Extraction of Cyclic Oligomer and Their Influence on Polyester Dyeing in a Silicone Waterless Dyeing System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oligomer Treatment Method and Dyeing of Polyester Fabric
2.2.1. Quantitative Analysis of Oligomers
2.2.2. Pretreatment of Polyester Sample
2.2.3. High-Performance Liquid Chromatography (HPLC) Analysis
2.2.4. Dyeing of Polyester in the Silicone Waterless Dyeing System
2.3. Characterization and Test Methods
2.3.1. Exhaustion Percent
2.3.2. Color Strength
2.3.3. Color Uniformity
2.3.4. Differential Scanning Calorimetry (DSC) Characterization
2.3.5. Dynamic Mechanical Analysis (DMA)
2.3.6. X-ray Diffraction Analysis (XRD) Analysis
2.3.7. Colorfastness
2.3.8. Scanning Electron Microscopy Analysis
3. Results and Discussion
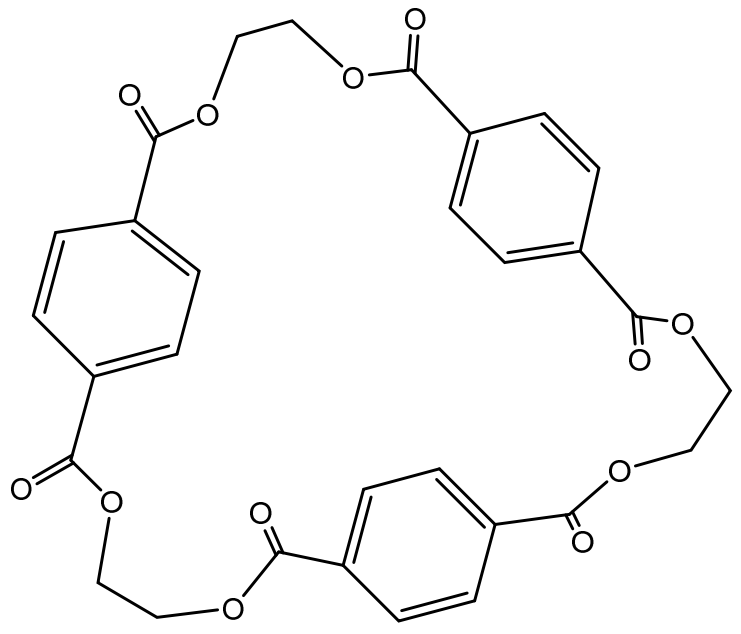
3.1. Oligomer Content in Different Samples
3.2. High-Performance Liquid Chromatography (HPLC) Analysis
3.3. Influence of Oligomers on the Dyeing Properties of Polyester
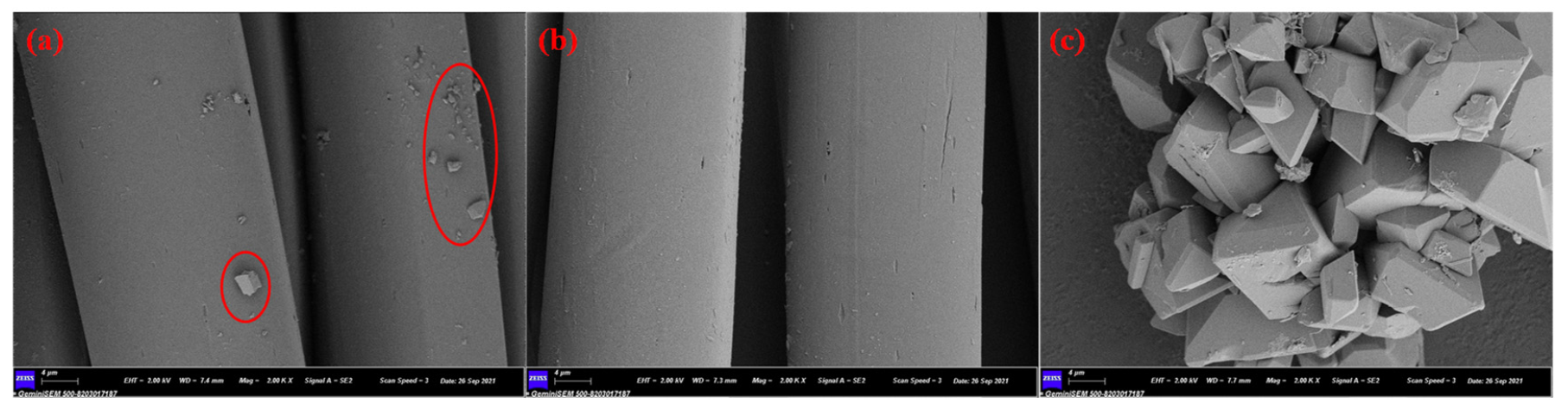
3.4. SEM Analysis
3.5. DSC Analysis
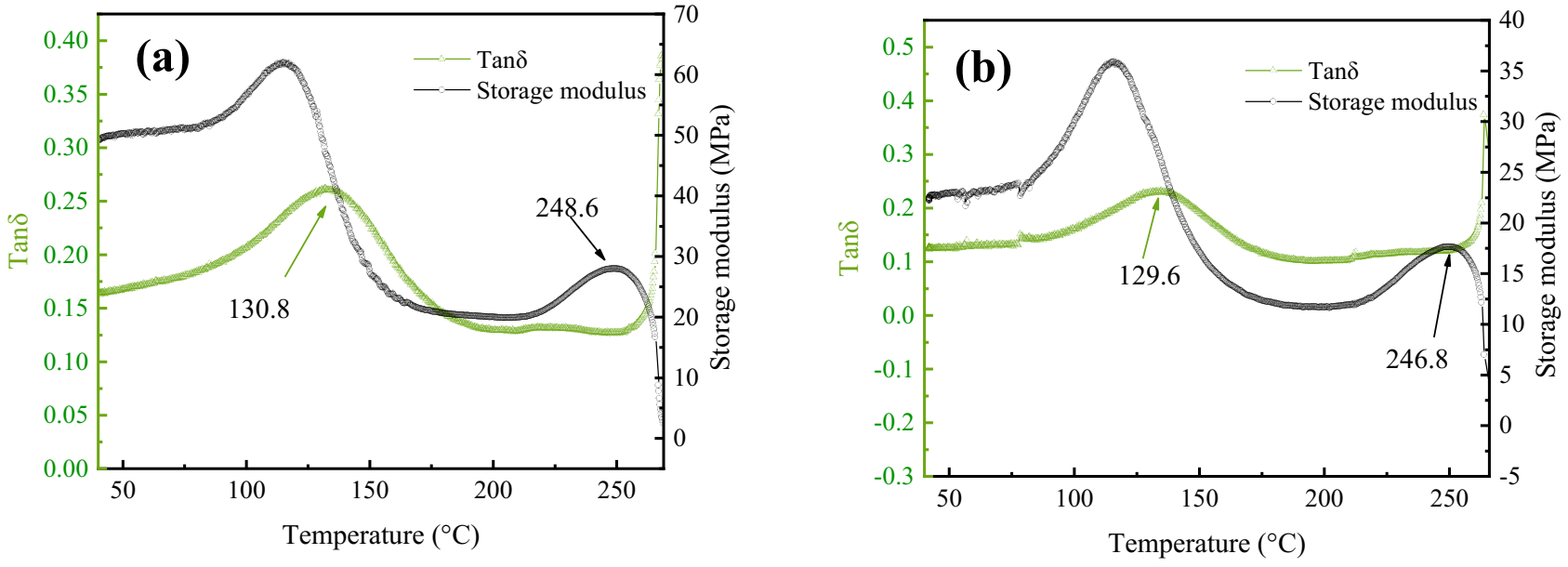
3.6. DMA Analysis
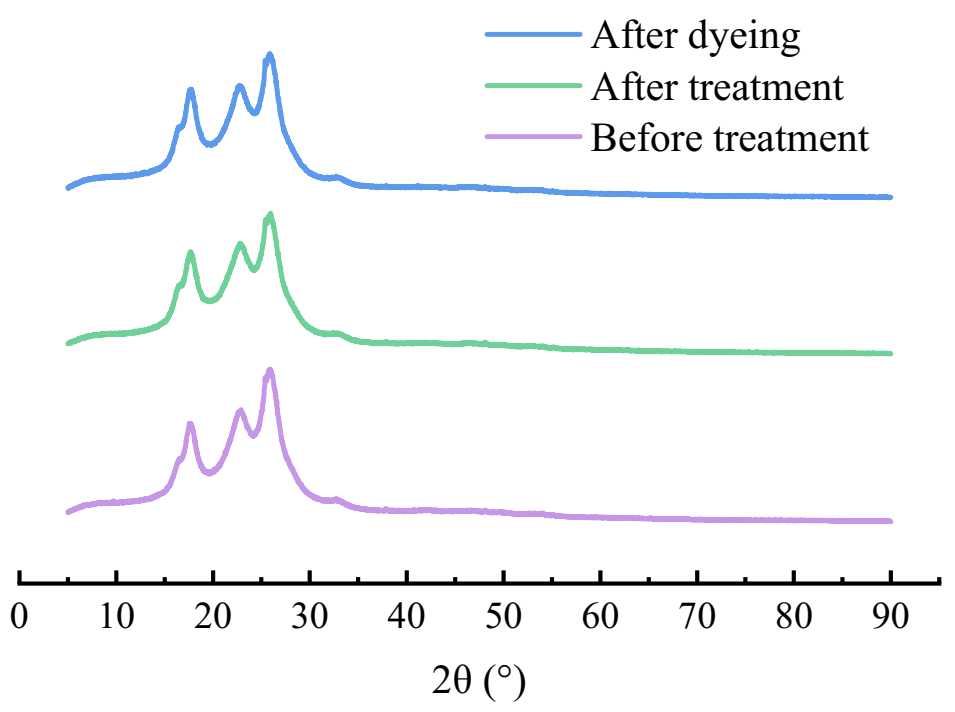
3.7. XRD Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Gao, L.; Hou, A.; Xie, K.; Song, X. Design, synthesis of novel bisazo disperse dyes: Structure analysis and dyeing performance on PET. Dyes Pigm. 2021, 196, 109761. [Google Scholar] [CrossRef]
- Royer, B.; Cardoso, N.F.; Lima, E.C.; Ruiz, V.S.O.; Macedo, T.R.; Airoldi, C. Organofunctionalized kenyaite for dye removal from aqueous solution. J. Colloid. Interf. Sci. 2009, 336, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-R.; Li, H.-B.; Wang, W.-H.; Gu, J.-D. Decolorization of dyes and textile wastewater by potassium permanganate. Chemosphere 2005, 59, 893–898. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A. Dyeing performance of disperse dyes on polyester fabrics using eco-friendly carrier and their antioxidant and anticancer activities. Int. J. Environ. Res. Public Health 2019, 16, 4603. [Google Scholar] [CrossRef] [Green Version]
