Identification of Potential Migrants in Polyethylene Terephthalate Samples of Ecuadorian Market
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Reagents
2.1.2. PET Samples
2.2. Methods
2.2.1. Extraction of Chemical Compounds from PET Samples
2.2.2. Analysis of PET Samples by GC-MS
2.2.3. Overall Migration Test
3. Results and Discussion
3.1. Lubricants
3.2. Plasticizers
3.3. Thermal Degradation Products
3.4. Degradation Products of Antioxidants
3.5. PET Recycling Indicators
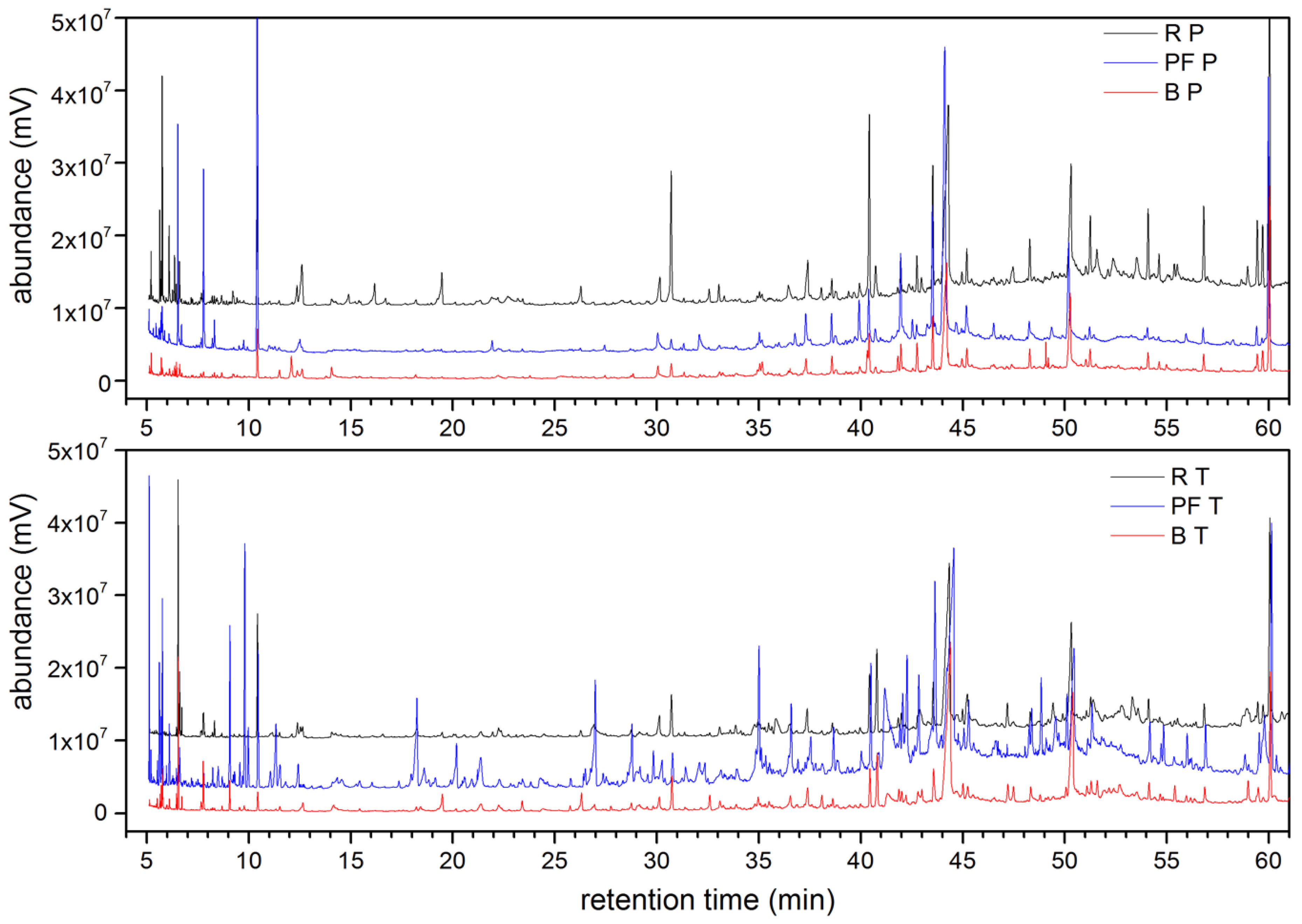
3.6. Chemical Compounds Identified at Different Phases of the Processing
3.7. Overall Migration Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welle, F. Twenty years of PET bottle to bottle recycling—An overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Welle, F. Food law compliance of Poly(ethylene Terephthalate) (PET) food packaging materials. In Food Additives and Packaging; American Chemical Society: Washington, DC, USA, 2014; pp. 167–195. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of oligomers in virgin and recycled polyethylene terephthalate (PET) samples by UPLC-MS-QTOF. Anal. Bioanal. Chem. 2018, 410, 2377–2384. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Bosnea, L. Migration of Substances from Food Packaging Materials to Foods. Crit. Rev. Food Sci. Nutr. 2004, 44, 63–76. [Google Scholar] [CrossRef]
- Bach, C.; Dauchy, X.; Chagnon, M.-C.; Etienne, S. Chemical compounds and toxicological assessments of drinking water stored in polyethylene terephthalate (PET) bottles: A source of controversy reviewed. Water Res. 2012, 46, 571–583. [Google Scholar] [CrossRef] [Green Version]
- European Committee for Standardization (CEN). EN 1186-3: Materials and Articles in Contact with Foodstuffs. Plastics. Part 3: Test Methods for Overall Migration into Aqueous Food Simulants by Total Immersion; European Committee for Standardization (CEN): Brussels, Belgium, 2002; p. 17. [Google Scholar]
- European Commitee for Standardization (CEN). EN 1186-1. Materials and Articles in Contact with Foodstuffs. Plastics. Part 1: Guide to the Selection of Conditions and Test Methods for Overall Migration; European Committee for Standardization (CEN): Brussels, Belgium, 2003; p. 50. [Google Scholar]
- Commission Regulation (EU) No. 10/2011. Commission Regulation (EU) No. 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food; Commission Regulation (EU): Brussels, Belgium, 2011; pp. 1–89. [Google Scholar]
