-protein-coated films and laminates at semi-industrial scale as novel recyclable food packaging materials with excellent barrier properties. Adv. Mater. Sci. Eng. 2013, 2013, 496207. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of whey-protein-coated films and laminates as novel recyclable food packaging materials with excellent barrier properties. Int. J. Polym. Sci. 2012, 2012, 562381. [Google Scholar] [CrossRef] [Green Version]
- Coltelli, M.B.; Aliotta, L.; Gigante, V.; Bellusci, M.; Cinelli, P.; Bugnicourt, E.; Schmid, M.; Staebler, A.; Lazzeri, A. Preparation and Compatibilization of PBS/Whey Protein Isolate Based Blends. Molecules 2020, 25, 3313. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Tongdeesoontorn, W.; Rawdkuen, S. Biodegradable protein-based films and their properties: A comparative study. Packag. Technol. Sci. 2016, 29, 77–90. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Pommet, M.; Redl, A.; Morel, M.H.; Guilbert, S. Study of wheat gluten plasticization with fatty acids. Polymer 2003, 44, 115–122. [Google Scholar] [CrossRef]
- Mo, X.; Sun, X. Plasticization of soy protein polymer by polyol-based plasticizers. J. Am. Oil Chem. Soc. 2002, 79, 197–202. [Google Scholar] [CrossRef]
- Schmid, M.; Reichert, K.; Hammann, F.; Stäbler, A. Storage time-dependent alteration of molecular interaction–property relationships of whey protein isolate-based films and coatings. J. Mater. Sci. 2015, 50, 4396–4404. [Google Scholar] [CrossRef]
- Schmid, M.; Prinz, T.K.; Stäbler, A.; Sängerlaub, S. Effect of sodium sulfite, sodium dodecyl sulfate, and urea on the molecular interactions and properties of whey protein isolate-based films. Front. Chem. 2017, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Sängerlaub, S.; Wege, L.; Stäbler, A. Properties of transglutaminase crosslinked whey protein isolate coatings and cast films. Packag. Technol. Sci. 2014, 27, 799–817. [Google Scholar] [CrossRef]
- Schmid, M.; Hinz, L.V.; Wild, F.; Noller, K. Effects of hydrolysed whey proteins on the techno-functional characteristics of whey protein-based films. Materials 2013, 6, 927–940. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M. Properties of cast films made from different ratios of whey protein isolate, hydrolysed whey protein isolate and glycerol. Materials 2013, 6, 3254–3269. [Google Scholar] [CrossRef] [PubMed]
- Kristo, E.; Biliaderis, C.G. Water sorption and thermo-mechanical properties of water/sorbitol-plasticized composite biopolymer films: Caseinate–pullulan bilayers and blends. Food Hydrocoll. 2006, 20, 1057–1071. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.F.; Martău, G.A.; Szabo, K.; Teleky, B.E.; Mureșan, V.; Rusu, A.V.; Socol, C.T.; Vodnar, D.C. Poly(vinyl alcohol)-Based Biofilms Plasticized with Polyols and Colored with Pigments Extracted from Tomato By-Products. Polymers 2020, 12, 532. [Google Scholar] [CrossRef] [Green Version]
- Coltelli, M.B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Eibl, I.; von der Haar, D.; Jesdinszki, M.; Stäbler, A.; Schmid, M.; Langowski, H. Adhesive based on micellar lupin protein isolate exhibiting oxygen barrier properties. J. Appl. Polym. Sci. 2018, 135, 46383. [Google Scholar] [CrossRef]
- Schmid, M.; Müller, K. Whey protein-based packaging films and coatings. In Whey Proteins; Elsevier: Amsterdam, The Netherlands, 2019; pp. 407–437. [Google Scholar]
- Letendre, M.; D’aprano, G.; Lacroix, M.; Salmieri, S.; St-Gelais, D. Physicochemical properties and bacterial resistance of biodegradable milk protein films containing agar and pectin. J. Agric. Food Chem. 2002, 50, 6017–6022. [Google Scholar] [CrossRef]
- Heredia, A. Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochim. Biophys. Acta BBA Gen. Subj. 2003, 1620, 1–7. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef]
- Pio, T.F.; Macedo, G.A. Optimizing the production of cutinase by Fusarium oxysporum using response surface methodology. Enzyme Microb. Technol. 2007, 41, 613–619. [Google Scholar] [CrossRef]
- Martin, L.B.; Rose, J.K. There’s more than one way to skin a fruit: Formation and functions of fruit cuticles. J. Exp. Bot. 2014, 65, 4639–4651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolattukudy, P.E. Polyesters in higher plants. In Biopolyesters; Springer: New York, NY, USA, 2001; pp. 1–49. [Google Scholar]
