High-Performanced Hemicellulose Based Organic-Inorganic Films with Polyethyleneimine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
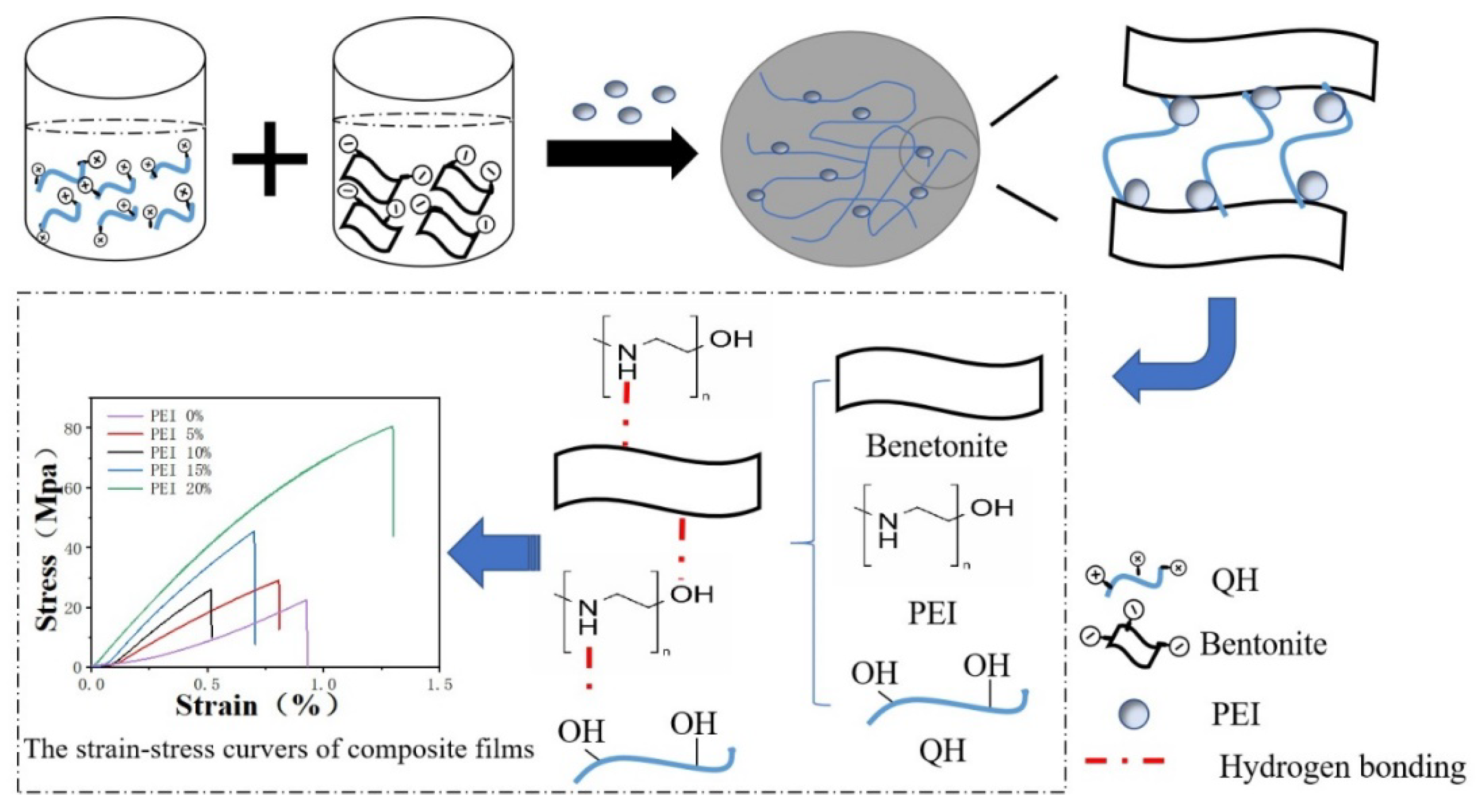
2.2. Preparation of Organic-Inorganic Film
2.3. Characterization of the Films
2.4. The Mechanical Properties of the Films
2.5. UV-Vis Transparency of the Composite Films
3. Results
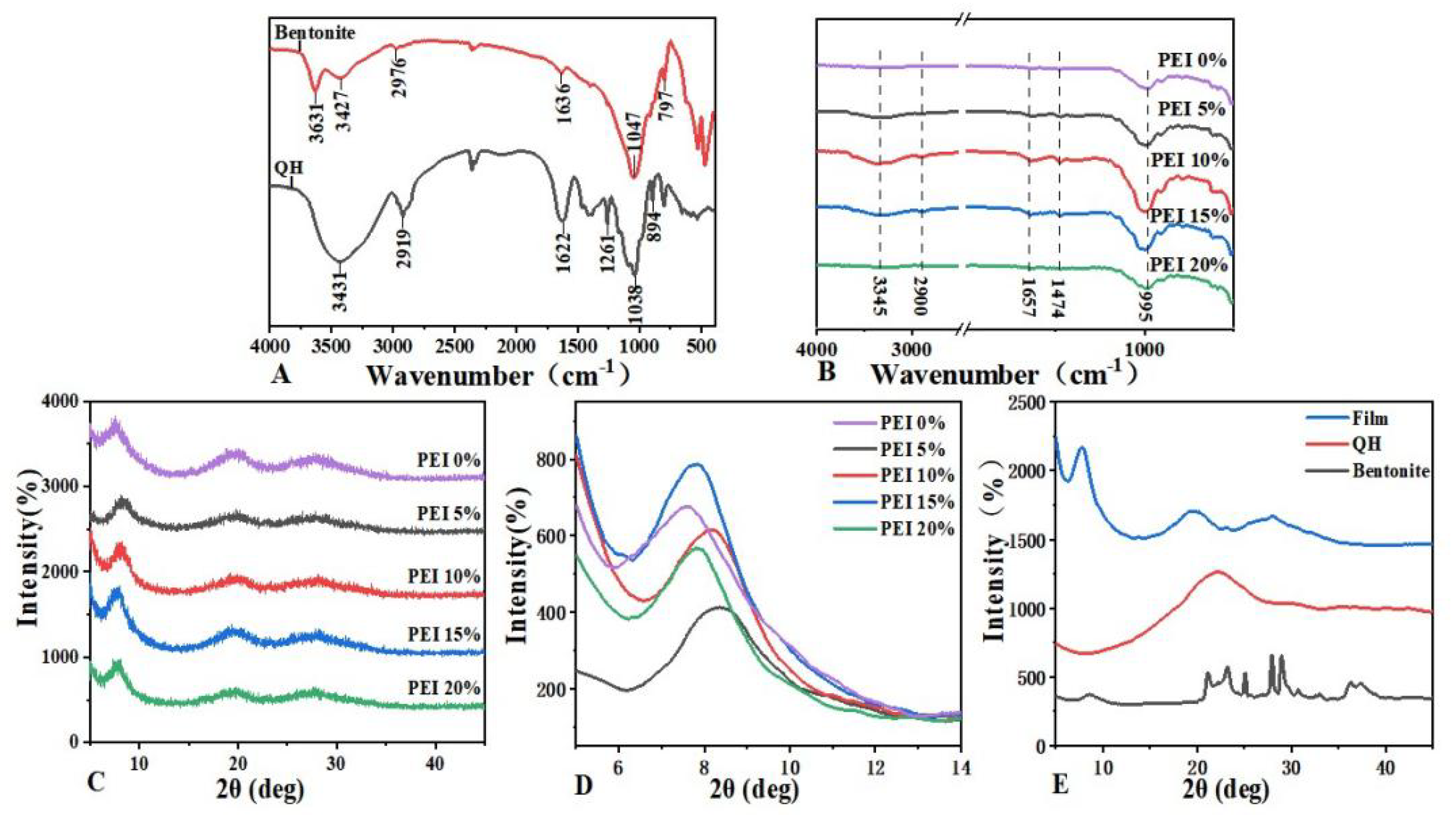
3.1. Structure of the Composite Films
3.2. Morphology of the Composite Films
3.3. Mechanical Properties of the Composite Films
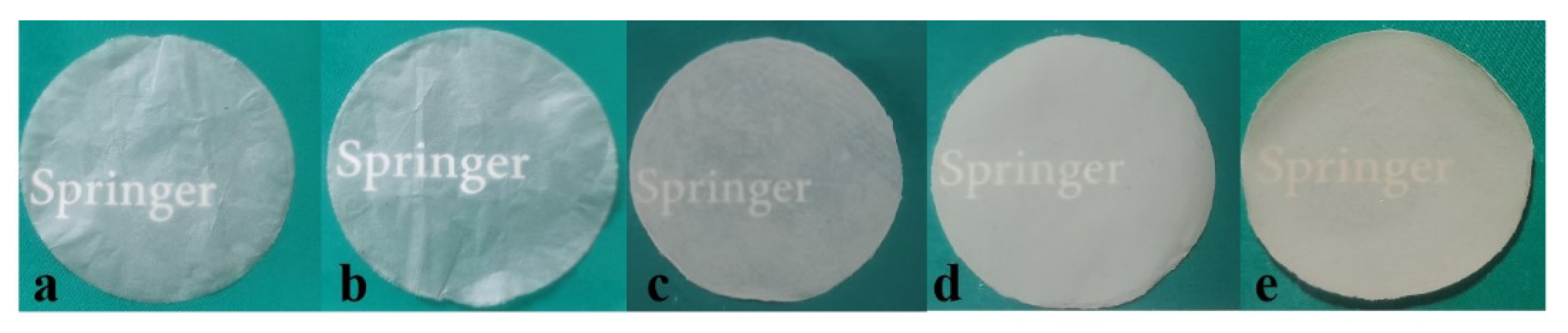
3.4. UV-Vis Transparency of the Composite Films
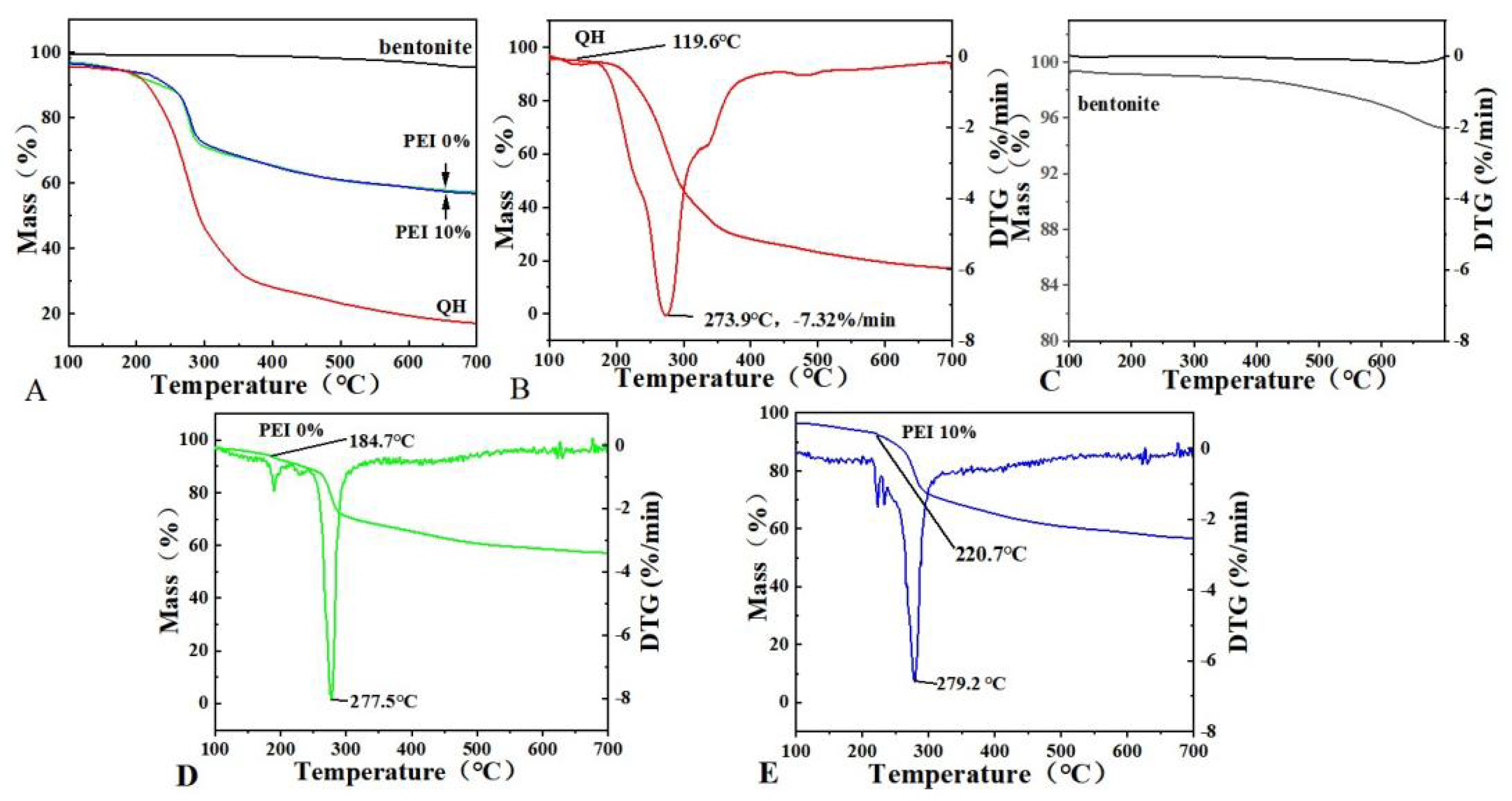
3.5. Thermal Properties of the Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.; Shen, Y.; Yang, H.; Li, J.; Guo, L. Underliquid Superlyophobic Copper-Coated Meshes for the Separation of Immiscible Organic Liquid Mixtures. ACS Appl. Mater. Interfaces 2019, 11, 28370–28376. [Google Scholar] [CrossRef]
- Mendes, F.R.; Bastos, M.S.; Mendes, L.G.; Silva, A.R.; Sousa, F.D.; Monteiro-Moreira, A.C.; Cheng, H.N.; Biswas, A.; Moreira, R.A. Preparation and evaluation of hemicellulose films and their blends. Food Hydrocoll. 2017, 70, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Pitkänen, L.; Douglade, J.; Tenkanen, M.; Remond, C.; Joly, C. Wheat bran arabinoxylans: Chemical structure and film properties of three isolated fractions. Carbohydr. Polym. 2011, 86, 852–859. [Google Scholar] [CrossRef]
