Construction of Self-Healing Disulfide-Linked Silicone Elastomers by Thiol Oxidation Coupling Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
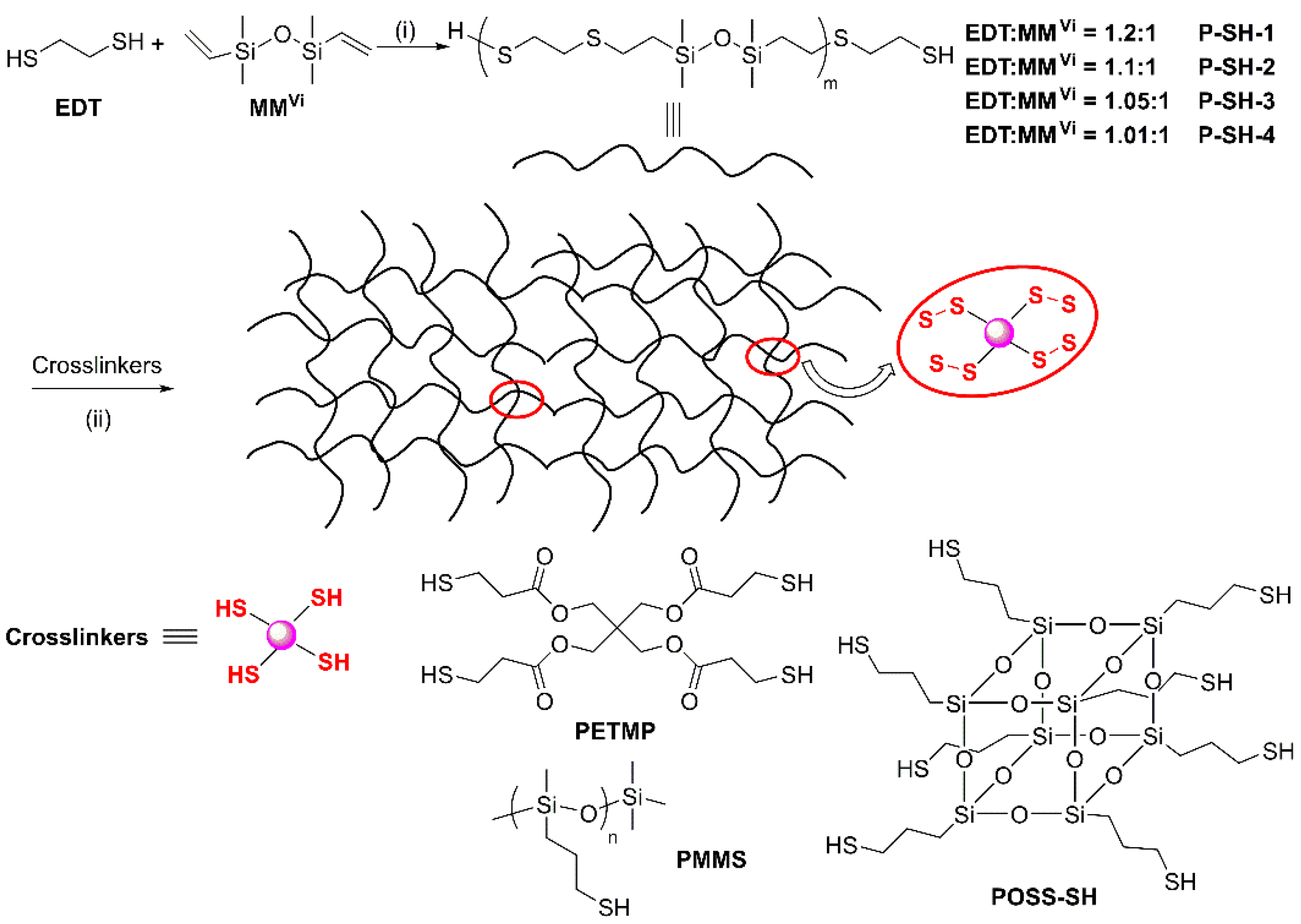
2.2. Synthesis of Thiol-Terminated Sulfur-Heterochain Polysiloxane (P-SH-1 to P-SH-4)
2.3. General Synthesis of Disulfide-Linked SEs
2.4. Lap Shear Adhesion
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Effects of Variables on the Formability and Mechanical Properties of SEs
3.3. Thermal Stability
3.4. Self-Healing Properties of SEs
3.5. Application as Adhesives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef] [Green Version]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Li, X.; Zhang, Z. Fabrication of advanced polydimethylsiloxane-based functional materials: Bulk modifications and surface functionalizations. Chem. Eng. J. 2020, 408, 127262. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, K.; Tian, G.; Jiang, B.; Huang, Y. Stretchable Electronics Based on PDMS Substrates. Adv. Mater. 2021, 33, 2003155. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Wang, S.; Hou, C.; Huang, X.; Cui, J.; Yao, X. Dynamic siloxane materials: From molecular engineering to emerging applications. Chem. Eng. J. 2021, 405, 127023. [Google Scholar] [CrossRef]
- Rus, D.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Lv, C.; Zhao, K.; Zheng, J. A Highly Stretchable Self-Healing Poly(dimethylsiloxane) Elastomer with Reprocessability and Degradability. Macromol. Rapid Commun. 2018, 39, 201700686. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-C.; Mei, J.-F.; Jia, X.-Y.; Li, C.-H.; You, X.-Z.; Bao, Z. A Stiff and Healable Polymer Based on Dynamic-Covalent Boroxine Bonds. Adv. Mater. 2016, 28, 8277–8282. [Google Scholar] [CrossRef]
- Kathan, M.; Kovaricek, P.; Jurissek, C.; Senf, A.; Dallmann, A.; Thuenemann, A.F.; Hecht, S. Control of Imine Exchange Kinetics with Photoswitches to Modulate Self-Healing in Polysiloxane Networks by Light Illumination. Ang. Chem. Inter. Ed. 2016, 55, 13882–13886. [Google Scholar] [CrossRef] [PubMed]
- Nasresfahani, A.; Zelisko, P.M. Synthesis of a self-healing siloxane-based elastomer cross-linked via a furan-modified polyhedral oligomeric silsesquioxane: Investigation of a thermally reversible silicon-based cross-link. Polym. Chem. 2017, 8, 2942–2952. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, C.; Liu, W.; Li, D.; Zhang, J.; Zhao, N.; Xu, J. Recyclable Polydimethylsiloxane Network Crosslinked by Dynamic Transesterification Reaction. Sci. Rep. 2017, 7, 11833. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, J.; Song, J.; Gao, C.; Wang, Z.; Song, C.; Wu, Y.; Liu, Y. Self-Healing Ti3C2 MXene/PDMS Supramolecular Elastomers Based on Small Biomolecules Modification for Wearable Sensors. ACS Appl. Mater. Interf. 2020, 12, 45306–45314. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, A.S.; Brook, M.A. Thermoplastic Silicone Elastomers through Self-Association of Pendant Coumarin Groups. Macromolecules 2014, 47, 1656–1663. [Google Scholar] [CrossRef]