- Elmaaty, T.A.; Sofan, M.; Kosbar, T.; Elsisi, H.; Negm, I. Green Approach to Dye PET and Nylon 6 Fabrics with Novel Pyrazole Disperse Dyes under Supercritical Carbon Dioxide and Its Aqueous Analogue. Fiber. Polym. 2019, 20, 2510–2521. [Google Scholar] [CrossRef]
- Hou, A.; Chen, B.; Dai, J.; Zhang, K. Using supercritical carbon dioxide as solvent to replace water in polyethylene terephthalate (PET) fabric dyeing procedures. J. Clean. Prod. 2010, 18, 1009–1014. [Google Scholar] [CrossRef]
- Hori, T.; Kongdee, A. Dyeing of PET/co-PP composite fibers using supercritical carbon dioxide. Dyes Pigm. 2014, 105, 163–166. [Google Scholar] [CrossRef]
- Li, S.Z.; Liu, J.Q.; Li, Y.Q.; Li, L.M. Study on the Dyeing of PET Fiber with Disperse Dyes in D5 Media. Adv. Mat. Res. 2011, 331, 334–337. [Google Scholar] [CrossRef]
- Pei, L.; Huang, Y.; Zhang, H.; Wang, J. Effect of dispersant on disperse dyeing for polyester fabric in silicone waterless dyeing system. J. Tex. Res. 2020, 6, 1–6. [Google Scholar]
- Yu, S.; Zhang, H.; Pei, L.; Liang, S.; Dong, A.; Wang, J. Substituent-Dyeing Properties Relationship of Disperse Dyes on Polyester in Low Pressure Waterless Dyeing System. Fiber. Polym. 2021, 8, 1–7. [Google Scholar]
- Wang, J.; Cheng, W.; Gao, Y.; Zhu, L.; Pei, L. Mechanism of Accelerant on Disperse Dyeing for PET Fiber in the Silicone Solvent Dyeing System. Polymers 2019, 11, 520. [Google Scholar] [CrossRef] [Green Version]
- Ferri, A.; Banchero, M.; Manna, L.; Sicardi, S. An experimental technique for measuring high solubilities of dyes in supercritical carbon dioxide. J. Supercrit. Fluid. 2004, 30, 41–49. [Google Scholar] [CrossRef]
- Saleem, M.A.; Pei, L.; Saleem, M.F.; Shahid, S.; Wang, J. Sustainable dyeing of nylon with disperse dyes in Decamethylcyclopentasiloxane waterless dyeing system. J. Clean. Prod. 2020, 276, 123258. [Google Scholar] [CrossRef]
- An, Y.; Miao, J.; Fan, J.; Li, M.; Hu, M.G.; Shao, M.; Shao, J. High-efficiency dispersant-free polyester dyeing using D5 non-aqueous medium. Dyes Pigm. 2021, 190, 109303. [Google Scholar] [CrossRef]