- Zhong, L.-X.; Peng, X.-W.; Yang, N.; Cao, X.-F.; Sun, R.-C. Long-Chain Anhydride Modification: A New Strategy for Preparing Xylan Films. J. Agric. Food Chem. 2013, 61, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Akkus, M.; Bahcegul, E.; Ozkan, N.; Bakir, U. Post-extrusion heat-treatment as a facile method to enhance the mechanical properties of extruded xylan-based polymeric materials. RSC Adv. 2014, 4, 62295–62300. [Google Scholar] [CrossRef]
- Hansen, N.M.L.; Plackett, D. Sustainable Films and Coatings from Hemicelluloses: A Review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Ann Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148. [Google Scholar] [CrossRef]
- Escalante, A.; Gonçalves, A.; Bodin, A.; Stepan, A.; Sandström, C.; Toriz, G.; Gatenholm, P. Flexible oxygen barrier films from spruce xylan. Carbohydr. Polym. 2012, 87, 2381–2387. [Google Scholar] [CrossRef]
- Peng, X.-W.; Ren, J.-L.; Zhong, L.-X.; Sun, R.-C. Nanocomposite Films Based on Xylan-Rich Hemicelluloses and Cellulose Nanofibers with Enhanced Mechanical Properties. Biomacromolecules 2011, 12, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Sun, R.; Tomkinson, J.; Fowler, P. Acetylation of wheat straw hemicellulose B in a new non-aqueous swelling system. Carbohydr. Polym. 1999, 41, 379–387. [Google Scholar] [CrossRef]