- Cigognini, I.; Montanari, A.; De la Torre Carreras, R.; Cardoso, G. Extraction Method of a Polyester Polymer or Cutin from the Wasted Tomato Peels and Polyester Polimer so Extracted. WO Patent WO2015028299, 3 May 2015. [Google Scholar]
- Montanari, A.; Bolzoni, L.; Cigognini, I.M.; Ciruelos, A.; Cardoso, M.G.; De La Torre, R. Tomato bio-based lacquer for sustainable metal packaging. In Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2017; Volume 1159, pp. 159–165. [Google Scholar]
- Benítez, J.J.; Heredia-Guerrero, J.A.; Guzmán-Puyol, S.; Barthel, M.J.; Domínguez, E.; Heredia, A. Polyhydroxyester Films Obtained by Non-Catalyzed Melt-Polycondensation of Natural Occurring Fatty Polyhydroxyacids. Front. Mater. 2015, 2, 59. [Google Scholar] [CrossRef] [Green Version]
- Tedeschi, G.; Benitez, J.J.; Ceseracciu, L.; Dastmalchi, K.; Itin, B.; Stark, R.E.; Heredia, A.; Athanassiou, A.; Heredia-Guerrero, J.A. Sustainable Fabrication of Plant Cuticle-Like Packaging Films from Tomato Pomace Agro-Waste, Beeswax, and Alginate. ACS Sustain. Chem. Eng. 2018, 6, 14955–14966. [Google Scholar] [CrossRef]
- Manrich, A.; Moreira, F.K.; Otoni, C.G.; Lorevice, M.V.; Martins, M.A.; Mattoso, L.H. Hydrophobic edible films made up of tomato cutin and pectin. Carbohydr. Polym. 2017, 164, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Benítez, J.J.; Heredia-Guerrero, J.A.; de Vargas-Parody, M.I.; Cruz-Carrillo, M.A.; Morales-Flórez, V.; de la Rosa-Fox, N.; Heredia, A. Biodegradable polyester films from renewable aleuritic acid: Surface modifications induced by melt-polycondensation in air. J. Phys. D Appl. Phys. 2016, 49, 175601. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview; Pignatello, R., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- He, P.; Davis, S.S.; Illum, L. Chitosan microspheres prepared by spray drying. Int. J. Pharm. 1999, 187, 53–65. [Google Scholar] [CrossRef]
- Muzzarelli, C.; Stanic, V.; Gobbi, L.; Tosi, G.; Muzzarelli, R.A. Spray-drying of solutions containing chitosan together with polyuronans and characterisation of the microspheres. Carbohydr. Polym. 2004, 57, 73–82. [Google Scholar] [CrossRef]
- Ngan, L.T.; Wang, S.L.; Hiep, Đ.M.; Luong, P.M.; Vui, N.T.; Đinh, T.M.; Dzung, N.A. Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Res. Chem. Intermed. 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T.; Krupa, A. Electrospray application to powder production and surface coating. J. Aerosol Sci. 2018, 125, 57–92. [Google Scholar] [CrossRef]
- Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu-Altan, O.I.; Basnett, P.; Cinelli, P.; De Clerck, K.; Roy, I.; Donnarumma, G.; Coltelli, M.B.; et al. Electrosprayed Chitin Nanofibril/Electrospun Polyhydroxyalkanoate Fiber Mesh as Functional Nonwoven for Skin Application. J. Funct. Biomater. 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Ricci, C.; Fusco, A.; Zavagna, L.; Linari, S.; Donnarumma, G.; Hadrich, A.; Cinelli, P.; Coltelli, M.B.; Danti, S. Electrosprayed Shrimp and Mushroom Nanochitins on Cellulose Tissue for Skin Contact Application. Molecules 2021, 26, 4374. [Google Scholar] [CrossRef]
- Susanna, G.; Salamandra, L.; Brown, T.M.; Di Carlo, A.; Brunetti, F.; Reale, A. Airbrush spray-coating of polymer bulk-heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 1775–1778. [Google Scholar] [CrossRef]
- Zhong, C.; Kapetanovic, A.; Deng, Y.; Rolandi, M. A Chitin Nanofiber Ink for Airbrushing, Replica Molding, and Microcontact Printing of Self-assembled Macro-, Micro-, and Nanostructures. Adv. Mater. 2011, 23, 4776–4781. [Google Scholar] [CrossRef]
- Benítez, J.J.; Osbild, S.; Guzman-Puyol, S.; Heredia, A.; Heredia-Guerrero, J.A. Bio-Based Coatings for Food Metal Packaging Inspired in Biopolyester Plant Cutin. Polymers 2020, 12, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, C.J. The mechanics of spin coating of polymer films. Phys. Fluids 1988, 31, 2786–2795. [Google Scholar] [CrossRef]
- Eichhorn, S.J. Cellulose nanowhiskers: Promising materials for advanced applications. Soft Matter. 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Ren, Y.; Babaie, E.; Bhaduri, S.B. Nanostructured amorphous magnesium phosphate/poly (lactic acid) composite coating for enhanced corrosion resistance and bioactivity of biodegradable AZ31 magnesium alloy. Prog. Org. Coat. 2018, 118, 1–8. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K. Manufacturing Process—Reinforced Rubber Sheet for Rubber Dam. In Hydraulic Rubber Dam; Plastics Design Library; Thomas, S., Rane, A.V., Abitha, V.K., Kanny, K., Dutta, A.B., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 37–46. ISBN 978-0-12-812210-5. [Google Scholar]