- Feigenbaum, A.; Scholler, D.; Bouquant, J.; Brigot, G.; Ferrier, D.; Franz, R.; Lillemark, L.; Riquet, A.M.; Petersen, J.H.; Van Lierop, B.; et al. Safety and quality of food contact materials. Part 1: Evaluation of analytical strategies to introduce migration testing into good manufacturing practice. Food Addit. Contam. 2002, 19, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, M.; Grob, K. Advantages of comprehensive two-dimensional gas chromatography for comprehensive analysis of potential migrants from food contact materials. Anal. Chim. Acta 2019, 1057, 11–17. [Google Scholar] [CrossRef]
- Salazar, R.; Domenek, S.; Plessis, C.; Ducruet, V. Quantitative determination of volatile organic compounds formed during Polylactide processing by MHS-SPME. Polym. Degrad. Stab. 2017, 136, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Sendon Garcia, R.; Sanches Silva, A.; Cooper, I.; Franz, R.; Paseiro Losada, P. Revision of analytical strategies to evaluate different migrants from food packaging materials. Trends Food Sci. Technol. 2006, 17, 354–366. [Google Scholar] [CrossRef]
- Coltro, L.; Pitta, J.B.; da Costa, P.A.; Fávaro Perez, M.Â.; de Araújo, V.A.; Rodrigues, R. Migration of conventional and new plasticizers from PVC films into food simulants: A comparative study. Food Control 2014, 44, 118–129. [Google Scholar] [CrossRef]
- Silva, A.S.; García, R.S.; Cooper, I.; Franz, R.; Losada, P.P. Compilation of analytical methods and guidelines for the determination of selected model migrants from plastic packaging. Trends Food Sci. Technol. 2006, 17, 535–546. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; De Quirós, A.R.-B.; Sendón, R.; Bustos, J.; Santillana, M.I.; Cruz, J.M.; Paseiro-Losada, P. Chromatographic Methods for the Determination of Polyfunctional Amines and Related Compounds Used as Monomers and Additives in Food Packaging Materials: A State-of-the-Art Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 676–694. [Google Scholar] [CrossRef] [PubMed]
- Lalanne, A.; Espino, E.; Salazar, R.; Domenek, S.; Ducruet, V. Identification of potential migrants in Poly(lactic acid) packagings. Ital. J. Food Sci. 2011, 23, 63–67. [Google Scholar]
- Nerin, C.; Albiñana, J.; Philo, M.R.; Castle, L.; Raffael, B.; Simoneau, C. Evaluation of some screening methods for the analysis of contaminants in recycled polyethylene terephthalate flakes. Food Addit. Contam. 2003, 20, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Franz, R.; Welle, F. Recycled poly(ethylene terephthalate) for direct food contact applications: Challenge test of an inline recycling process. Food Addit. Contam. 2002, 19, 502–511. [Google Scholar] [CrossRef]
