Green Synthesis of Silver Nanoparticles as an Effective Antibiofouling Material for Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of AgNPs
Preparation of Aqueous P. argentea Extract
Preparation of AgNPs
2.2.2. Polyvinylidene Fluoride (PVDF) Casting Process
2.2.3. Characterization
2.3. Antibacterial Activity
2.3.1. Antibacterial Activity for AgNPs
2.3.2. Membrane Antibacterial Activity
Preparation of Culture Media and Solutions
Preparation of Membrane Samples
3. Results and Discussion
3.1. Optimization AgNPs Synthesis
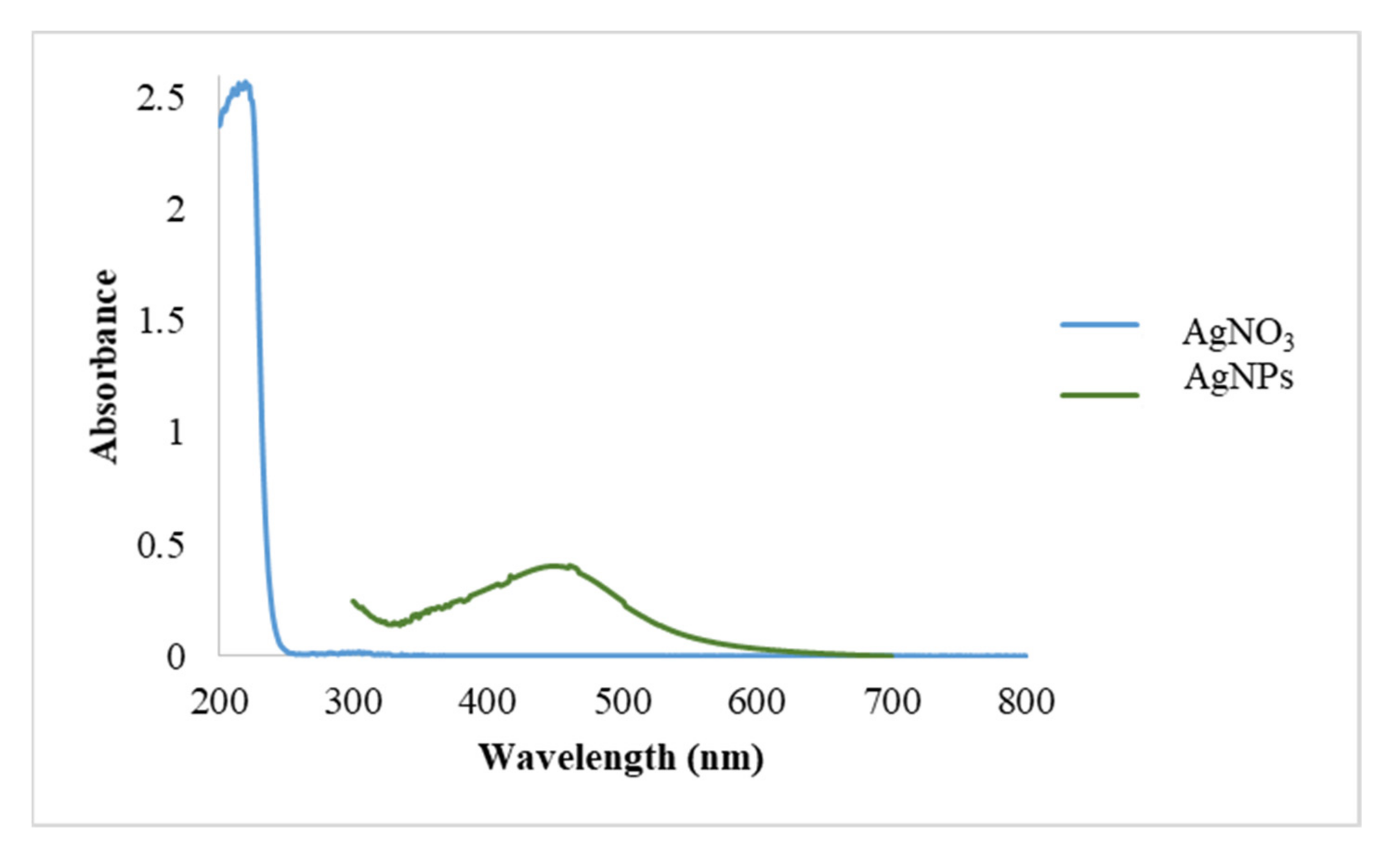
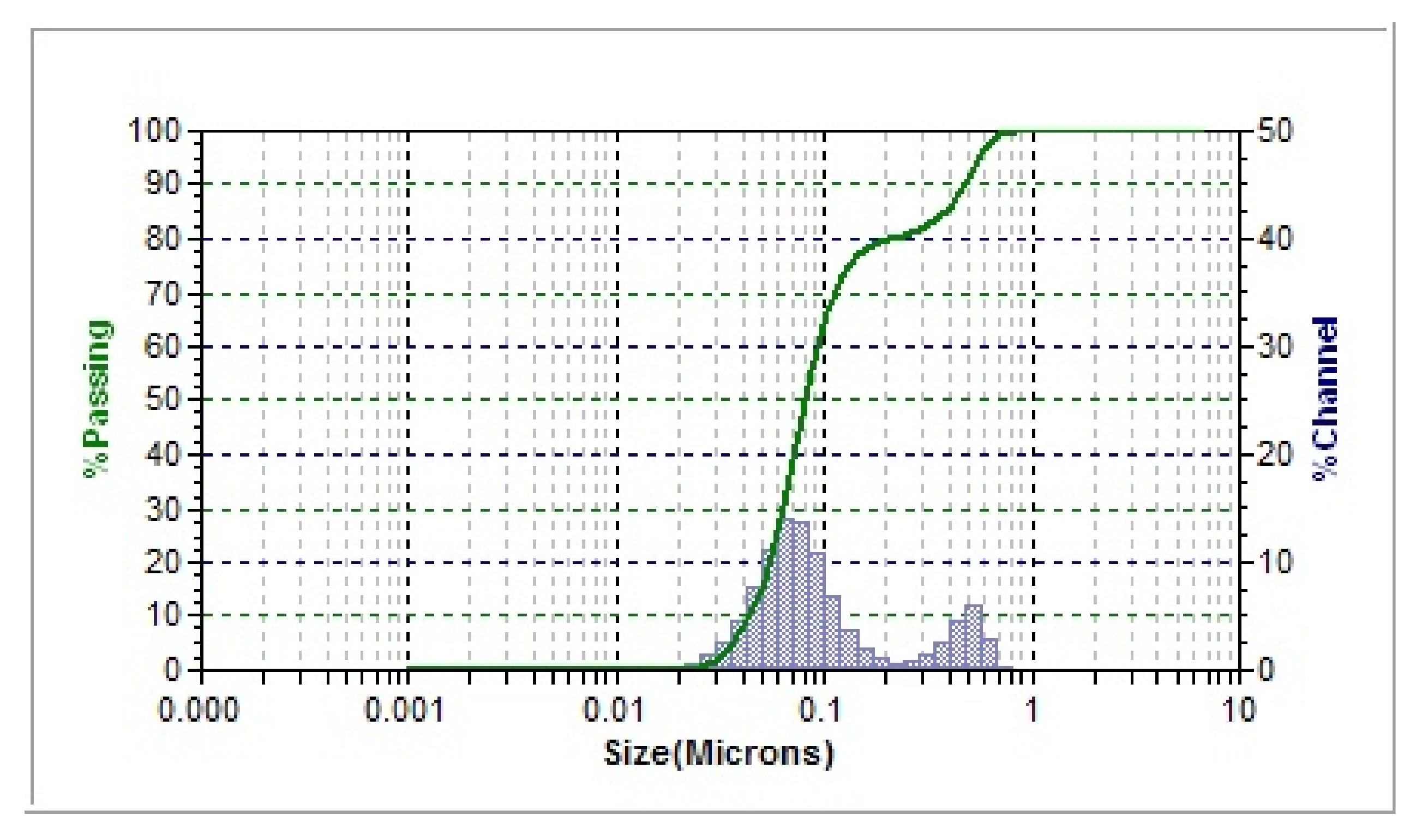
3.1.1. UV-Vis and DLS Measurements
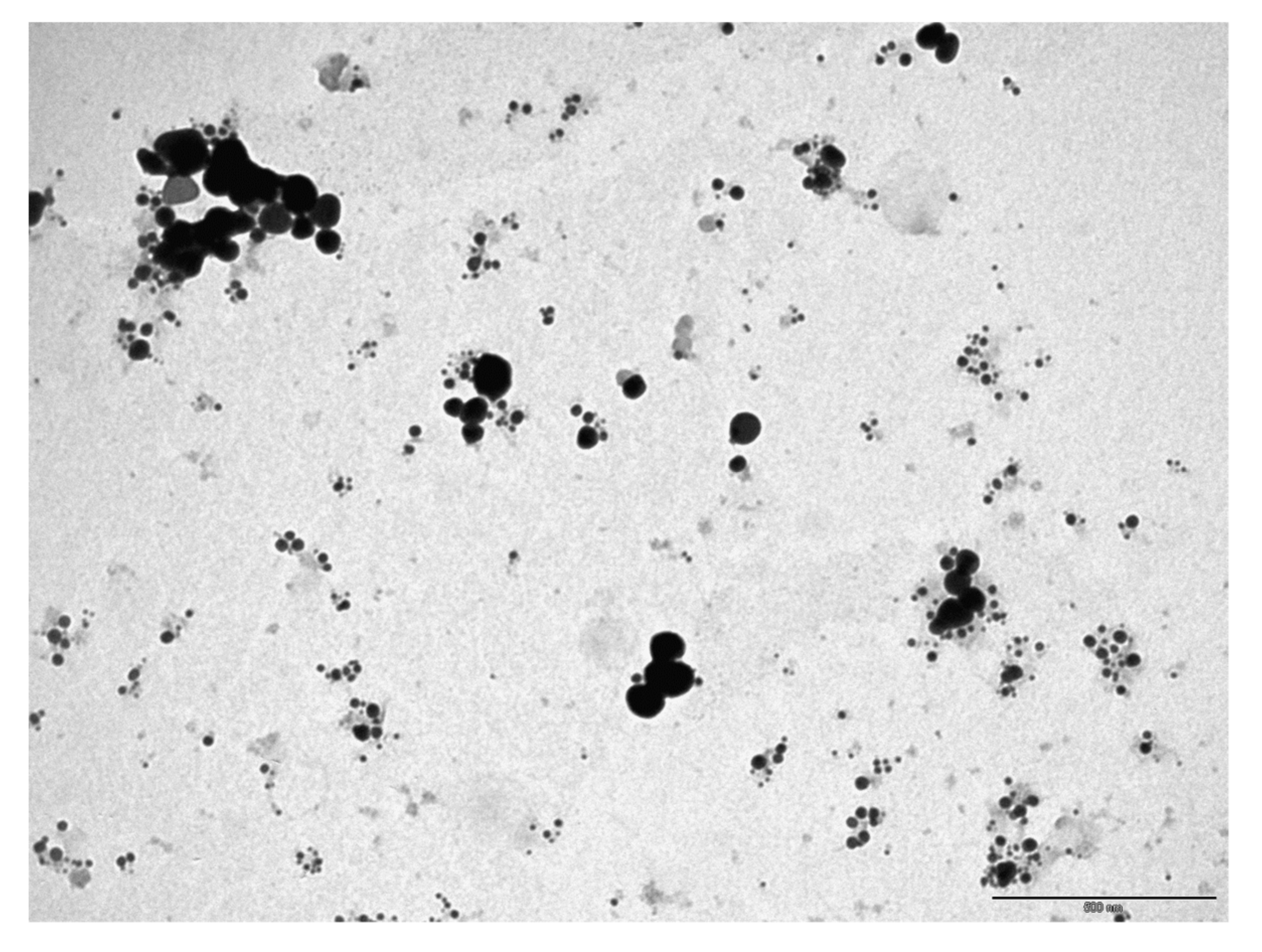
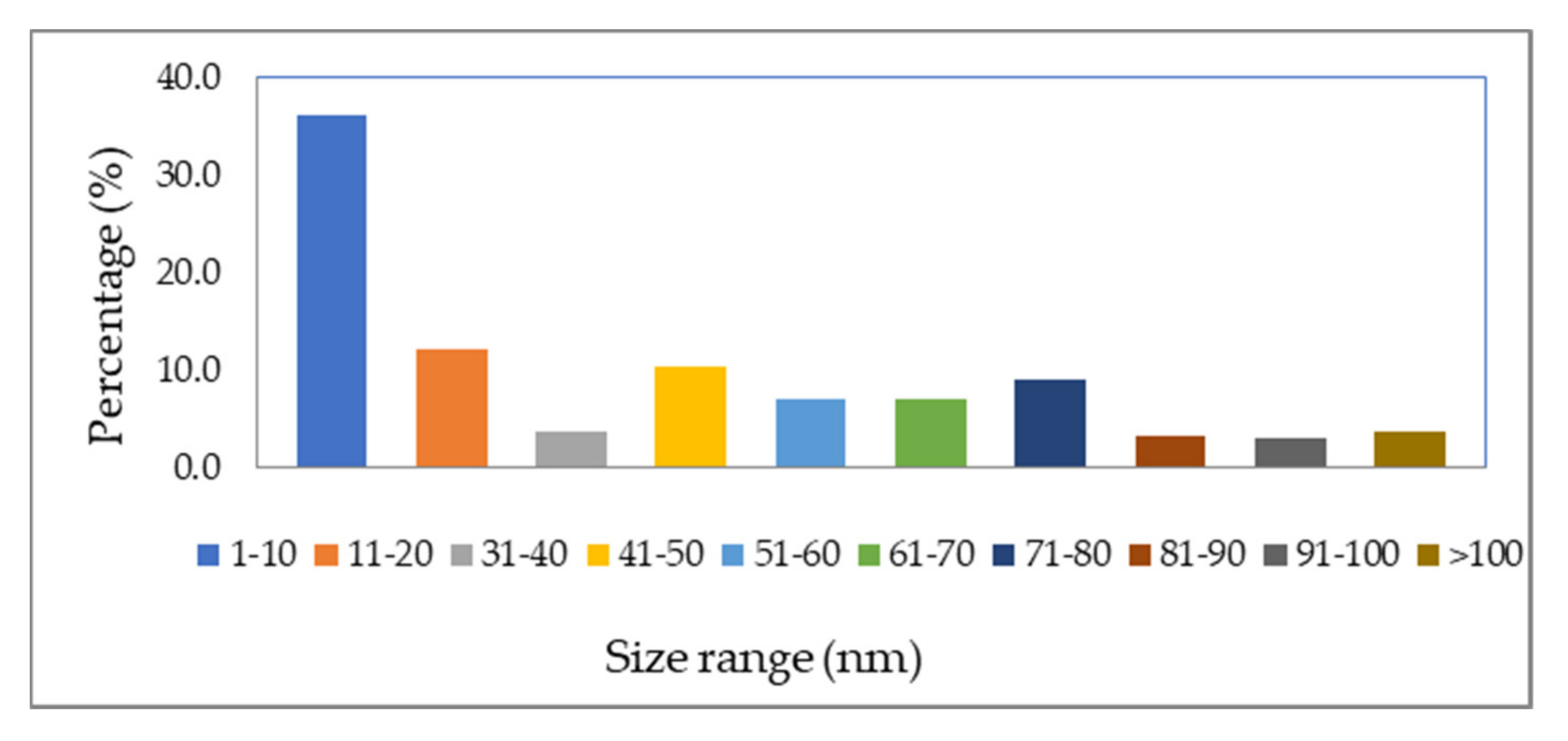
3.1.2. Transmission Electron Microscope (TEM)
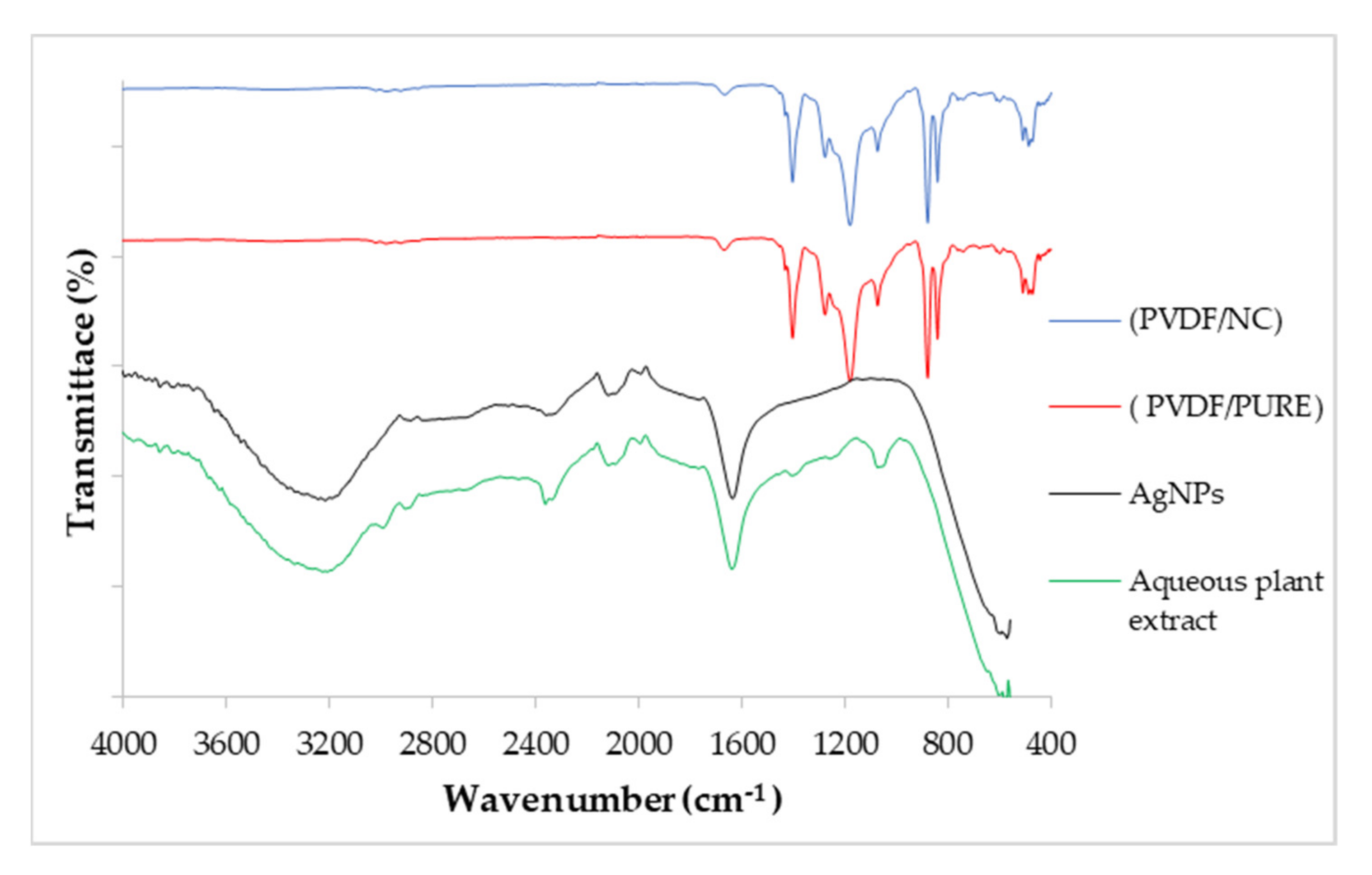
3.1.3. Fourier Transform Infrared Spectroscopy (FTIR)
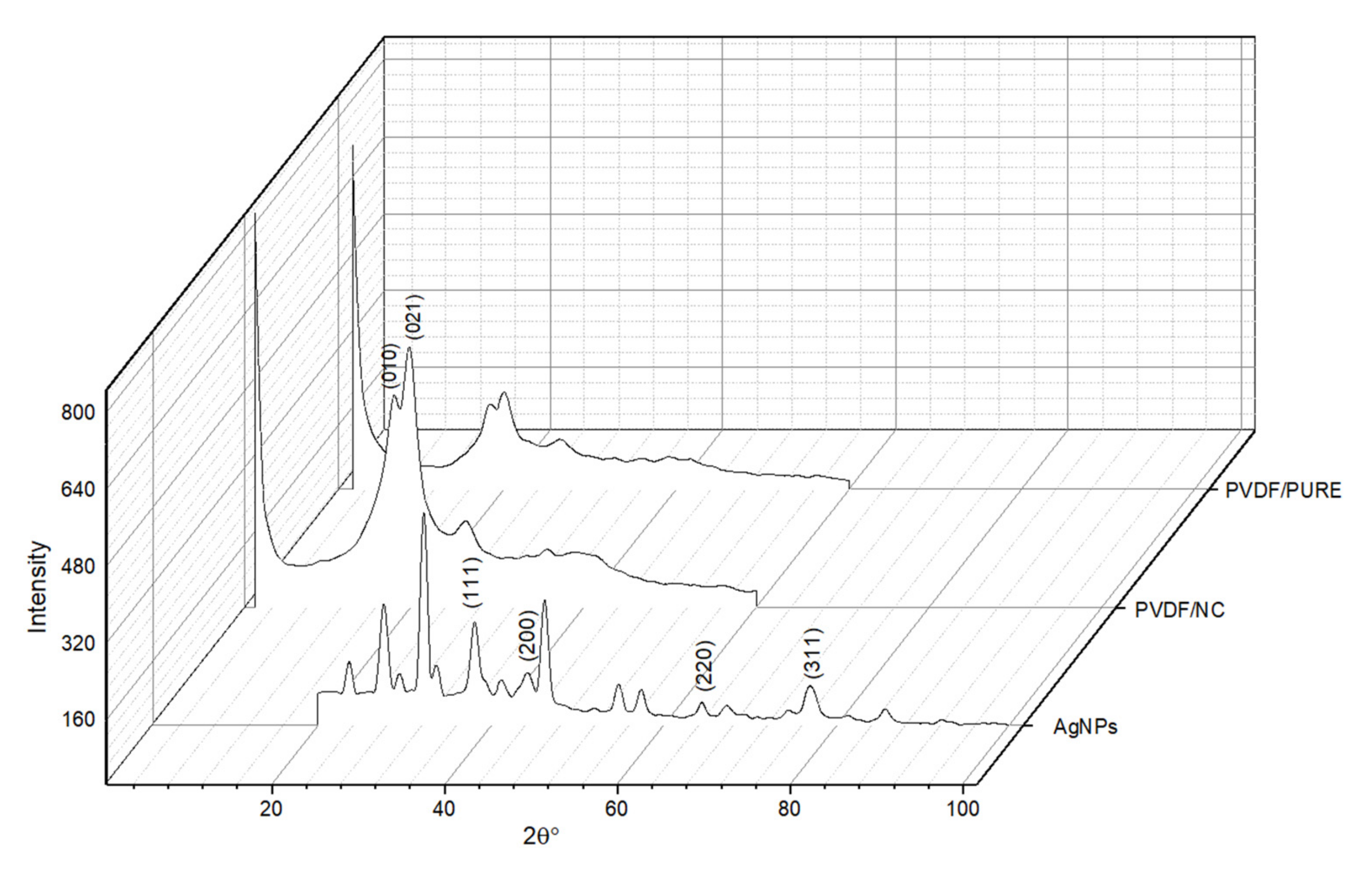
3.1.4. X-ray Diffraction (XRD)
3.2. Antibacterial Activity
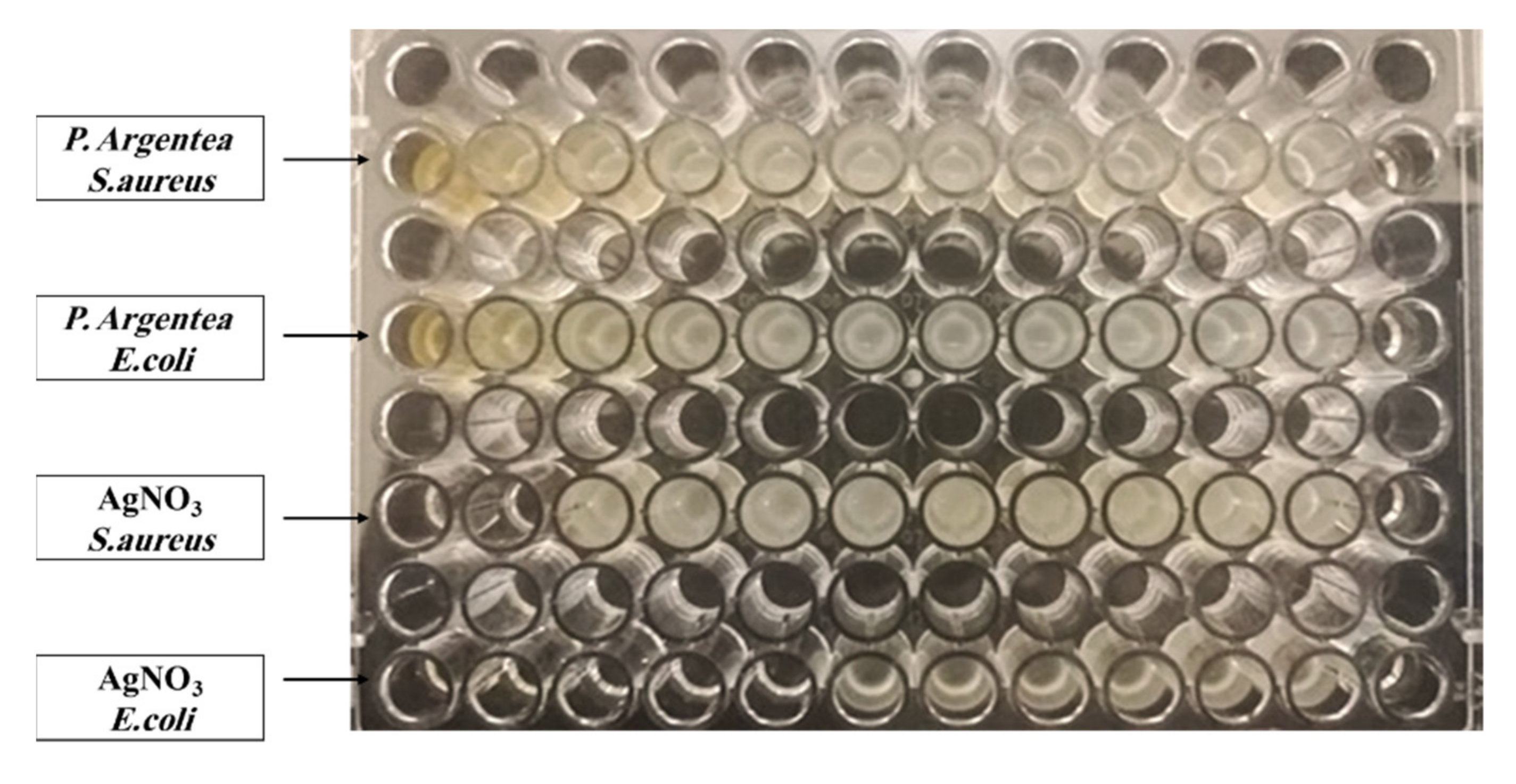
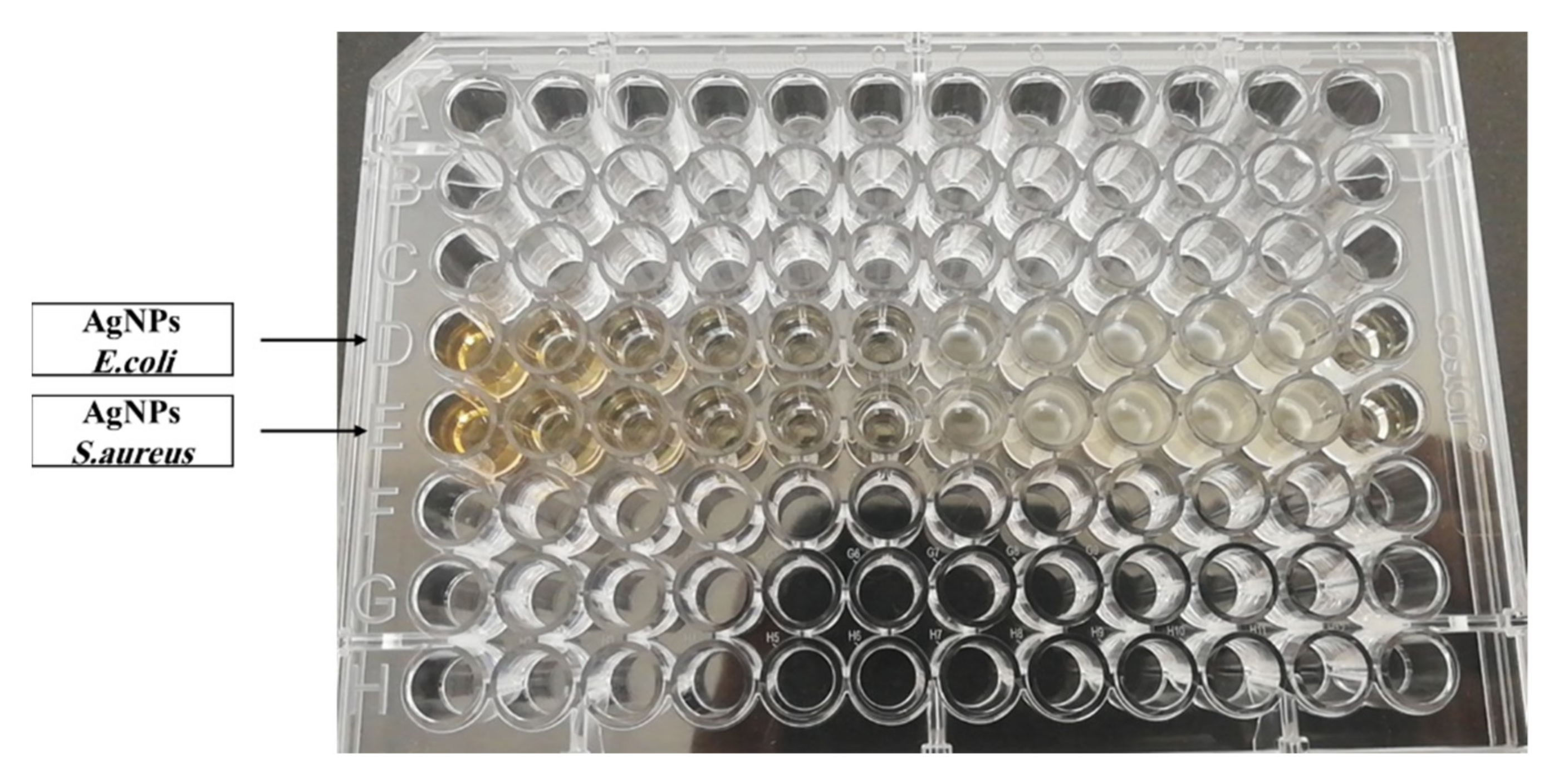
3.2.1. Microdilution Assay
3.2.2. Antibacterial Test for PVDF Membrane
3.2.3. SEM
3.2.4. The Hydrophilicity of Membranes
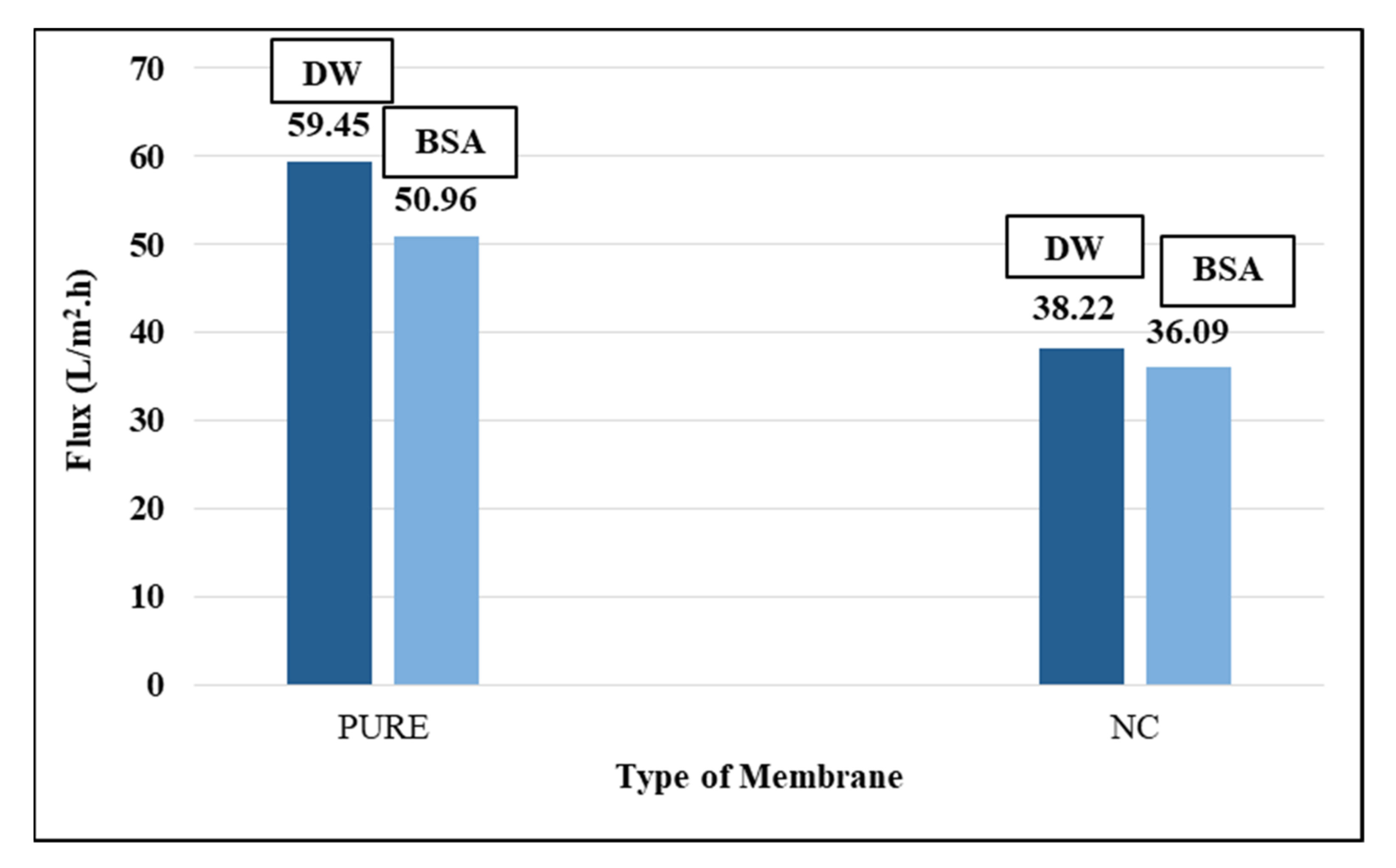
3.2.5. PVDF Membranes Permeation Experiments
3.2.6. AgNPs Release from Membranes
3.2.7. Porosity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arfanuzzaman, M.; Rahman, A.A. Sustainable Water Demand Management in the Face of Rapid Urbanization and Ground Water Depletion for Social–Ecological Resilience Building. Glob. Ecol. Conserv. 2017, 10, 9–22. [Google Scholar] [CrossRef]
- Surwade, S.P.; Smirnov, S.N.; Vlassiouk, I.V.; Unocic, R.R.; Veith, G.M.; Dai, S.; Mahurin, S.M. Water Desalination Using Nanoporous Single-Layer Graphene. Nat. Nanotechnol. 2015, 10, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zreig, M.; Ababneh, F.; Abdullah, F. Assessment of Rooftop Rainwater Harvesting in Northern Jordan. Phys. Chem. Earth Parts ABC 2019, 102794. [Google Scholar] [CrossRef]
- Hadadin, N.; Qaqish, M.; Akawwi, E.; Bdour, A. Water Shortage in Jordan—Sustainable Solutions. Desalination 2010, 250, 197–202. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Membrane Technology for Water Production in Agriculture: Desalination and Wastewater Reuse. Desalination 2015, 364, 17–32. [Google Scholar] [CrossRef]