- Lindblad, M.S.; Ranucci, E.; Albertsson, A.-C. Biodegradable Polymers from Renewable Sources. New Hemicellulose-Based Hydrogels. Macromol. Rapid Commun. 2001, 22, 962–967. [Google Scholar] [CrossRef]
- Salam, A.; Pawlak, J.J.; Venditti, R.A.; El-Tahlawy, K. Incorporation of carboxyl groups into xylan for improved absorbency. Cellulose 2011, 18, 1033–1041. [Google Scholar] [CrossRef]
- Zhu Ryberg, Y.; Edlund, U.; Albertsson, A.C. Conceptual approach to renewable barrier film design based on wood hydroly-sate. Biomacromolecules 2011, 12, 1355–1362. [Google Scholar] [CrossRef]
- Haack, V.; Heinze, T.; Oelmeyer, G.; Kulicke, W.-M. Starch Derivatives of High Degree of Functionalization, 8. Synthesis and Flocculation Behavior of Cationic Starch Polyelectrolytes. Macromol. Mater. Eng. 2002, 287, 495–502. [Google Scholar] [CrossRef]
- Zhang, L.-M. Preparation and anti-clay-swelling ability of new water-soluble cellulose derivatives containing quaternary ammonium groups. J. Appl. Polym. Sci. 2000, 79, 1416–1422. [Google Scholar] [CrossRef]
- Thanou, M.; Kotzé, A.; Scharringhausen, T.; Lueßen, H.; de Boer, A.; Verhoef, J.; Junginger, H. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Control. Release 2000, 64, 15–25. [Google Scholar] [CrossRef]