- Franz, R.; Mauer, A.; Welle, F. European survey on post-consumer poly (ethylene terephthalate)(PET) materials to determine contamination levels and maximum consumer exposure from food packages made from recycled PET. Food Addit. Contam. 2004, 21, 265–286. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Migration measurement and modelling from poly(ethylene terephthalate) (PET) into soft drinks and fruit juices in comparison with food simulants. Food Addit. Contam.—Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1033–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassouf, A.; Maalouly, J.; Chebib, H.; Rutledge, D.N.; Ducruet, V. Chemometric tools to highlight non-intentionally added substances (NIAS) in polyethylene terephthalate (PET). Talanta 2013, 115, 928–937. [Google Scholar] [CrossRef]
- Balafas, D.; Shaw, K.J.; Whitfield, F.B. Phthalate and adipate esters in Australian packaging materials. Food Chem. 1999, 65, 279–287. [Google Scholar] [CrossRef]
- Farhoodi, M.; Emam-Djomeh, Z.; Ehsani, M.R.; Oromiehie, A. Migration of model contaminants (ethylene glycol, DEHA and DEHP) from PET bottles into Iranian yogurt drink. e-Polymers 2008, 8, 3–4. [Google Scholar] [CrossRef]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Al-Odaini, N.A.; Song, Y.K.; Hong, S.H. Qualitative Analysis of Additives in Plastic Marine Debris and Its New Products. Arch. Environ. Contam. Toxicol. 2015, 69, 352–366. [Google Scholar] [CrossRef]
- Selke, S.E.M.; Culter, J.D. Plastics Packaging: Properties, Processing, Applications, and Regulations; Carl Hanser Verlag GmbH Co. KG: Munich, Germany, 2016; ISBN 3446437193. [Google Scholar]
- Kim, H.; Gilbert, S.G.; Johnson, J.B. Determination of potential migrants from commercial amber polyethylene terephthalate bottle wall. Pharm. Res. 1990, 7, 176–179. [Google Scholar] [CrossRef]
- Kassouf, A. Sécurité Sanitaire des Denrées au Contact de Matériau D’emballage: Proposition D’une Démarche Méthodologique; L’Université Libanaise: Beirut, Lebanon, 2015. [Google Scholar]
- Marklund, A.; Andersson, B.; Haglund, P. Organophosphorus flame retardants and plasticizers in Swedish sewage treatment plants. Environ. Sci. Technol. 2005, 39, 7423–7429. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jeong, W.; Kannan, K.; Moon, H.B. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res. 2016, 103, 182–188. [Google Scholar] [CrossRef]