- Aloui, H.; Ghazouani, Z.; Khwaldia, K. Bioactive Coatings Enriched with Cuticle Components from Tomato Wastes for Cherry Tomatoes Preservation. Waste Biomass Valoriz. 2021, 12, 6155–6163. [Google Scholar] [CrossRef]
- Fooladi, S.; Kiahosseini, S.R. Creation and investigation of chitin/HA double-layer coatings on AZ91 magnesium alloy by dipping method. J. Mater. Res. 2017, 32, 2532–2541. [Google Scholar] [CrossRef]
- Siemann, U. Solvent cast technology—A versatile tool for thin film production BT—Scattering Methods and the Properties of Polymer Materials. In Scattering Methods and the Properties of Polymer Materials; Stribeck, N., Smarsly, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–14. ISBN 978-3-540-31510-0. [Google Scholar]
- Garrido, T.; Leceta, I.; Cabezudo, S.; Guerrero, P.; de la Caba, K. Tailoring soy protein film properties by selecting casting or compression as processing methods. Eur. Polym. J. 2016, 85, 499–507. [Google Scholar] [CrossRef]
- Taylor, J.; Taylor, J.R.; Dutton, M.F.; de Kock, S. Identification of kafirin film casting solvents. Food Chem. 2005, 90, 401–408. [Google Scholar] [CrossRef]
- Fan, Y.; Fukuzumi, H.; Saito, T.; Isogai, A. Comparative characterization of aqueous dispersions and cast films of different chitin nanowhiskers/nanofibers. Int. J. Biol. Macromol. 2012, 50, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Maeda, T.; Hotta, A. Polymer Surface Modifications by Coating. In Printing on Polymers; Izdebska, J., Thomas, S.B., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 143–160. ISBN 978-0-323-37468-2. [Google Scholar]
- Bautista, L.; Molina, L.; Niembro, S.; García, J.M.; López, J.; Vílchez, A. Chapter Eight—Coatings and Inks for Food Packaging Including Nanomaterials. In Emerging Nanotechnologies in Food Science; Busquets, R., Ed.; Micro and Nano Technologies; Elsevier: Boston, MA, USA, 2017; pp. 149–173. ISBN 978-0-323-42980-1. [Google Scholar]
- Webster, D.C.; Ryntz, R.A. Pigments, Paints, Polymer Coatings, Lacquers, and Printing Inks. In Handbook of Industrial Chemistry and Biotechnology; Kent, J.A., Bommaraju, T.V., Barnicki, S.D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 805–822. ISBN 978-3-319-52287-6. [Google Scholar]
- Kapur, N.; Hewson, R.; Sleigh, P.A.; Summers, J.L.; Thompson, H.M.; Abbott, S.J. A review of gravure coating systems. Mater. Sci. 2011, 1, 56–60. [Google Scholar]
- Tambe, C.; Graiver, D.; Narayan, R. Moisture resistance coating of packaging paper from biobased silylated soybean oil. Prog. Org. Coat. 2016, 101, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Piergiovanni, L.; Li, F.; Farris, S. Coatings of Bio-based Materials on Flexible Food Packaging: Opportunities for Problem Solving and Innovations. In Advances in Industrial Biotechnology; Publishing House Pvt. Ltd, Ed.; IK International: Mumbai, India, 2014; pp. 233–251. [Google Scholar]
- Spieser, H.; Denneulin, A.; Deganello, D.; Gethin, D.; Koppolu, R.; Bras, J. Cellulose nanofibrils and silver nanowires active coatings for the development of antibacterial packaging surfaces. Carbohydr. Polym. 2020, 240, 116305. [Google Scholar] [CrossRef]
- Kallio, H.; Nieminen, R.; Tuomasjukka, S.; Hakala, M. Cutin composition of five Finnish berries. J. Agric. Food Chem. 2006, 54, 457–462. [Google Scholar] [CrossRef]
- Picard, E.; Gauthier, H.; Gérard, J.F.; Espuche, E. Influence of the intercalated cations on the surface energy of montmorillonites: Consequences for the morphology and gas barrier properties of polyethylene/montmorillonites nanocomposites. J. Colloid Interface Sci. 2007, 307, 364–376. [Google Scholar] [CrossRef]
- Burnett, D.J.; Thielmann, F.; Ryntz, R.A. Correlating thermodynamic and mechanical adhesion phenomena for thermoplastic polyolefins. J. Coat. Technol. Res. 2007, 4, 211–215. [Google Scholar] [CrossRef]