- Peng, X.; Ren, J.; Sun, R. An efficient method for the synthesis of hemicellulosic derivatives with bifunctional groups in butanol/water medium and their rheological properties. Carbohydr. Polym. 2011, 83, 1922–1928. [Google Scholar] [CrossRef]
- Rao, J.; Gao, H.; Guan, Y.; Li, W.Q.; Liu, Q. Fabrication of hemicelluloses films with enhanced mechanical properties by gra-phene oxide for humidity sensing. Carbohydr. Polym. 2019, 208, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Vijayendra, S.V.N.; Shamala, T.R. Film forming microbial biopolymers for commercial applications—A review. Crit. Rev. Biotechnol. 2013, 34, 338–357. [Google Scholar] [CrossRef]
- Khodaeimehr, R.; Peighambardoust, S.J.; Peighambardoust, S.H. Preparation and characterization of corn starch/clay nano-composite films: Effect of clay content and surface modification. Starch-Stärke 2018, 70, 1700251–1700262. [Google Scholar] [CrossRef]
- Massaro, M.; Iborra, C.V.; Cavallaro, G.; Colletti, C.; García-Villén, F.; Lazzara, G.; Riela, S. Synthesis and Characterization of Nanomaterial Based on Halloysite and Hectorite Clay Minerals Covalently Bridged. Nanomaterials 2021, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Coo, J. Hydraulic conductivity of clay mixed with nanomaterials. Can. Geotech. J. 2015, 52, 808–811. [Google Scholar] [CrossRef]