- Sheftel, V.O. Indirect Food Additives and Polymers; CRC Press: London, UK, 2000; ISBN 9780429076756. [Google Scholar]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Cincotta, F.; Verzera, A.; Tripodi, G.; Condurso, C. Non-intentionally added substances in PET bottled mineral water during the shelf-life. Eur. Food Res. Technol. 2018, 244, 433–439. [Google Scholar] [CrossRef]
- Mihucz, V.G.; Záray, G. Occurrence of antimony and phthalate esters in polyethylene terephthalate bottled drinking water. Appl. Spectrosc. Rev. 2016, 51, 163–189. [Google Scholar] [CrossRef]
- Dziȩcioł, M.; Trzeszczyński, J. Volatile products of poly(ethylene terephthalate) thermal degradation in nitrogen atmosphere. J. Appl. Polym. Sci. 2000, 77, 1894–1901. [Google Scholar] [CrossRef]
- Botelho, G.; Queirós, A.; Liberal, S.; Gijsman, P. Studies on thermal and thermo-oxidative degradation of poly(ethylene terephthalate) and poly(butylene terephthalate). Polym. Degrad. Stab. 2001, 74, 39–48. [Google Scholar] [CrossRef]
- Badia, J.D.; Martinez-Felipe, A.; Santonja-Blasco, L.; Ribes-Greus, A. Thermal and thermo-oxidative stability of reprocessed poly(ethylene terephthalate). J. Anal. Appl. Pyrolysis 2013, 99, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Romão, W.; Franco, M.F.; Corilo, Y.E.; Eberlin, M.N.; Spinacé, M.A.S.; De Paoli, M.A. Poly (ethylene terephthalate) thermo-mechanical and thermo-oxidative degradation mechanisms. Polym. Degrad. Stab. 2009, 94, 1849–1859. [Google Scholar] [CrossRef]
- Nerin, C.; Alfaro, P.; Aznar, M.; Domeño, C. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Anal. Chim. Acta 2013, 775, 14–24. [Google Scholar] [CrossRef]
- Welle, F.; Mauer, A.; Franz, R. Migration and sensory changes of packaging materials caused by ionising radiation. Radiat. Phys. Chem. 2002, 63, 841–844. [Google Scholar] [CrossRef]
- Bourges, F.; Bureau, G.; Pascat, B. Effects of electron beam irradiation on commercial polypropylene: Kinetic study of antioxidant degradation. Packag. Technol. Sci. 1992, 5, 197–204. [Google Scholar] [CrossRef]
- Dopico-García, M.S.; López-Vilariñó, J.M.; González-Rodríguez, M.V. Antioxidant content of and migration from commercial polyethylene, polypropylene, and polyvinyl chloride packages. J. Agric. Food Chem. 2007, 55, 3225–3231. [Google Scholar] [CrossRef] [PubMed]
- Karayannidis, G.P.; Sideridou, I.D.; Zamboulis, D.X.; Stalidis, G.A.; Bikiaris, D.N.; Wilmes, A. Effect of some current antioxidants on the thermo-oxidative stability of poly(ethylene terephthalate). Polym. Degrad. Stab. 1994, 44, 9–15. [Google Scholar] [CrossRef]