- Rambarran, T.; Bertrand, A.; Gonzaga, F.; Boisson, F.; Bernard, J.; Fleury, E.; Ganachaud, F.; Brook, M.A. Sweet supramolecular elastomers from [small alpha], [small omega]-([small beta]-cyclodextrin terminated) PDMS. Chem. Commun. 2016, 52, 6681–6684. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; Mackay, M.E.; Char, K.; Pyun, J. Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur for Healable Infrared Optical Materials. ACS Macro Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Michal, B.T.; Spencer, E.J.; Rowan, S.J. Stimuli-Responsive Reversible Two-Level Adhesion from a Structurally Dynamic Shape-Memory Polymer. ACS Appl. Mater. Interf. 2016, 8, 11041–11049. [Google Scholar] [CrossRef]
- Michal, B.T.; Jaye, C.A.; Spencer, E.J.; Rowan, S.J. Inherently Photohealable and Thermal Shape-Memory Polydisulfide Networks. ACS Macro Lett. 2013, 2, 694–699. [Google Scholar] [CrossRef]
- Xu, W.M.; Rong, M.Z.; Zhang, M.Q. Sunlight driven self-healing, reshaping and recycling of a robust, transparent and yellowing-resistant polymer. J. Mater. Chem. A 2016, 4, 10683–10690. [Google Scholar] [CrossRef]
- Amamoto, Y.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Self-Healing of Covalently Cross-Linked Polymers by Reshuffling Thiuram Disulfide Moieties in Air under Visible Light. Adv. Mater. 2012, 24, 3975–3980. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.Q.; Xiang, H.P.; Yuan, Y.J.; Rong, M.Z.; Zhang, M.Q. Room-Temperature Self-Healable and Remoldable Cross-linked Polymer Based on the Dynamic Exchange of Disulfide Bonds. Chem. Mater. 2014, 26, 2038–2046. [Google Scholar] [CrossRef]
- Xiang, H.P.; Rong, M.Z.; Zhang, M.Q. A facile method for imparting sunlight driven catalyst-free self-healability and recyclability to commercial silicone elastomer. Polymer 2017, 108, 339–347. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, J.; Zhang, K.; Guo, K.; Yuan, L.; Wu, Y.; Gao, C. A novel type of self-healing silicone elastomers with reversible cross-linked network based on the disulfide, hydrogen and metal-ligand bonds. Prog. Org. Coat. 2020, 144, 105661. [Google Scholar] [CrossRef]
- Shan, S.; Lin, Y.; Zhang, A. Stretchable, robust and reprocessable poly(siloxane-urethanes) elastomers based on exchangeable aromatic disulfides. Polymer 2021, 221, 123588. [Google Scholar] [CrossRef]
- Zhao, L.; Yin, Y.; Jiang, B.; Guo, Z.; Qu, C.; Huang, Y. Fast room-temperature self-healing siloxane elastomer for healable stretchable electronics. J. Colloid Interf. Sci. 2020, 573, 105–114. [Google Scholar] [CrossRef]