- Le, N.L.; Nunes, S.P. Materials and Membrane Technologies for Water and Energy Sustainability. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhao, S.; Wang, Z.; Wu, J.; Wang, J.; Wang, S. In Situ Immobilization of Silver Nanoparticles for Improving Permeability, Antifouling and Anti-Bacterial Properties of Ultrafiltration Membrane. J. Membr. Sci. 2016, 499, 269–281. [Google Scholar] [CrossRef]
- Li, Z.; Rana, D.; Matsuura, T.; Lan, C.Q. The Performance of Polyvinylidene Fluoride-Polytetrafluoroethylene Nanocomposite Distillation Membranes: An Experimental and Numerical Study. Sep. Purif. Technol. 2019, 226, 192–208. [Google Scholar] [CrossRef]
- Oskoui, S.A.; Vatanpour, V.; Khataee, A. Effect of Different Additives on the Physicochemical Properties and Performance of NLDH/PVDF Nanocomposite Membrane. Sep. Purif. Technol. 2019, 209, 921–935. [Google Scholar] [CrossRef]
- Sun, C.; Feng, X. Enhancing the Performance of PVDF Membranes by Hydrophilic Surface Modification via Amine Treatment. Sep. Purif. Technol. 2017, 185, 94–102. [Google Scholar] [CrossRef]
- Hashaikeh, R.; Lalia, B.S.; Kochkodan, V.; Hilal, N. A Novel in Situ Membrane Cleaning Method Using Periodic Electrolysis. J. Membr. Sci. 2014, 471, 149–154. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C. One-Step Fabricated Bionic PVDF Ultrafiltration Membranes Exhibiting Innovative Antifouling Ability to the Cake Fouling. J. Membr. Sci. 2016, 515, 29–35. [Google Scholar] [CrossRef]
- Yuan, X.-T.; Xu, C.-X.; Geng, H.-Z.; Ji, Q.; Wang, L.; He, B.; Jiang, Y.; Kong, J.; Li, J. Multifunctional PVDF/CNT/GO Mixed Matrix Membranes for Ultrafiltration and Fouling Detection. J. Hazard. Mater. 2020, 384, 120978. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; He, G.; Li, H.; Zhao, R.; Han, Y.; Deng, Y. Antifouling Enhancement of Poly (Vinylidene Fluoride) Microfiltration Membrane by Adding Mg (OH)2 Nanoparticles. J. Membr. Sci. 2012, 387, 40–47. [Google Scholar] [CrossRef]
- Fan, T.; Miao, J.; Li, Z.; Cheng, B. Bio-Inspired Robust Superhydrophobic-Superoleophilic Polyphenylene Sulfide Membrane for Efficient Oil/Water Separation under Highly Acidic or Alkaline Conditions. J. Hazard. Mater. 2019, 373, 11–22. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Xu, Z.-L.; Shen, H.-M.; Yang, H. Preparation and Characterization of PVDF–SiO2 Composite Hollow Fiber UF Membrane by Sol–Gel Method. J. Membr. Sci. 2009, 337, 257–265. [Google Scholar] [CrossRef]
- Yuan, X.-S.; Guo, Z.-Y.; Geng, H.-Z.; Rhen, D.S.; Wang, L.; Yuan, X.-T.; Li, J. Enhanced Performance of Conductive Polysulfone/MWCNT/PANI Ultrafiltration Membrane in an Online Fouling Monitoring Application. J. Membr. Sci. 2019, 575, 160–169. [Google Scholar] [CrossRef]