- Dhakal, H.N.; Zhang, Z.Y.; Bennett, N. Influence of fibre treatment and glass fibre hybridisation on thermal degradation and surface energy characteristics of hemp/unsaturated polyester composites. Compos. Part B Eng. 2012, 43, 2757–2761. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Blayo, A.; Gandini, A. Surface Characterization of Polysaccharides, Lignins, Printing Ink Pigments, and Ink Fillers by Inverse Gas Chromatography. J. Colloid Interface Sci. 1996, 182, 431–436. [Google Scholar] [CrossRef]
- Kendall, K. The adhesion and surface energy of elastic solids. J. Phys. D Appl. Phys. 1971, 4, 1186–1195. [Google Scholar] [CrossRef]
- Packham, D.E. Surface energy, surface topography and adhesion. Int. J. Adhes. Adhes. 2003, 23, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Watts, J.F.; Critchlow, G.W.; Packham, D.E.; Kneafsey, B.; Sherriff, M.; Shanahan, M.E.; Cope, B.C.; Pascoe, M.W.; Sagar, A.J.; Allen, K.W.; et al. Handbook of Adhesion. In Handbook of Adhesion; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2005; pp. 1–58. ISBN 9780470014226. [Google Scholar]
- Bogdanova, Y.G.; Dolzhikova, V.D.; Summ, B.D. Wetting of Solids by Aqueous Solutions of Surfactant Binary Mixtures: 2. Wetting of High-Energy Surface. Colloid J. 2003, 65, 290–294. [Google Scholar] [CrossRef]
- Mourougou-Candoni, N.; Prunet-Foch, B.; Legay, F.; Vignes-Adler, M.; Wong, K. Retraction Phenomena of Surfactant Solution Drops upon Impact on a Solid Substrate of Low Surface Energy. Langmuir 1999, 15, 6563–6574. [Google Scholar] [CrossRef]
- Kiani, S.; Rogers, S.E.; Sagisaka, M.; Alexander, S.; Barron, A.R. A New Class of Low Surface Energy Anionic Surfactant for Enhanced Oil Recovery. Energy Fuels 2019, 33, 3162–3175. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.H.; Gell, M. Effect of solution concentration on splat formation and coating microstructure using the solution precursor plasma spray process. Surf. Coat. Technol. 2008, 202, 2132–2138. [Google Scholar] [CrossRef]
- Schubert, D.W.; Dunkel, T. Spin coating from a molecular point of view: Its concentration regimes, influence of molar mass and distribution. Mater. Res. Innov. 2003, 7, 314–321. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of Coating Thickness on Viscosity of Coating Solution Applied to Fruits and Vegetables by Dipping Method. J. Food Sci. 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The correlation between the surface energy, the contact angle and the spreading parameter, and their relevance for the wetting behaviour of DLC with lubricating oils. Tribol. Int. 2013, 66, 225–233. [Google Scholar] [CrossRef]
- Nakae, H.; Inui, R.; Hirata, Y.; Saito, H. Effects of surface roughness on wettability. Acta Mater. 1998, 46, 2313–2318. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Encinas, N.; Pantoja, M.; Abenojar, J.; Martínez, M.A. Control of Wettability of Polymers by Surface Roughness Modification. J. Adhes. Sci. Technol. 2010, 24, 1869–1883. [Google Scholar] [CrossRef] [Green Version]
- Al-Turaif, H.; Bousfield, D.W. The influence of substrate absorbency on coating surface energy. Prog. Org. Coat. 2004, 49, 62–68. [Google Scholar] [CrossRef]
- Al-Turaif, H.; Bousfield, D.W.; LePoutre, P. The influence of substrate absorbency on coating surface chemistry. Prog. Org. Coat. 2002, 44, 307–315. [Google Scholar] [CrossRef]
- Zheng, C.G.; Gall, B.L.; Gao, H.W.; Miller, A.E.; Bryant, R.S. Effects of Polymer Adsorption and Flow Behavior on Two-Phase Flow in Porous Media. SPE Reserv. Eval. Eng. 2000, 3, 216–223. [Google Scholar] [CrossRef]
- Ogihara, H.; Xie, J.; Saji, T. Factors determining wettability of superhydrophobic paper prepared by spraying nanoparticle suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 35–41. [Google Scholar] [CrossRef]
- Holman, R.K.; Cima, M.J.; Uhland, S.A.; Sachs, E. Spreading and Infiltration of Inkjet-Printed Polymer Solution Droplets on a Porous Substrate. J. Colloid Interface Sci. 2002, 249, 432–440. [Google Scholar] [CrossRef]
- Bose, S.; Keller, S.S.; Alstrøm, T.S.; Boisen, A.; Almdal, K. Process Optimization of Ultrasonic Spray Coating of Polymer Films. Langmuir 2013, 29, 6911–6919. [Google Scholar] [CrossRef]
- Rowe, R.C. The measurement of the adhesion of film coatings to tablet surfaces: The effect of tablet porosity, surface roughness and film thickness. J. Pharm. Pharmacol. 1978, 30, 343–346. [Google Scholar] [CrossRef]