- Yan, J.; Cao, J.; Xue, L.; Feng, S.; Zhang, H.; Wang, D. Thiol Oxidative Coupling Synthesis of Silicone Foams for Oil/Water Separation. ACS Appl. Polym. Mater. 2020, 2, 1634–1643. [Google Scholar] [CrossRef]
- Li, L.; Liu, H. Rapid Preparation of Silsesquioxane-Based Ionic Liquids. Chem. Eur. J. 2016, 22, 4713–4716. [Google Scholar] [CrossRef]








| Entry | P-SHs a | THF/mL | H2O2/mL b | NaI mL c | Crosslinkers (g) | Formability |
|---|---|---|---|---|---|---|
| SE-1 | P-SH-1 | 8 | 0.1 | 0.05 | PETMP(0) | × (tacky) |
| SE-2 | P-SH-1 | 8 | 0.1 | 0.05 | PETMP(0.016) | × (tacky) |
| SE-3 | P-SH-1 | 8 | 0.1 | 0.05 | PETMP(0.036) | √ |
| SE-4 | P-SH-1 | 8 | 0.1 | 0.05 | PETMP(0.065) | √ |
| SE-5 | P-SH-1 | 8 | 0.1 | 0.05 | PETMP(0.1) | √ (cracks inside) |
| SE-6 | P-SH-1 | 8 | 0.1 | 0.05 | POSS-SH(0.01) | √ |
| SE-7 | P-SH-1 | 8 | 0.1 | 0.05 | POSS-SH(0.02) | √ |
| SE-8 | P-SH-1 | 8 | 0.1 | 0.05 | POSS-SH(0.04) | √ |
| SE-9 | P-SH-1 | 8 | 0.1 | 0.05 | POSS-SH(0.06) | √ |
| SE-10 | P-SH-1 | 8 | 0.1 | 0.05 | PMMS(0.10) | × |
| SE-11 | P-SH-2 | 8 | 0.1 | 0.05 | PETMP(0.0168) | × (tacky, bubbles inside) |
| SE-12 | P-SH-2 | 8 | 0.1 | 0.05 | PETMP(0.036) | √ |
| SE-13 | P-SH-2 | 8 | 0.1 | 0.05 | PETMP(0.068) | √ |
| SE-14 | P-SH-2 | 8 | 0.1 | 0.05 | POSS-SH(0.06) | √ |
| SE-15 | P-SH-3 | 8 | 0.1 | 0.05 | PETMP(0.0168) | × (tacky, bubbles inside) |
| SE-16 | P-SH-3 | 8 | 0.1 | 0.05 | PETMP(0.036) | √ (bubbles inside) |
| SE-17 | P-SH-3 | 8 | 0.05 | 0.05 | PETMP(0.036) | √ |
| SE-18 | P-SH-3 | 8 | 0.05 | 0.05 | PETMP(0.068) | √ (bubbles inside) |
| SE-19 | P-SH-3 | 8 | 0.05 | 0.05 | POSS-SH(0.02) | × (tacky, bubbles inside) |
| SE-20 | P-SH-3 | 8 | 0.05 | 0.05 | POSS-SH(0.04) | √ |
| SE-21 | P-SH-3 | 8 | 0.05 | 0.05 | POSS-SH(0.06) | √ (cracks inside) |
| SE-22 | P-SH-4 | 8 | 0.1 | 0.05 | PETMP/POSS-SH | × (tacky) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Yan, J.; Wang, D.; Feng, S.; Zhou, C. Construction of Self-Healing Disulfide-Linked Silicone Elastomers by Thiol Oxidation Coupling Reaction. Polymers 2021, 13, 3729. https://doi.org/10.3390/polym13213729
Huang Y, Yan J, Wang D, Feng S, Zhou C. Construction of Self-Healing Disulfide-Linked Silicone Elastomers by Thiol Oxidation Coupling Reaction. Polymers. 2021; 13(21):3729. https://doi.org/10.3390/polym13213729
Chicago/Turabian StyleHuang, Yanhua, Jianpan Yan, Dengxu Wang, Shengyu Feng, and Chuanjian Zhou. 2021. "Construction of Self-Healing Disulfide-Linked Silicone Elastomers by Thiol Oxidation Coupling Reaction" Polymers 13, no. 21: 3729. https://doi.org/10.3390/polym13213729
APA StyleHuang, Y., Yan, J., Wang, D., Feng, S., & Zhou, C. (2021). Construction of Self-Healing Disulfide-Linked Silicone Elastomers by Thiol Oxidation Coupling Reaction. Polymers, 13(21), 3729. https://doi.org/10.3390/polym13213729