- Laohaprapanon, S.; Vanderlipe, A.D.; Doma, B.T., Jr.; You, S.-J. Self-Cleaning and Antifouling Properties of Plasma-Grafted Poly (Vinylidene Fluoride) Membrane Coated with ZnO for Water Treatment. J. Taiwan Inst. Chem. Eng. 2017, 70, 15–22. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, H.; Zhou, X.; Liu, R.; Wu, Y.; Li, D.; Wang, Q.; Shi, X.; Deng, H. Acrylic Acid-Grafted Pre-Plasma Nanofibers for Efficient Removal of Oil Pollution from Aquatic Environment. J. Hazard. Mater. 2019, 371, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Shi, J.-Y.; Wang, J.-L.; Li, Y.-L.; Gao, N.-N.; Liu, Z.-X.; Lian, W.-T. Preparation and Characterization of PEG-g-MWCNTs/PSf Nano-Hybrid Membranes with Hydrophilicity and Antifouling Properties. RSC Adv. 2015, 5, 84746–84753. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Z.; Wang, J.; Wang, S. A Novel Method of Surface Modification to Polysulfone Ultrafiltration Membrane by Preadsorption of Citric Acid or Sodium Bisulfite. Membr. Water Treat. 2012, 3, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.-Y.; Yuan, X.-S.; Geng, H.-Z.; Wang, L.-D.; Jing, L.-C.; Gu, Z.-Z. High Conductive PPy–CNT Surface-Modified PES Membrane with Anti-Fouling Property. Appl. Nanosci. 2018, 8, 1597–1606. [Google Scholar] [CrossRef]
- Mehta, R.; Brahmbhatt, H.; Bhojani, G.; Mukherjee, M.; Bhattacharya, A. Poly (Piperizinamide) with Copper Ion Composite Membranes: Application for Mitigation of Hexaconazole from Water and Combat Microbial Contamination. J. Hazard. Mater. 2019, 376, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Wang, H.; Song, X.; Wang, T.; He, B.; Liang, X.; Ngo, H.H. An Electrocatalytic Membrane Reactor with Self-cleaning Function for Industrial Wastewater Treatment. Angew. Chem. 2011, 123, 2196–2198. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Hussein, M.A.; Khaleel, M.A.; Khraisheh, M.; Hilal, N. Can Carbon-Based Nanomaterials Revolutionize Membrane Fabrication for Water Treatment and Desalination? Desalination 2016, 391, 69–88. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M. A Review of Water Treatment Membrane Nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J. Nanotechnology for a Safe and Sustainable Water Supply: Enabling Integrated Water Treatment and Reuse. Acc. Chem. Res. 2013, 46, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Varma, R.S.; Zafarnia, N.; Yaghoobi, H.; Sarani, M.; Kumar, V.G. Applications of Green Synthesized Ag, ZnO and Ag/ZnO Nanoparticles for Making Clinical Antimicrobial Wound-Healing Bandages. Sustain. Chem. Pharm. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Maddinedi, S.; Mandal, B.K.; Maddili, S.K. Biofabrication of Size Controllable Silver Nanoparticles–a Green Approach. J. Photochem. Photobiol. B 2017, 167, 236–241. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green Synthesis of Silver Nanoparticles from Leaf Extract of Mimusops Elengi, Linn. for Enhanced Antibacterial Activity against Multi Drug Resistant Clinical Isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Czernel, G.; Bloch, D.; Matwijczuk, A.; Cieśla, J.; Kędzierska-Matysek, M.; Florek, M.; Gagoś, M. Biodirected Synthesis of Silver Nanoparticles Using Aqueous Honey Solutions and Evaluation of Their Antifungal Activity against Pathogenic Candida Spp. Int. J. Mol. Sci. 2021, 22, 7715. [Google Scholar] [CrossRef] [PubMed]
- Jebril, S.; Jenana, R.K.B.; Dridi, C. Green Synthesis of Silver Nanoparticles Using Melia Azedarach Leaf Extract and Their Antifungal Activities: In Vitro and In Vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Mittal, A.K.; Kaler, A.; Banerjee, U.C. Free Radical Scavenging and Antioxidant Activity of Silver Nanoparticles Synthesized from Flower Extract of Rhododendron Dauricum. Nano Biomed. Eng. 2012, 4, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Abduraimova, A.; Molkenova, A.; Duisembekova, A.; Mulikova, T.; Kanayeva, D.; Atabaev, T.S. Cetyltrimethylammonium Bromide (CTAB)-Loaded SiO2–Ag Mesoporous Nanocomposite as an Efficient Antibacterial Agent. Nanomaterials 2021, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Roco, M.C.; Li, X.; Lin, Y. Trends in Nanotechnology Patents. Nat. Nanotechnol. 2008, 3, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Khadri, H.; Alzohairy, M.; Janardhan, A.; Kumar, A.P.; Narasimha, G. Green Synthesis of Silver Nanoparticles with High Fungicidal Activity from Olive Seed Extract. Adv. Nanopart. 2013, 2, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Benakashani, F.; Allafchian, A.; Jalali, S.A.H. Green Synthesis, Characterization and Antibacterial Activity of Silver Nanoparticles from Root Extract of Lepidium Draba Weed. Green Chem. Lett. Rev. 2017, 10, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Padalia, H.; Moteriya, P.; Chanda, S. Green Synthesis of Silver Nanoparticles from Marigold Flower and Its Synergistic Antimicrobial Potential. Arab. J. Chem. 2015, 8, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Kaur, G.; Sharma, P.; Singh, S.; Ikram, S. Fruit Waste (Peel) as Bio-Reductant to Synthesize Silver Nanoparticles with Antimicrobial, Antioxidant and Cytotoxic Activities. J. Appl. Biomed. 2018, 16, 221–231. [Google Scholar]