- Collins, G.W.; Letts, S.A.; Fearon, E.M.; McEachern, R.L.; Bernat, T.P. Surface Roughness Scaling of Plasma Polymer Films. Phys. Rev. Lett. 1994, 73, 708–711. [Google Scholar] [CrossRef]
- Mesic, B.; Cairns, M.; Järnstrom, L.; Joo Le Guen, M.; Parr, R. Film formation and barrier performance of latex based coating: Impact of drying temperature in a flexographic process. Prog. Org. Coat. 2019, 129, 43–51. [Google Scholar] [CrossRef]
- Wang, J.; Law, C.L.; Nema, P.K.; Zhao, J.H.; Liu, Z.L.; Deng, L.Z.; Gao, Z.J.; Xiao, H.W. Pulsed vacuum drying enhances drying kinetics and quality of lemon slices. J. Food Eng. 2018, 224, 129–138. [Google Scholar] [CrossRef]
- Alibas, I. Microwave, Vacuum, and Air Drying Characteristics of Collard Leaves. Dry. Technol. 2009, 27, 1266–1273. [Google Scholar] [CrossRef]
- Signori, F.; Coltelli, M.B.; Bronco, S. Thermal degradation of poly(lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Jawaid, M.; Lee, C.H. Effect of Modified Tapioca Starch on Mechanical, Thermal, and Morphological Properties of PBS Blends for Food Packaging. Polymers 2018, 10, 1187. [Google Scholar] [CrossRef] [Green Version]
- Montano-Herrera, L.; Pratt, S.; Arcos-Hernandez, M.V.; Halley, P.J.; Lant, P.A.; Werker, A.; Laycock, B. In-line monitoring of thermal degradation of PHA during melt-processing by Near-Infrared spectroscopy. New Biotechnol. 2014, 31, 357–363. [Google Scholar] [CrossRef]
- Brömme, H.J.; Peschke, E.; Israel, G. Photo-degradation of melatonin: Influence of argon, hydrogen peroxide, and ethanol. J. Pineal Res. 2008, 44, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Nonell, S.; Moncayo, L.; Trull, F.; Amat-Guerri, F.; Lissi, E.A.; Soltermann, A.T.; Criado, S.; García, N.A. Solvent influence on the kinetics of the photodynamic degradation of trolox, a water-soluble model compound for vitamin E. J. Photochem. Photobiol. B Biol. 1995, 29, 157–162. [Google Scholar] [CrossRef]
- Auria, R.; Aycaguer, A.C.; Devinny, J.S. Influence of Water Content on Degradation Rates for Ethanol in Biofiltration. J. Air Waste Manag. Assoc. 1998, 48, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Yu, B.; Yue, Z.; Wang, T.; Wen, X.; Liu, Z.; Zhao, C. Preparation of porous chitosan gel beads for copper(II) ion adsorption. J. Hazard. Mater. 2007, 147, 67–73. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G. Chitin nanofibrils for advanced cosmeceuticals. Clin. Dermatol. 2008, 26, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Trujillo, M.A.; Filomena-Ambrosio, A.; Quintanilla-Carvajal, M.X.; Sotelo-Díaz, L.I. Stability of low-fat oil in water emulsions obtained by ultra turrax, rotor-stator and ultrasound homogenization methods. Int. J. Gastron. Food Sci. 2018, 13, 58–64. [Google Scholar] [CrossRef]
- Ganta, S.; Paxton, J.W.; Baguley, B.C.; Garg, S. Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery. Int. J. Pharm. 2009, 367, 179–186. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Z.; Cai, J.; Tian, J. Conductive biomass-based composite wires with cross-linked anionic nanocellulose and cationic nanochitin as scaffolds. Int. J. Biol. Macromol. 2020, 156, 1183–1190. [Google Scholar] [CrossRef]
- Lv, S.; Zhou, H.; Bai, L.; Rojas, O.J.; McClements, D.J. Development of food-grade Pickering emulsions stabilized by a mixture of cellulose nanofibrils and nanochitin. Food Hydrocoll. 2021, 113, 106451. [Google Scholar] [CrossRef]
- Lin, D.; Li, R.; Lopez-Sanchez, P.; Li, Z. Physical properties of bacterial cellulose aqueous suspensions treated by high pressure homogenizer. Food Hydrocoll. 2015, 44, 435–442. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chun, S.J.; Kang, I.A.; Park, J.Y. Preparation of cellulose nanofibrils by high-pressure homogenizer and cellulose-based composite films. J. Ind. Eng. Chem. 2009, 15, 50–55. [Google Scholar] [CrossRef]
- Youssef, N.H.; Duncan, K.E.; Nagle, D.P.; Savage, K.N.; Knapp, R.M.; McInerney, M.J. Comparison of methods to detect biosurfactant production by diverse microorganisms. J. Microbiol. Methods 2004, 56, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Md, F. Biosurfactant: Production and Application. J. Pet. Environ. Biotechnol. 2012, 3, 124. [Google Scholar] [CrossRef] [Green Version]
- He, Q. Development of Waterborne Bio-Based Primers for Metal Application. Master’s Thesis, KTH, School of Engineering Sciences in Chemistry, Biotechnology and Health (CBH), Stockholm, Sweden, 2020. [Google Scholar]