- Castillo, R.; Biedermann, M.; Riquet, A.-M.; Grob, K. Comprehensive on-line HPLC-GC for screening potential migrants from polypropylene into food: The effect of pulsed light decontamination as an example. Polym. Degrad. Stab. 2013, 98, 1679–1687. [Google Scholar] [CrossRef]
- Dimitrov, N.; Krehula, L.K.; Siročić, A.P.; Hrnjak-Murgić, Z. Analysis of recycled PET bottles products by pyrolysis-gas chromatography. Polym. Degrad. Stab. 2013, 98, 972–979. [Google Scholar] [CrossRef]
- García Ibarra, V.; Rodríguez Bernaldo de Quirós, A.; Paseiro Losada, P.; Sendón, R. Identification of intentionally and non-intentionally added substances in plastic packaging materials and their migration into food products. Anal. Bioanal. Chem. 2018, 410, 3789–3803. [Google Scholar] [CrossRef]
- Mancini, S.D.; Nogueira, A.R.; Schwartzman, J.A.S.; Kagohara, D.A. Characterization of Post-Consumer PET after Removal of the Original Surface: Influence of Raw Material. Int. J. Polym. Mater. 2010, 59, 407–423. [Google Scholar] [CrossRef]
- Bayer, F.L. Polyethylene terephthalate recycling for food-contact applications: Testing, safety and technologies: A global perspective. Food Addit. Contam. 2002, 19, 111–134. [Google Scholar] [CrossRef]
- Ecuador Official Registry—No 354. Ley Orgánica Para la Racionalización, Reutilización y Reducción de Plásticos de un Solo Uso; Ecuador Official Registry: Quito, Ecuador, 2020; p. año II-No 354. [Google Scholar]
- Maddalena, R.; Schwarzman, M.; Carrick, T.; Kokai, A.; Russell, M.; Papagni, C.; Lee, W. Experimental Comparison of Chemical Migration from Petrochemical Plastic and Bioplastic Bottles into Drinking Water; Contractor’s Report DRRR-2013-1486; California Department of Resources Recycling and Recovery: Sacramento, CA, USA, 2013. [Google Scholar]
- Ashby, R. Migration from polyethylene terephthalate under all conditions of use. Food Addit. Contam. 1988, 5, 485–492. [Google Scholar] [CrossRef]
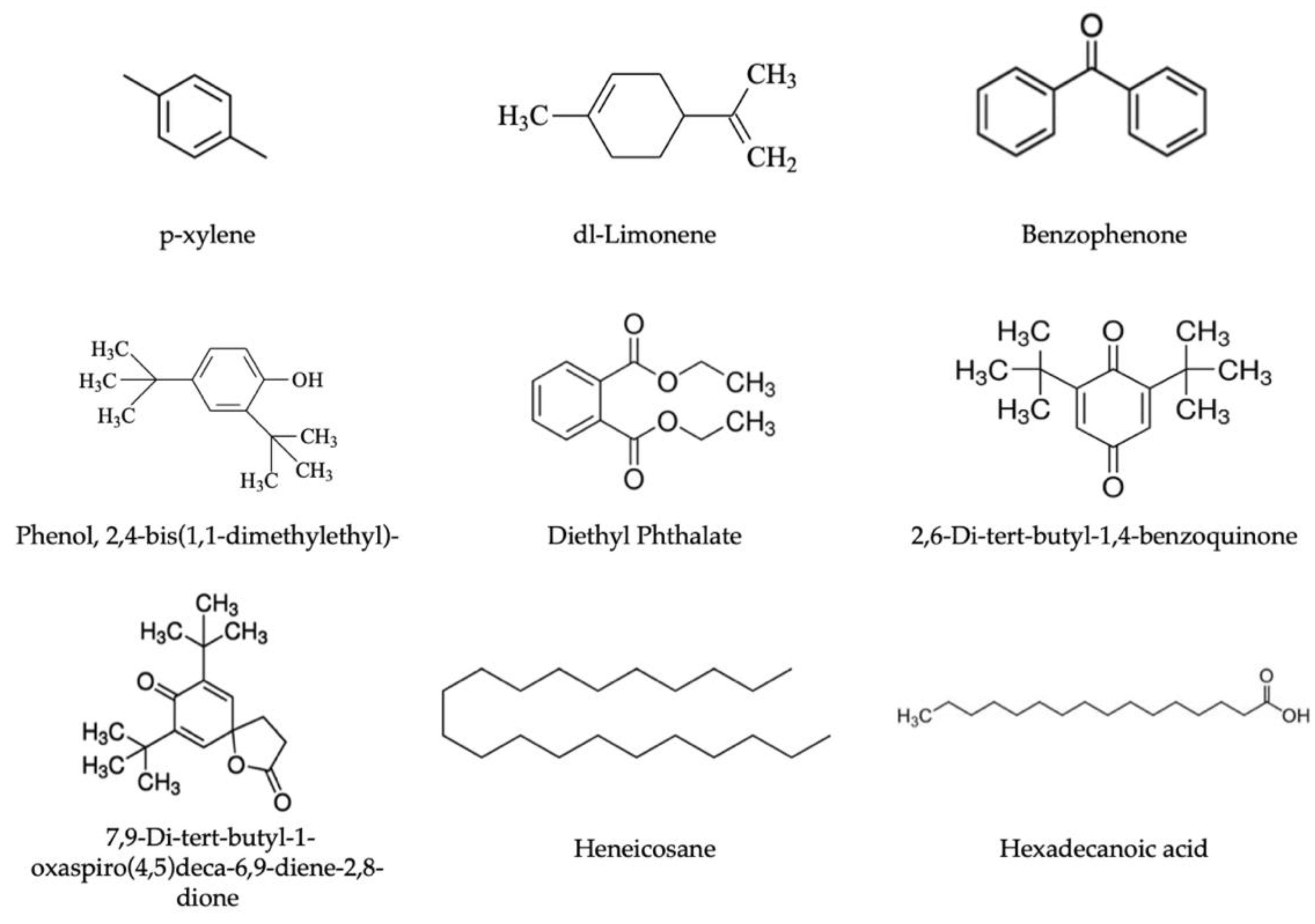
| Tr (min) | Compounds | Function/Origin | Mw | Formula | CAS | KIcal b | KI Ref c | Pellets | Preforms | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | T | P | T | ||||||||
| 5.64 | p-Xylene | Thermal degradation product | 106 | C8H10 | 106-42-3 | 902 | 860 | - | - | - | 2.39 ± 0.22 |
| 7.40 | Benzaldehyde | PET recycled indicator | 106 | C7H6O | 100-52-7 | 966 | 970 | - | - | - | 0.36 ± 0.14 |