- Schoonheydt, R.A. Reflections on the material science of clay minerals. Appl. Clay Sci. 2016, 131, 107–112. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, B.; Tan, X.; Qi, X.-M.; Bian, J.; Peng, F.; Sun, R.-C. Organic–Inorganic Composite Films Based on Modified Hemicelluloses with Clay Nanoplatelets. ACS Sustain. Chem. Eng. 2014, 2, 1811–1818. [Google Scholar] [CrossRef]
- Pandey, S. A comprehensive review on recent developments in bentonite-based materials used as adsorbents for wastewater treatment. J. Mol. Liq. 2017, 241, 1091–1113. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mustafa, A.B.M.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Elkawash, H.; Tirkes, S.; Hacioglu, F.; Tayfun, U. Physical and mechanical performance of bentonite and barite loaded low density polyethylene composites: Influence of surface silanization of minerals. J. Compos. Mater. 2020, 54. [Google Scholar] [CrossRef]
- Chen, G.-G.; Qi, X.-M.; Li, M.-P.; Guan, Y.; Bian, J.; Peng, F.; Yao, C.-L.; Sun, R.-C. Hemicelluloses/montmorillonite hybrid films with improved mechanical and barrier properties. Sci. Rep. 2015, 5, 16405–16416. [Google Scholar] [CrossRef]
- Guan, Y.; Qi, X.M.; Chen, G.G.; Peng, F.; Sun, R.C. Facile approach to preparedrug-loading film from hemicelluloses and chi-tosan. Carbohydr. Polym. 2016, 153, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, C.; Zhang, X.; Sun, C.; Huang, Y. Hydrogen bond and surface stress relaxation by aldehydic and formic acidic molecular solvation. J. Mol. Liq. 2017, 249, 494–500. [Google Scholar] [CrossRef]