- Lakshmanan, G.; Sathiyaseelan, A.; Kalaichelvan, P.T.; Murugesan, K. Plant-Mediated Synthesis of Silver Nanoparticles Using Fruit Extract of Cleome viscosa L.: Assessment of Their Antibacterial and Anticancer Activity. Karbala Int. J. Mod. Sci. 2018, 4, 61–68. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; Mendoza, E.G.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green Synthesis of Silver Nanoparticles Using Lysiloma Acapulcensis Exhibit High-Antimicrobial Activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Oh, H.-J.; Dao, V.-D.; Choi, H.-S. Electromagnetic Shielding Effectiveness of a Thin Silver Layer Deposited onto PET Film via Atmospheric Pressure Plasma Reduction. Appl. Surf. Sci. 2018, 435, 7–15. [Google Scholar] [CrossRef]
- Xuan, L.; Tian, L.-J.; Tian, T.; Wang, X.-M.; Yang, D.-H.; Yu, H.-Q. In Situ Synthesizing Silver Nanoparticels by Bio-Derived Gallic Acid to Enhance Antimicrobial Performance of PVDF Membrane. Sep. Purif. Technol. 2020, 251, 117381. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Derese, S.; Gutierrez, L.; Verliefde, A.R.; Mamba, B.B.; Barnard, T.G.; Mhlanga, S.D. Green Synthesis of Silver Nanoparticles Using One-Pot and Microwave-Assisted Methods and Their Subsequent Embedment on PVDF Nanofibre Membranes for Growth Inhibition of Mesophilic and Thermophilic Bacteria. New J. Chem. 2019, 43, 4168–4180. [Google Scholar] [CrossRef]
- Fahrina, A.; Arahman, N.; Mulyati, S.; Aprilia, S.; Mat Nawi, N.I.; Aqsha, A.; Bilad, M.R.; Takagi, R.; Matsuyama, H. Development of Polyvinylidene Fluoride Membrane by Incorporating Bio-Based Ginger Extract as Additive. Polymers 2020, 12, 2003. [Google Scholar] [CrossRef]
- Fahrina, A.; Yusuf, M.; Muchtar, S.; Fitriani, F.; Mulyati, S.; Aprilia, S.; Rosnelly, C.M.; Bilad, M.R.; Ismail, A.F.; Takagi, R.; et al. Development of Anti-Microbial Polyvinylidene Fluoride (PVDF) Membrane Using Bio-Based Ginger Extract-Silica Nanoparticles (GE-SiNPs) for Bovine Serum Albumin (BSA) Filtration. J. Taiwan Inst. Chem. Eng. 2021, 125, 323–331. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Antibacterial and Catalytic Activities of Green Synthesized Silver Nanoparticles. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 135, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Li, X.; Zhao, X.; Liu, D.; He, C. Engineering a Highly Hydrophilic PVDF Membrane via Binding TiO 2 Nanoparticles and a PVA Layer onto a Membrane Surface. ACS Appl. Mater. Interfaces 2015, 7, 8427–8436. [Google Scholar] [CrossRef]