| 9.06 | dl-Limonene | PET recycled indicator | 136 | C10H16 | 138-86-3 | 1025 | 1032 | - | - | - | 7.86 ± 0.68 |
| 14.16 | Benzoic acid | PET thermal degradation product | 150 | C9H10O2 | 93-89-0 | 1177 | 1160 | - | - | - | 4.75 ± 0.83 |
| 30.13 | Dodecanoic acid | Lubricant | 200 | C12H24O2 | 143-07-7 | 1500 | 1562 | 4.36 ± 0.33 | - | 6.19 ± 0.37 | - |
| 30.74 | Diethyl phthalate | Plasticizer | 222 | C12H14O4 | 84-66-2 | 1571 | 1546 | 18.73 ± 5.14 | 7.32 ± 4.48 | - | - |
| 36.77 | 3,5-Di-tert-butyl-4-hydroxybenzaldehyde | BHT oxidation product | 234 | C15H22O2 | 1620-98-0 | 1701 | 1774 | - | - | 2.63 ± 1.50 | - |
| 37.40 | Tetradecanoic acid | Lubricant | 228 | C14H28O2 | 544-63-8 | 1750 | 1761 | 6.87 ± 0.10 | 3.79 ± 0.50 | 5.64 ± 0.68 | 4.78 ± 1.50 |
| 40.84 | Pentadecanoic acid | Lubricant | 242 | C15H30O2 | 1002-84-2 | 1842 | 1855 | 1.17 ± 0.87 | - | - | - |
| 44.35 | Hexadecanoic acid | Lubricant | 256 | C16H32O2 | 57-10-3 | 1961 | 1957 | 52.74 ± 2.21 | 59.07 ± 7.71 | 68.16 ± 0.79 | 55.30 ± 3.70 |
| 50.36 | Octadecanoic acid | Lubricant | 284 | C18H36O2 | 57-11-4 | 2147 | 2170 | 16.13 ± 4.41 | 29.83 ± 3.31 | 17.39 ± 1.98 | 24.56 ± 1.30 |
| Tr (min) | Compounds | Function/Origin | Mw | Formula | CAS | KIcal b | KI Ref c | P | T | A | D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5.64 | p-Xylene | Thermal degradation product | 106 | C8H10 | 106-42-3 | 903 | 860 | 1.68 ± 0.69 | - | - | 1.69 ± 0.60 |
| 9.06 | dl-Limonene | PET recycled indicator | 136 | C10H16 | 138-86-3 | 1026 | 1020 | - | 1.94 ± 0.14 | - | 1.08 ± 0.05 |
| 11.53 | Nonanal | Thermal degradation product from PE waxes (lubricants) | 142 | C9H18O | 124-19-6 | 1104 | 1071 | 2.14 ± 1.25 | - | 1.03 ± 0.51 | 0.41 ± 0.30 |
| 12.13 | Triethyl phosphate | Plasticizer | 182 | C6H15O4P | 78-40-0 | 1122 | 1120 | 10.06 ± 5.24 | - | - | 0.92 ± 0.19 |
| 14.16 | Benzoic acid | PET thermal degradation product | 122 | C7H6O2 | 65-85-0 | 1172 | 1170 | - | 1.72 ± 0.69 | - | - |
| 15.41 | Decanal | Thermal degradation product from PE waxes (lubricants) | 156 | C10H20O | 112-31-2 | 1206 | 1214 | - | - | - | 0.26 ± 0.10 |
| 18.52 | Nonanoic acid | Lubricant | 158 | C9H18O2 | 112-05-0 | 1270 | 1278 | - | - | - | 1.15 ± 0.21 |
| 25.76 | 2,6-Di-tert-butyl-1,4-benzoquinone | Irganox degradation product | 212 | C14H20O2 | 719-22-2 | 1449 | 1458 | - | 0.50 ± 0.12 | - | 0.46 ± 0.19 |
| 27.75 | Phenol, 2,4-bis(1,1-dimethylethyl)- | Degradation product from phosphite-based antioxidant–process stabilizer | 206 | C14H22O | 96-76-4 | 1495 | 1502 | 1.45 ± 1.12 | 0.43 ± 0.12 | - | 0.97 ± 0.14 |