- Atta, A.M.; Al-Hodan, H.A.; Hameed, R.S.; Ezzat, A.O. Preparation of green cardanol-based epoxy and hardener as primer coatings for petroleum and gas steel in marine environment. Prog. Org. Coat. 2017, 111, 283–293. [Google Scholar] [CrossRef]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Van Savage, G.; Rhodes, C.T. The sustained release coating of solid dosage forms: A historical review. Drug Dev. Ind. Pharm. 1995, 21, 93–118. [Google Scholar] [CrossRef]
- Paul, C.W. Hot-melt adhesives. Mrs Bull. 2003, 28, 440–444. [Google Scholar] [CrossRef]
- Park, Y.J.; Joo, H.S.; Kim, H.J.; Lee, Y.K. Adhesion and rheological properties of EVA-based hot-melt adhesives. Int. J. Adhes. Adhes. 2006, 26, 571–576. [Google Scholar] [CrossRef]
- Moody, V.; Needles, H.L. Hot Melt Coating. In Tufted Carpet: Textile Fibers, Dyes, Finishes and Processes; William Andrew Publishing: Norwich, NY, USA, 2004; p. 202. ISBN 0815519400. [Google Scholar]
- Li, W.; Bouzidi, L.; Narine, S.S. Current research and development status and prospect of hot-melt adhesives: A review. Ind. Eng. Chem. Res. 2008, 47, 7524–7532. [Google Scholar] [CrossRef]
- Gilvary, G.C.; Ammar, A.; Li, S.; Senta-Loys, Z.; Tian, Y.; Kelleher, J.F.; Healy, A.M.; Jones, D.S.; Andrews, G.P. Hot-melt co-extrusion technology as a manufacturing platform for anti-hypertensive fixed-dose combinations. Br. J. Pharm. 2019, 4, S14–S15. [Google Scholar] [CrossRef]
- Vynckier, A.K.; Dierickx, L.; Voorspoels, J.; Gonnissen, Y.; Remon, J.P.; Vervaet, C. Hot-melt co-extrusion: Requirements, challenges and opportunities for pharmaceutical applications. J. Pharm. Pharmacol. 2014, 66, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Farris, S. Main Manufacturing Processes for Food Packaging Materials. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–9. ISBN 978-0-08-100596-5. [Google Scholar]
- Sarraf, A.G.; Tissot, H.; Tissot, P.; Alfonso, D.; Gurny, R.; Doelker, E. Influence of hot-melt extrusion and compression molding on polymer structure organization, investigated by differential scanning calorimetry. J. Appl. Polym. Sci. 2001, 81, 3124–3132. [Google Scholar] [CrossRef]
- Barletta, M.; Bolelli, G.; Guarino, S.; Lusvarghi, L. Development of matte finishes in electrostatic (EFB) and conventional hot dipping (CHDFB) fluidized bed coating process. Prog. Org. Coat. 2007, 59, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Barletta, M.; Gisario, A.; Rubino, G. Scratch response of high-performance thermoset and thermoplastic powders deposited by the electrostatic spray and ‘hot dipping’fluidised bed coating methods: The role of the contact condition. Surf. Coat. Technol. 2011, 205, 5186–5198. [Google Scholar] [CrossRef]
- Kang, D.; Kim, J.; Kim, I.; Choi, K.H.; Lee, T.M. Experimental qualification of the process of electrostatic spray deposition. Coatings 2019, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, K.; Zhang, S.; Chauhan, R.S.; Ishizuka, N.; Yamamoto, M.; Masaoka, Y.; Kataoka, M.; Yamashita, S.; Sakuma, S. Preparation of fenofibrate solid dispersion using electrospray deposition and improvement in oral absorption by instantaneous post-heating of the formulation. Int. J. Pharm. 2013, 450, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shin, K.; Lee, C. Roll-to-roll coating technology and its applications: A review. Int. J. Precis. Eng. Manuf. 2016, 17, 537–550. [Google Scholar] [CrossRef]
- Moustafa, A.F. Release of a cohesively strong, general purpose hot-melt pressure sensitive adhesive from a silicone liner. Int. J. Adhes. Adhes. 2014, 50, 65–69. [Google Scholar] [CrossRef]
- Achanta, A.S.; Adusumilli, P.S.; James, K.W.; Rhodes, C.T. Development of hot melt coating methods. Drug Dev. Ind. Pharm. 1997, 23, 441–449. [Google Scholar] [CrossRef]
- Bose, S.; Bogner, R.H. Solventless pharmaceutical coating processes: A review. Pharm. Dev. Technol. 2007, 12, 115–131. [Google Scholar] [CrossRef]
- Jones, D.M.; Percel, P.J. Coating of Multiparticulates Using Molten Materials. Formulation and Process Considerations; Ghebre-Sellasie, I., Ed.; Taylor & Francis Inc: Abingdon, UK, 1994; Volume 65. [Google Scholar]
- Khobragade, D.S.; Wankar, J.; Patil, A.T.; Potbhare, M.S.; Lakhotiya, C.L.; Umathe, S.N. A novel practical approach for enhancement of bioavailability of a poorly water soluble drug by hot melt coating technique. Int. J. Pharm. Sci. Rev. Res 2014, 26, 258–263. [Google Scholar]