- Sivakumar, M.; Mohanasundaram, A.; Mohan, D.; Balu, K.; Rangarajan, R. Modification of Cellulose Acetate: Its Characterization and Application as an Ultrafiltration Membrane. J. Appl. Polym. Sci. 1998, 67, 1939–1946. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Ge, Q.; Wang, H.; Xu, T. Preparation of Polyethersulfone/Carbon Nanotube Substrate for High-Performance Forward Osmosis Membrane. Desalination 2013, 330, 70–78. [Google Scholar] [CrossRef]
- Marinho, R.; Horiuchi, L.; Pires, C.A. Effect of stirring speed on conversion and time to particle stabilization of poly (vinyl chloride) produced by suspension polymerization process at the beginning of reaction. Braz. J. Chem. Eng. 2018, 35, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Rolim, W.R.; Pelegrino, M.T.; de Araújo Lima, B.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.B. Green Tea Extract Mediated Biogenic Synthesis of Silver Nanoparticles: Characterization, Cytotoxicity Evaluation and Antibacterial Activity. Appl. Surf. Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- AbuDalo, M.A.; Al-Mheidat, I.R.; Al-Shurafat, A.W.; Grinham, C.; Oyanedel-Craver, V. Synthesis of Silver Nanoparticles Using a Modified Tollens’ Method in Conjunction with Phytochemicals and Assessment of Their Antimicrobial Activity. PeerJ 2019, 7, e6413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atarod, M.; Nasrollahzadeh, M.; Mohammad Sajadi, S. Euphorbia Heterophylla Leaf Extract Mediated Green Synthesis of Ag/TiO2 Nanocomposite and Investigation of Its Excellent Catalytic Activity for Reduction of Variety of Dyes in Water. J. Colloid Interface Sci. 2016, 462, 272–279. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of Silver Nanoparticles Using Marine Macroalgae padina sp. and Its Antibacterial Activity towards Pathogenic Bacteria. Beni-Suef. Univ. J. Basic Appl. Sci. 2020, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Hess, W.; Frisch, H.L.; Klein, R. On the Hydrodynamic Behavior of Colloidal Aggregates. Zeitschrift Für Physik B Condensed Matter 1986, 64, 65–67. [Google Scholar] [CrossRef]
- Odeh, F.; Heldt, N.; Gauger, M.; Slack, G.; Li, Y. PFG-NMR Investigation of Liposome Systems Containing Hydrotrope. J. Dispers. Sci. Technol. 2006, 27, 665–669. [Google Scholar] [CrossRef]