- Dolan, G.K.; Cartwright, B.; Bonilla, M.R.; Gidley, M.J.; Stokes, J.R.; Yakubov, G.E. Probing adhesion between nanoscale cel-lulose fibres using AFM lateral force spectroscopy: The effect of hemicelluloses on hydrogen bonding. Carbohydr. Polym. 2019, 208, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Christidis, G.; Blum, A.E.; Eberl, D. Influence of layer charge and charge distribution of smectites on the flow behaviour and swelling of bentonites. Appl. Clay Sci. 2006, 34, 125–138. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. Twenty Years of Polymer-Clay Nanocomposites. Macromol. Mater. Eng. 2006, 291, 1449–1476. [Google Scholar] [CrossRef]
- Xu, L.; Chu, Z.; Wang, H.; Cai, L.; Tu, Z.; Liu, H.; Zhu, C.; Shi, H.; Pan, D.; Pan, J.; et al. Electrostatically Assembled Multilayered Films of Biopolymer Enhanced Nanocapsules for on-Demand Drug Release. ACS Appl. Bio Mater. 2019, 2, 3429–3438. [Google Scholar] [CrossRef]
- Kačuráková, M.; Ebringerová, A.; Hirsch, J.; Hromádková, Z. Infrared study of arabinoxylans. J. Sci. Food Agric. 1994, 66, 423–427. [Google Scholar] [CrossRef]
- Aroke, U.; Abdulkarim, A.; Ogubunka, R. Fourier-transform infrared characterization of kaolin, granite, bentonite and barite. ATBU J. Environ. Technol. 2013, 6, 42–53. [Google Scholar]