- Jannin, V.; Berard, V.; N’diaye, A.; Andres, C.; Pourcelot, Y. Comparative study of the lubricant performance of Compritol 888 ATO either used by blending or by hot melt coating. Int. J. Pharm. 2003, 262, 39–45. [Google Scholar] [CrossRef]
- Lopes, D.G.; Salar-Behzadi, S.; Zimmer, A. Designing optimal formulations for hot-melt coating. Int. J. Pharm. 2017, 533, 357–363. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A review of hot-melt extrusion: Process technology to pharmaceutical products. Int. Sch. Res. Not. 2012, 12, 27. [Google Scholar] [CrossRef] [Green Version]
- Maniruzzaman, M.; Rana, M.M.; Boateng, J.S.; Mitchell, J.C.; Douroumis, D. Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers. Drug Dev. Ind. Pharm. 2013, 39, 218–227. [Google Scholar] [CrossRef]
- Correlo, V.M.; Boesel, L.F.; Bhattacharya, M.; Mano, J.F.; Neves, N.M.; Reis, R.L. Properties of melt processed chitosan and aliphatic polyester blends. Mater. Sci. Eng. A 2005, 403, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, Y.; Sawada, T.; Handa, T.; Watanuki, Y.; Kudo, T. Influence of air-cooling time on physical properties of thermoplastic polyester powder coatings. Prog. Org. Coat. 2012, 75, 584–589. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Z. Powder Coating Compositions Containing Reactive Nanoparticles. U.S. Patent 10/450,399, 1 April 2004. [Google Scholar]
- Kage, H.; Dohzaki, M.; Ogura, H.; Matsuno, Y. Powder coating efficiency of small particles and their agglomeration in circulating fluidized bed. Korean J. Chem. Eng. 1999, 16, 630–634. [Google Scholar] [CrossRef]
- Takeshita, Y.; Miwa, T.; Ishii, A.; Sawada, T. Innovative thermoplastic powder coatings in telecommunication fields. J. Curr. Issues Media Telecommun. 2017, 9, 289–3131. [Google Scholar]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Van Haveren, J.; Oostveen, E.A.; Miccichè, F.; Noordover, B.A.; Koning, C.E.; Van Benthem, R.A.; Frissen, A.E.; Weijnen, J.G. Resins and additives for powder coatings and alkyd paints, based on renewable resources. J. Coat. Technol. Res. 2007, 4, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Dorigato, A.; Perin, D.; Pegoretti, A. Effect of the Temperature and of the Drawing Conditions on the Fracture Behaviour of Thermoplastic Starch Films for Packaging Applications. J. Polym. Environ. 2020, 28, 3244–3255. [Google Scholar] [CrossRef]
- Miletić, A.; Ristić, I.; Coltelli, M.B.; Pilić, B. Modification of PLA-Based Films by Grafting or Coating. J. Funct. Biomater. 2020, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Herbst, C.; Müller, K.; Stäbler, A.; Schlemmer, D.; Coltelli, M.B.; Lazzeri, A. Effect of Potato Pulp Filler on the Mechanical Properties and Water Vapor Transmission Rate of Thermoplastic WPI/PBS Blends. Polym. Plast. Technol. Eng. 2016, 55, 510–517. [Google Scholar] [CrossRef]

| Bio-Based Polymer | Preparation | Application Method | Properties Improved and Main Results | REF |
|---|---|---|---|---|
| Chitin | 0–2 wt.% chtin nanowhiskers dissolved in H2SO4 and glycerol. | Casting method on maize-starch films. | Evident antimicrobial resistance vs. Gram-positive Listeria monocytogenes. | [30] |
| 2 wt.% of water suspension of nanofibrils dispersed in PEG 8000. | Spray dryer on bioplastics films. | Antimicrobial and skin-regenerative improvements. | [31] | |
| Chitosan | Chitosan (2 wt.%) and glycerol (2 wt.%) dissolved in a 1% (vol/vol) aqueous solution of acetic acid. | Chromatography plate coater application onto PP films. corona-treated | Evident antimicrobial resistance vs. Listeria monocytogenes, Staphylococcus aureus, and Escherichia coli. | [32] |
| Chitosan concentration of 0.02 g/mL in acetic acid mixed in equal volumes with hydroxypropyl methylcellulose. | Thin-layer chromatography plate coater on plastic films. | Excellent long-term antilisterial effect. | [33] | |
| Lignin | Dissolution in acetone of different amounts of softwood kraft lignin and evaporation of the solvent. | Erichsen coater on to a paperboard substrate. | Evident decrease in Oxygen Transmission Rate (OTR) value and a stable contact angle with respect to paperboard alone. | [34] |