- Singh, S.; Bharti, A.; Meena, V.K. Structural, Thermal, Zeta Potential and Electrical Properties of Disaccharide Reduced Silver Nanoparticles. J. Mater. Sci. Mater. Electron. 2014, 25, 3747–3752. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of Environmental Conditions (PH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.W.; Midmore, B.R.; Lamb, A.; Hunter, R.J. Electroacoustic Studies of Moderately Concentrated Colloidal Suspensions. Faraday Discuss. Chem. Soc. 1990, 90, 301–312. [Google Scholar] [CrossRef]
- Edison, T.J.I.; Sethuraman, M.G. Instant Green Synthesis of Silver Nanoparticles Using Terminalia Chebula Fruit Extract and Evaluation of Their Catalytic Activity on Reduction of Methylene Blue. Process Biochem. 2012, 47, 1351–1357. [Google Scholar] [CrossRef]
- Al-Bakri, A.G.; Afifi, F.U. Evaluation of Antimicrobial Activity of Selected Plant Extracts by Rapid XTT Colorimetry and Bacterial Enumeration. J. Microbiol. Methods 2007, 68, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; Bader, A.; Siciliano, T.; De Tommasi, N. Secondary Metabolites from Paronychia argentea. Magn. Reson. Chem. 2008, 46, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Alenizi, A.; Shibli, R.A.; Tahtamouni, R.W.; Al-Qudah, T.S.; Abu-Iramaileh, B. In Vitro Propagation and Enhancement of Quercetins and Isorhamnetin Production in Wild Paronychia argentea L. Jordan J. Pharm. Sci. 2020, 13, 65–75. [Google Scholar]
- Bulut, E.; Özacar, M. Rapid, Facile Synthesis of Silver Nanostructure Using Hydrolyzable Tannin. Ind. Eng. Chem. Res. 2009, 48, 5686–5690. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Alinezhad, H.; Nasrollahzadeh, M.; Kamali, T.A. Green Synthesis of the Ag/HZSM-5 Nanocomposite by Using Euphorbia Heterophylla Leaf Extract: A Recoverable Catalyst for Reduction of Organic Dyes. J. Alloys Compd. 2016, 685, 258–265. [Google Scholar] [CrossRef]
- Al-Khateeb, F.I. Studying the Influence of Salinity Stress on Growth, Physiological Changes, and Phenolic Compounds Production of In Vitro Grown Paronychia argentea. 2018. Available online: http://hip.jopuls.org.jo/c/portal/layout?p_l_id=PUB.1016.1&p_p_id=search_WAR_fusion&p_p_action=1&p_p_state=normal&p_p_mode=view&p_p_col_id=column-1&p_p_col_pos=0&p_p_col_count=1&_search_WAR_fusion_action=navigate&_search_WAR_fusion_navigationData=full%7E%3D1%7E%217028410%7E%21442%7E%21442%7E%211%7E%212147483647%7E%21 (accessed on 18 October 2021).