- Eckardt, M.; Schneider, J.; Simat, T.J. In vitro intestinal digestibility of cyclic aromatic polyester oligomers from polyethylene terephthalate (PET) and polybutylene terephthalate (PBT). Food Addit. Contam. Part A-Chem. 2019, 36, 1882–1894. [Google Scholar] [CrossRef]
- Vermylen, V.; Lodefier, P.; Devaux, J.; Legras, R.; Hoffmann, E.D. Study of the thermal evolution of the cyclic-oligomer formation in a cyclic-oligomer-free pet. J. Poly. Sci. Poly. Chem. 2015, 38, 416–422. [Google Scholar] [CrossRef]
- Wang, Q.F.; Keffer, D.J.; Petrovan, S.; Thomas, J.B. Molecular Dynamics Simulation of Poly(ethylene terephthalate) Oligomers. J. Phys. Chem. B 2010, 114, 786–795. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Alberto Lopes, J.; Kappenstein, O.; Tietz, T.; Hoekstra, E.J. Quantification of PET cyclic and linear oligomers in teabags by a validated LC-MS method—In silico toxicity assessment and consumer’s exposure. Food Chem. 2020, 317, 126427. [Google Scholar] [CrossRef]
- Montero, G.; Hinks, D.; Hooker, J. Reducing problems of cyclic trimer deposits in supercritical carbon dioxide polyester dyeing machinery. J. Supercrit. Fluid. 2003, 26, 47–54. [Google Scholar] [CrossRef]
- Peets, P.; Vahur, S.; Kruve, A.; Haljasorg, T.; Herodes, K.; Pagano, T.; Leito, I. Instrumental techniques in the analysis of natural red textile dyes. J. Cult. Herit. 2020, 42, 19–27. [Google Scholar] [CrossRef]
- de Alvarenga Freire, M.T.; Damant, A.; Castle, L.; Reyes, F. Thermal stability of polyethylene terephthalate (PET): Oligomer distribution and formation of volatiles. Packag. Technol. Sci. 1999, 12, 29–36. [Google Scholar] [CrossRef]
- Costley, C.; Newton, I.; Carroll, J. Extraction of Oligomers From Poly(ethylene terephthalate) by Microwave-assisted Extraction. Anal. Commun. 1997, 34, 89–91. [Google Scholar] [CrossRef]
- Cai, X.; Li, S.; Jia, P.; Run, M. Melting and Crystallization Properties of Crystalline/Crystalline Blends of Poly(Trimethylene Terephthalate) and Poly(Ethylene Terephthalate). Appl. Mech. Mater. 2014, 665, 331–334. [Google Scholar] [CrossRef]