- Qureshi, D.; Behera, K.P.; Mohanty, D.; Mahapatra, S.K.; Verma, S.; Sukyai, P.; Banerjee, I.; Pal, S.K.; Mohanty, B.; Kim, D.; et al. Synthesis of novel poly (vinyl alcohol)/tamarind gum/bentonite-based composite films for drug delivery applications. Colloid Surf. A 2021, 613, 126043–126060. [Google Scholar] [CrossRef]
- Eminoğlu, E.M.; Beypınar, F.; Kahraman, M.V.; Durmuş, A. Fabrication of photo-cross-linked polyethyleneimine-based barriers for CO2 capture. Polym. Adv. Technol. 2015, 26, 1053–1058. [Google Scholar] [CrossRef]
- Liang, C.Y.; Marchessault, R.H.J. Infrared spectra of crystalline polysaccharides. II. Native celluloses in the region from 640 to 1700 cm−1. Polym. Sci. 1959, 37, 385–395. [Google Scholar] [CrossRef]
- Mangiacapra, P.; Gorrasi, G.; Sorrentino, A.; Vittoria, V. Biodegradable nanocomposites obtained by ball milling of pectin and montmorillonites. Carbohydr. Polym. 2006, 64, 516–523. [Google Scholar] [CrossRef]
- Tezcan, F.; Günister, E.; Özen, G.; Erim, F.B. Biocomposite films based on alginate and organically modified clay. Int. J. Biol. Macromol. 2012, 50, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Costafreda, J.L.; Martín, D.A. Bentonites in Southern Spain. Characterization and Applications. Crystals 2021, 11, 706. [Google Scholar] [CrossRef]
- Alther, G.R. Thermal stability of some industrial bentonites. Appl. Clay Sci. 1991, 5, 469–488. [Google Scholar] [CrossRef]

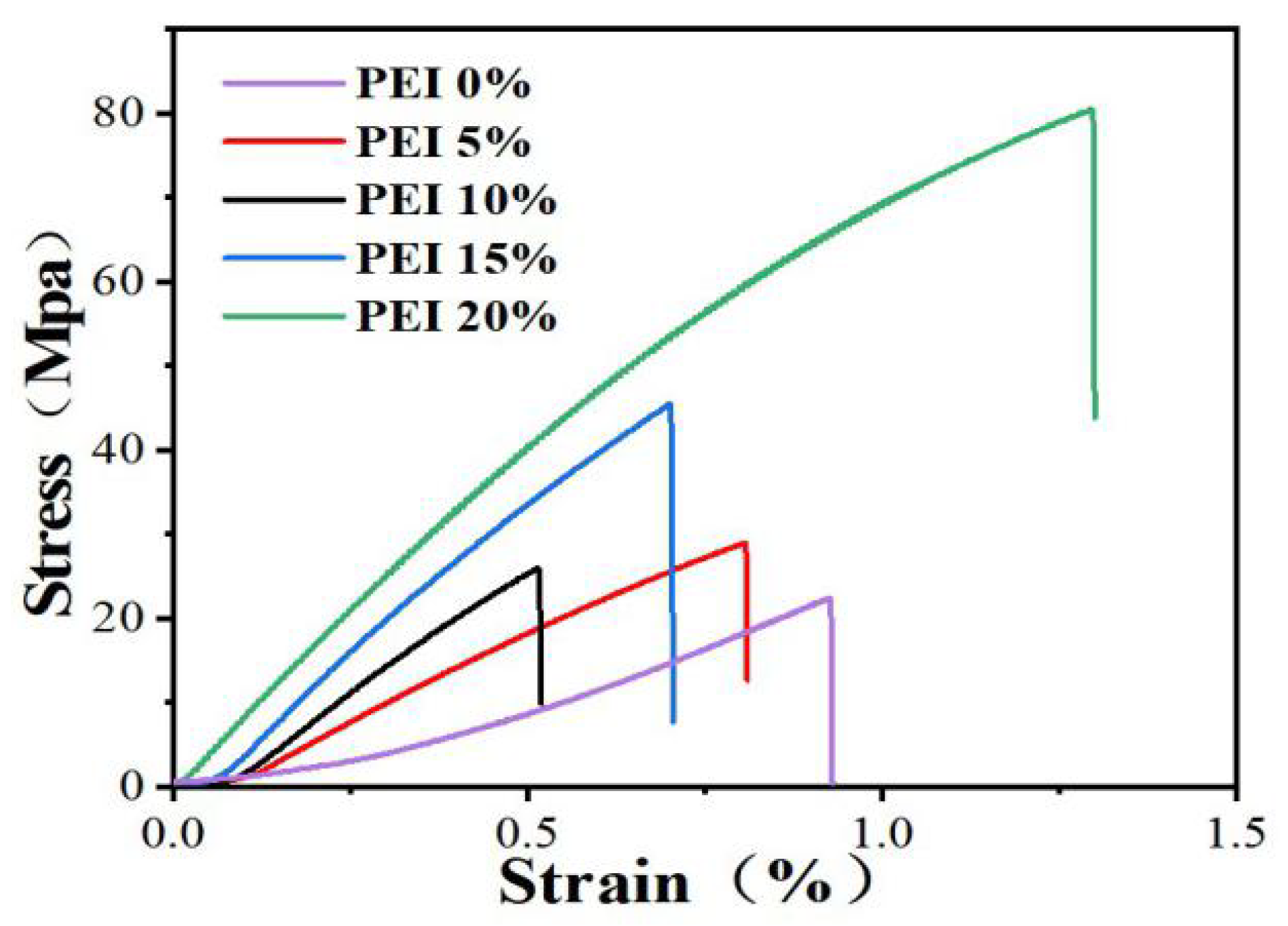