| 30.13 | Dodecanoic acid | Lubricant | 200 | C12H24O2 | 2305-05-7 | 1554 | 1562 | 3.36 ± 1.65 | 1.69 ± 0.21 | 5.96 ± 0.94 | 2.40 ± 1.52 |
| 30.74 | Diethyl phthalate | Plasticizer | 222 | C12H14O4 | 84-66-2 | 1572 | 1546 | 2.65 ± 0.43 | 4.08 ± 0.92 | 24.74 ± 10.74 | 1.16 ± 0.61 |
| 31.34 | Hexadecane | From paraffine wax (lubricant) | 226 | C16H34 | 544-76-3 | 1588 | 1603 | 0.77 ± 0.12 | - | - | - |
| 32.12 | Benzophenone | PET recycled indicator | 182 | C13H10O | 119-61-9 | 1608 | 1644 | 0.74 ± 0.15 | 0.54 ± 0.04 | - | 0.47 ± 0.16 |
| 37.40 | Tetradecanoic acid | Lubricant | 228 | C14H28O2 | 544-63-8 | 1773 | 1756 | 4.88 ± 0.44 | 2.74 ± 0.47 | - | 4.98 ± 1.46 |
| 40.84 | Pentadecanoic acid | Lubricant | 242 | C15H30O2 | 1002-84-2 | 1842 | 1855 | - | - | - | 4.70 ± 2.48 |
| 41.81 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | Possible degradation product from Irgafox 168 | 276 | C17H24O3 | 82304-66-3 | 1873 | 1916 | - | - | 2.97 ± 0.39 | - |
| 44.35 | Hexadecanoic acid | Lubricant | 256 | C16H32O2 | 57-10-3 | 1981 | 1972 | 44.54 ± 3.88 | 56.67 ± 0.87 | 46.59 ± 8.63 | 45.80 ± 8.08 |
| 47.22 | Heptadecanoic acid | Lubricant | 270 | C17H34O2 | 506-1 2-7 | 2043 | 2080 | - | 2.49 ± 0.35 | - | - |
| 48.34 | Heneicosane | From paraffine wax (lubricant) | 296 | C21H44 | 629-94-7 | 2088 | 2100 | 2.96 ± 0.95 | 1.47 ± 0.72 | 7.52 ± 2.05 | - |
| 50.36 | Octadecanoic acid | Lubricant | 284 | C18H36O2 | 57-11-4 | 2144 | 2158 | 25.14 ± 2.11 | 26.36 ± 3.47 | 11.21 ± 6.21 | 33.54 ± 7.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Morocho, K.; Domenek, S.; Salazar, R. Identification of Potential Migrants in Polyethylene Terephthalate Samples of Ecuadorian Market. Polymers 2021, 13, 3769. https://doi.org/10.3390/polym13213769
Marín-Morocho K, Domenek S, Salazar R. Identification of Potential Migrants in Polyethylene Terephthalate Samples of Ecuadorian Market. Polymers. 2021; 13(21):3769. https://doi.org/10.3390/polym13213769
Chicago/Turabian StyleMarín-Morocho, Karina, Sandra Domenek, and Rómulo Salazar. 2021. "Identification of Potential Migrants in Polyethylene Terephthalate Samples of Ecuadorian Market" Polymers 13, no. 21: 3769. https://doi.org/10.3390/polym13213769
APA StyleMarín-Morocho, K., Domenek, S., & Salazar, R. (2021). Identification of Potential Migrants in Polyethylene Terephthalate Samples of Ecuadorian Market. Polymers, 13(21), 3769. https://doi.org/10.3390/polym13213769