- Sterzyński, T.; Tomaszewska, J.; Piszczek, K.; Skórczewska, K. The influence of carbon nanotubes on the PVC glass transition temperature. Compos. Sci. Technol. 2010, 70, 966–969. [Google Scholar] [CrossRef]
- Ravikumar, H.B.; Ranganathaiah, C.; Kumaraswamy, G.N.; Thomas, S. Positron annihilation and differential scanning calorimetric study of poly(trimethylene terephthalate)/EPDM blends. Polymer 2005, 46, 2372–2380. [Google Scholar] [CrossRef]
- Schick, C.; Androsch, R. The origin of annealing peaks in semicrystalline polymers: Enthalpy recovery or melting? Macromolecules 2020, 53, 8751–8756. [Google Scholar] [CrossRef]
- Alarcon, R.T.; Gaglieri, C.; de Oliveira, A.R.; Bannach, G. Use of DSC in degree of conversion of dimethacrylate polymers: Easier and faster than MIR technique. J. Therm. Anal. Calorim. 2018, 132, 1423–1427. [Google Scholar] [CrossRef] [Green Version]
- Morgan, D.R.; Kalachandra, S.; Shobha, H.K.; Gunduz, N.; Stejskal, E.O. Analysis of a dimethacrylate copolymer (Bis-GMA and TEGDMA) network by DSC and 13C solution and solid-state NMR spectroscopy. Biomaterials 2000, 21, 1897–1903. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Bin, Y. The investigation of the growth and perfection of the poly(ethylene terephthalate) crystalline region from amorphous state during annealing using a controlled temperature gradient. Polym. Cryst. 2021, 4, e10178. [Google Scholar]
- Rahman, M.S.; Al-Marhubi, I.M.; Al-Mahrouqi, A. Measurement of glass transition temperature by mechanical (DMTA), thermal (DSC and MDSC), water diffusion and density methods: A comparison study. Chem. Phys. Let. 2007, 440, 372–377. [Google Scholar] [CrossRef]
- Yoon, B.; Cho, S.-H.; Lee, S.K.; Cho, K.; Tabe, C.A.; Giese, U.; Nam, J.-D.; Suhr, J. Natural cork/potato periderm derivatives enabled interface engineering of elastomer composites for tunable energy-absorbing capabilities. Ind. Crops. Prod. 2021, 170, 113763. [Google Scholar] [CrossRef]
- Young, E.; McDonald, A. Preparation and Characterization of Biobased Lignin-Co-Polyester/Amide Thermoplastics. Molecules 2021, 26, 2437. [Google Scholar] [CrossRef] [PubMed]