- Ye, R.; Ni, M.; Chen, H.; Li, S. Photochemical Synthesis of Silver Nanoparticles in H2O/Triton X-100/[Bmim]PF 6 Ionic Liquid Microemulsions and Their Antimicrobial Activity. Mater. Express 2020, 10, 267–271. [Google Scholar] [CrossRef]
- Ali, K.; Ahmed, B.; Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Microwave Accelerated Green Synthesis of Stable Silver Nanoparticles with Eucalyptus Globulus Leaf Extract and Their Antibacterial and Antibiofilm Activity on Clinical Isolates. PLoS ONE 2015, 10, e0131178. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green Synthesis of Silver Nanoparticles Using the Plant Extract of Salvia Spinosa Grown in Vitro and Their Antibacterial Activity Assessment. J. Nanostructure Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Cabrera, J.; Zhong, L.; Wang, W.; Duan, D.; Wang, X.; Liu, S.; Xie, Y.F. Using Loose Nanofiltration Membrane for Lake Water Treatment: A Pilot Study. Front. Environ. Sci. Eng. 2020, 15, 69. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β and γ Phases in Poly (Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Mehta, B.; Chhajlani, M.; Shrivastava, B. Green Synthesis of Silver Nanoparticles and Their Characterization by XRD; IOP Publishing: Bristol, UK, 2017; Volume 836, p. 12050. [Google Scholar]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Tripathy, M.; Paliwal, N. Green Synthesis, Characterization, Antibacterial, Antioxidant and Photocatalytic Activity of Parkia Speciosa Leaves Extract Mediated Silver Nanoparticles. Results Phys. 2019, 15, 102565. [Google Scholar] [CrossRef]
- Kim, J.; Cho, W.; Ha, C. Morphology, Crystalline Structure, and Properties of Poly (Vinylidene Fluoride)/Silica Hybrid Composites. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 19–30. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Liu, L.; Zhang, Y.; Wang, Z.; Zhang, Q. Functional Poly(Vinylidene Fluoride) Membrane Anchored with Silver Nanoparticle with Antibacterial Activity. Synth. Met. 2013, 174, 1–5. [Google Scholar] [CrossRef]
- Fu, G.; Vary, P.S.; Lin, C.-T. Anatase TiO2 Nanocomposites for Antimicrobial Coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial Activity of the Metals and Metal Oxide Nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Prasher, P.; Singh, M.; Mudila, H. Oligodynamic Effect of Silver Nanoparticles: A Review. BioNanoScience 2018, 8, 951–962. [Google Scholar] [CrossRef]
- Wani, I.A.; Khatoon, S.; Ganguly, A.; Ahmed, J.; Ahmad, T. Structural Characterization and Antimicrobial Properties of Silver Nanoparticles Prepared by Inverse Microemulsion Method. Colloids Surf. B Biointerfaces 2013, 101, 243–250. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.; Xiao, T.; Zhao, W.; Li, J.; Zhao, C. Tannic Acid-Inspiration and Post-Crosslinking of Zwitterionic Polymer as a Universal Approach towards Antifouling Surface. Chem. Eng. J. 2018, 337, 122–132. [Google Scholar] [CrossRef]
- Li, J.-H.; Shao, X.-S.; Zhou, Q.; Li, M.-Z.; Zhang, Q.-Q. The Double Effects of Silver Nanoparticles on the PVDF Membrane: Surface Hydrophilicity and Antifouling Performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.; Matsuura, T.; Kassim, M.; Abdullah, M. Effect of Modified PVDF Hollow Fiber Submerged Ultrafiltration Membrane for Refinery Wastewater Treatment. Desalination 2011, 283, 214–220. [Google Scholar] [CrossRef]
- Cui, A.; Liu, Z.; Xiao, C.; Zhang, Y. Effect of Micro-Sized SiO2-Particle on the Performance of PVDF Blend Membranes via TIPS. J. Membr. Sci. 2010, 360, 259–264. [Google Scholar] [CrossRef]





| The Aqueous P. argentea Extract Volume (mL) | The Conversion Percentage of Ag+ to AgNPs |
|---|---|
| 6.0 | 57.4% |
| 8.0 | 83.2% |
| 10.0 | 67.8% |
| 12.0 | 69.0% |
| The Aqueous P. argentea Extract Volume (mL) | The Magnetic Stirrer Rotation Velocity (rpm) | The Conversion Percentage of Ag+ to AgNPs |
|---|---|---|
| 8.0 | 50 | 78.2% |
| 8.0 | 750 | 83.20% |
| 8.0 | 1000 | 96.50% |
| 8.0 | 1500 | 67.40% |
| Bacteria | The Extract of P. argentea | AgNO3 | AgNPs | |||
|---|---|---|---|---|---|---|
| MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MBC (μL/mL) | |
| S. aureus ATCC no. 25193 | N.D | N.D | 80.9 | 80.9 | 4.9 | 4.9 |
| E. coli ATCC no. 8739 | N.D | N.D | 5.05 | 5.05 | 4.9 | 19.9 |
| Method of Synthesis AgNPs | Average Size (nm) of AgNPs | Antimicrobial Activity | Reference |
|---|---|---|---|
| Photoreduction | 10 nm by TEM. | By the solution of 1.56 μg/mL, AgNPs were killed over 99% of (E. coli) BL21. | [69] |
| A commercial green tea extract | 34.68 ± 4.95 nm by DLS. | Various concentrations of AgNPs (2-fold dilution: 1000–0.1 µg/mL) were used in each well. S. aureus; ATCC 29213→ MIC = 250 µg/mL. MBC = 250 µg/mL. E. coli; ATCC 25922→ AgNPs: MIC = 15 µg/mL. MBC = 15 µg/mL. | [53] |
| Microemulsion method | The TEM average size of AgNPs was (8–40) nm. | E. coli; MTCC 443→ 40 nm AgNPs MIC = 100 µg/mL. 30 nm AgNPs MIC = 75 µg/mL. 8 nm AgNPs MIC = 50 µg/mL. | [81] |
| Our Work Green synthesis of AgNPs by the aqueous extract of P. argentea | The average sizes were of DLS 71.1 nm. The results of TEM showed that the highest percentage of diameter is less than 10 nm. | The solution of AgNPs (312.5 µL/mL) S. aureus; ATCC 25193 → MIC = 4.9 µg/mL. MBC = 4.9 µg/mL. E. coli; ATCC 8739 → MIC = 4.9 µg/mL. MBC = 19.9 µL/mL. |
| Membrane | Type | (E. coli) Bacteria (ATCC no. 8739) | (S. aureus) Bacteria (ATCC no. 25913) |
|---|---|---|---|
| PVDF | Control (PVDF-PURE) | 48.9 × 106 Bacteria/cm2 | 1.7 × 106 Bacteria/cm2 |
| PVDF | Treatment with AgNPs (PVDF-NC) | 10 Bacteria/cm2 | 10 Bacteria/cm2 |
| Sterilization ratio | 99.9% | 99.9% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnairat, N.; Abu Dalo, M.; Abu-Zurayk, R.; Abu Mallouh, S.; Odeh, F.; Al Bawab, A. Green Synthesis of Silver Nanoparticles as an Effective Antibiofouling Material for Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane. Polymers 2021, 13, 3683. https://doi.org/10.3390/polym13213683
Alnairat N, Abu Dalo M, Abu-Zurayk R, Abu Mallouh S, Odeh F, Al Bawab A. Green Synthesis of Silver Nanoparticles as an Effective Antibiofouling Material for Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane. Polymers. 2021; 13(21):3683. https://doi.org/10.3390/polym13213683
Chicago/Turabian StyleAlnairat, Nour, Muna Abu Dalo, Rund Abu-Zurayk, Saida Abu Mallouh, Fadwa Odeh, and Abeer Al Bawab. 2021. "Green Synthesis of Silver Nanoparticles as an Effective Antibiofouling Material for Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane" Polymers 13, no. 21: 3683. https://doi.org/10.3390/polym13213683
APA StyleAlnairat, N., Abu Dalo, M., Abu-Zurayk, R., Abu Mallouh, S., Odeh, F., & Al Bawab, A. (2021). Green Synthesis of Silver Nanoparticles as an Effective Antibiofouling Material for Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane. Polymers, 13(21), 3683. https://doi.org/10.3390/polym13213683