| Sample | Thickness (um) | Tensile Strength (MPa) | Tensile Strain at Fracture (%) | Young’s Modulus (GPa) |
|---|---|---|---|---|
| PEI 0% | 71 ± 0.87 | 22.67 ± 0.65 | 0.92 ± 0.22 | 2.41 ± 0.29 |
| PEI 5% | 73 ± 0.93 | 25.94 ± 0.71 | 0.51 ± 0.12 | 4.31 ± 0.59 |
| PEI 10% | 76 ± 0.62 | 28.96 ± 0.42 | 0.81 ± 0.31 | 6.08 ± 0.13 |
| PEI 15% | 78 ± 0.53 | 45.54 ± 0.53 | 0.70 ± 0.31 | 7.15 ± 0.17 |
| PEI 20% | 80 ± 0.24 | 80.52 ± 0.33 | 1.30 ± 0.84 | 8.14 ± 0.04 |
| Sample | Tonset/°C | T1/°C | Residual Mass/% |
|---|---|---|---|
| PEI 0% | 184.7 ± 0.97 | 277.5 ± 0.42 | 55.6 ± 0.57 |
| PEI 10% | 220.7 ± 0.77 | 279.2 ± 0.39 | 57.3 ± 0.54 |
| QH | 119.6 ± 0.52 | 273.9 ± 0.48 | 17.3 ± 0.27 |
| bentonite | - | - | 95.3 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Li, J.; Wu, Y.; Gao, H.; Guan, Y. High-Performanced Hemicellulose Based Organic-Inorganic Films with Polyethyleneimine. Polymers 2021, 13, 3777. https://doi.org/10.3390/polym13213777
Wu H, Li J, Wu Y, Gao H, Guan Y. High-Performanced Hemicellulose Based Organic-Inorganic Films with Polyethyleneimine. Polymers. 2021; 13(21):3777. https://doi.org/10.3390/polym13213777
Chicago/Turabian StyleWu, Han, Jing Li, Yule Wu, Hui Gao, and Ying Guan. 2021. "High-Performanced Hemicellulose Based Organic-Inorganic Films with Polyethyleneimine" Polymers 13, no. 21: 3777. https://doi.org/10.3390/polym13213777