- Türk, C.T.; Hasçiçek, C.; Gönül, N. Evaluation of drug-polymer interaction in polymeric microspheres containing diltiazem hydrochloride. J. Therm. Anal. Calorim. 2009, 95, 865–869. [Google Scholar] [CrossRef]



| Retention Time (min) | A% (Acetonitrile) | B% (Water) |
|---|---|---|
| 0 | 30 | 70 |
| 15 | 100 | 0 |
| 20 | 100 | 0 |
| 21 | 30 | 70 |
| Polyester Sample | Oligomer Content | Standard Deviation |
|---|---|---|
| Fabric | 2.15% | 0.578% |
| Filament, 188D | 2.05% | 0.432% |
| Filament, 160D | 2.03% | 0.378% |
| Spun, 225D | 1.65% | 0.063% |
| Spun, 135D | 1.18% | 0.476% |
| Dye Concentration (% o.w.f.) | Before Treatment | After Treatment | ||
|---|---|---|---|---|
| Dry Rubbing Fastness | Wet Rubbing Fastness | Dry Rubbing Fastness | Wet Rubbing Fastness | |
| 0.3 | 4/5 | 5 | 5 | 5 |
| 0.5 | 4 | 4/5 | 4/5 | 5 |
| 0.7 | 3/4 | 4 | 4 | 4 |
| 1.0 | 2–3 | 3 | 3 | 3–4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Pei, L.; Zhang, H.; Wang, Z.; Saleem, M.A.; Alebeid, O.K.; Wang, J. Extraction of Cyclic Oligomer and Their Influence on Polyester Dyeing in a Silicone Waterless Dyeing System. Polymers 2021, 13, 3687. https://doi.org/10.3390/polym13213687
Li H, Pei L, Zhang H, Wang Z, Saleem MA, Alebeid OK, Wang J. Extraction of Cyclic Oligomer and Their Influence on Polyester Dyeing in a Silicone Waterless Dyeing System. Polymers. 2021; 13(21):3687. https://doi.org/10.3390/polym13213687
Chicago/Turabian StyleLi, Hao, Liujun Pei, Hongjuan Zhang, Zhiwen Wang, Muhammad Asad Saleem, Omer Kamal Alebeid, and Jiping Wang. 2021. "Extraction of Cyclic Oligomer and Their Influence on Polyester Dyeing in a Silicone Waterless Dyeing System" Polymers 13, no. 21: 3687. https://doi.org/10.3390/polym13213687
APA StyleLi, H., Pei, L., Zhang, H., Wang, Z., Saleem, M. A., Alebeid, O. K., & Wang, J. (2021). Extraction of Cyclic Oligomer and Their Influence on Polyester Dyeing in a Silicone Waterless Dyeing System. Polymers, 13(21), 3687. https://doi.org/10.3390/polym13213687