| Lignin estereified with palitic and lauric acid chloride in a mixture 3:1 ethanol/water. | Erichsen coater on a commercial paperboard substrate. | Good barrier properties against O2 and H2O | [35] | |
| Cellulose derivates | Cellulose nitrate ester (CMCN) were dissolved in mixed solvents systems in different amounts. | Solvent casting method. | Gas and water barrier optimized. | [36] |
| Hydroxypropyl methylcellulose acetate succinate plasticized with triethyl citrate and acetylated monoglyceride | Centrifugal granulator for feeding the coating powder and spraying simultaneously the plasticized. | Improved gastric resistance, coating efficiency, and processing stability | [37] | |
| Proteins | Whey proteins with hydrolysed lactose at different contents | “Bird-type” applicator onto paperboard substrates | Good grease resistance and minimization of plasticizer migration | [38] |
| 12 g of whey proteins in 6 g of glycerol and 30 g of deionized water | Compression molding onto cellulosic substrates | Gas-barrier properties improvements | [39] |
| LIQUID COATINGS | ||
|---|---|---|
| Technique | Description | Meaningful Applications in Liquid Bio-Based Coatings |
| Spray Drying | Transformation of a solution in which are dispersed particles into dried ones, thanks to a gaseous hot drying medium [108]. | [109,110,111] |
| Electrospray | Liquid atomization through commanding electrical forces on the flow of a liquid injection from a cilindric die. This technique gaurantees uniform droplets generation [112]. | [113,114] |
| Airbrush Spraying | Polymer solutions are sprayed through an airbrush supplied by a nitrogen line and fixed on a mechanic arm over a hot plate [115]. | [116,117] |
| Spin Coating | The material used to coat is present at the centre of the substrate, then it is rotated at high speed until centrifugal force spreads the coating material [118]. | [119,120] |
| Dipping | The solution substrate is immersed in the coating for effective formation of the complete material [121]. | [122,123] |
| Solution Casting | A polymer is dissolved in a solution into which an inner diameter mold is immersed. The solvent is removed to leave a solid cast layer. This layer can be laminated or coated before being stripped from the mold [124]. | [125,126,127] |
| Flexography | Flexographic assumes the possibility to widespread liquid inks with a low viscosity on paper, cardboard, or plastic films [128]. | [129,130] |
| Gravure Roll Coater | Coating is introduced onto the surface of an engraved roll, then it is partially submersed in or by an enclosed applicator head that holds the coating against the roll [131]. | [132,133,134] |
| Biomolecule | Liquid | Solid |
|---|---|---|
| Chitin/chitosan | Antimicrobial coatings for cellulose, bioplastic and textile substrates. | Potentially antimicrobial and water barrier coatings for cellulose, plastic and textile substrates. |
| Protein | High oxygen barrier coatings for plastic and cellulose. | In blend with polyesters, oxygen barrier coatings for cellulose and plastic. |
| Cutin | Hydrorepellent coatings and potentially for cellulose, bioplastic, and textile substrates. | Potentially hydrorepellent coatings for cellulose, bioplastic, and textile substrates. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gigante, V.; Panariello, L.; Coltelli, M.-B.; Danti, S.; Obisesan, K.A.; Hadrich, A.; Staebler, A.; Chierici, S.; Canesi, I.; Lazzeri, A.; et al. Liquid and Solid Functional Bio-Based Coatings. Polymers 2021, 13, 3640. https://doi.org/10.3390/polym13213640
Gigante V, Panariello L, Coltelli M-B, Danti S, Obisesan KA, Hadrich A, Staebler A, Chierici S, Canesi I, Lazzeri A, et al. Liquid and Solid Functional Bio-Based Coatings. Polymers. 2021; 13(21):3640. https://doi.org/10.3390/polym13213640
Chicago/Turabian StyleGigante, Vito, Luca Panariello, Maria-Beatrice Coltelli, Serena Danti, Kudirat Abidemi Obisesan, Ahdi Hadrich, Andreas Staebler, Serena Chierici, Ilaria Canesi, Andrea Lazzeri, and et al. 2021. "Liquid and Solid Functional Bio-Based Coatings" Polymers 13, no. 21: 3640. https://doi.org/10.3390/polym13213640
APA StyleGigante, V., Panariello, L., Coltelli, M.-B., Danti, S., Obisesan, K. A., Hadrich, A., Staebler, A., Chierici, S., Canesi, I., Lazzeri, A., & Cinelli, P. (2021). Liquid and Solid Functional Bio-Based Coatings. Polymers, 13(21), 3640. https://doi.org/10.3390/polym13213640












