An Insight into a Sustainable Removal of Bisphenol A from Aqueous Solution by Novel Palm Kernel Shell Magnetically Induced Biochar: Synthesis, Characterization, Kinetic, and Thermodynamic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Magnetic Biochar
2.3. Characterization of Neat and Magnetic Biochar
2.4. Analysis of Surface Chemistry of Biochar (pHpzc)
2.5. Adsorption Experiment
2.6. Statistical Analysis
2.7. Reusability and Regeneration of MBC
2.8. Adsorption Isotherm
2.9. Langmuir Adsorption Isotherm
2.10. Freundlich Adsorption Isotherm
3. Results
3.1. Results and Discussion
Characterization and Analysis of Synthesized Biochar Adsorbents
3.2. Morphological Analysis of Synthesized Biochar
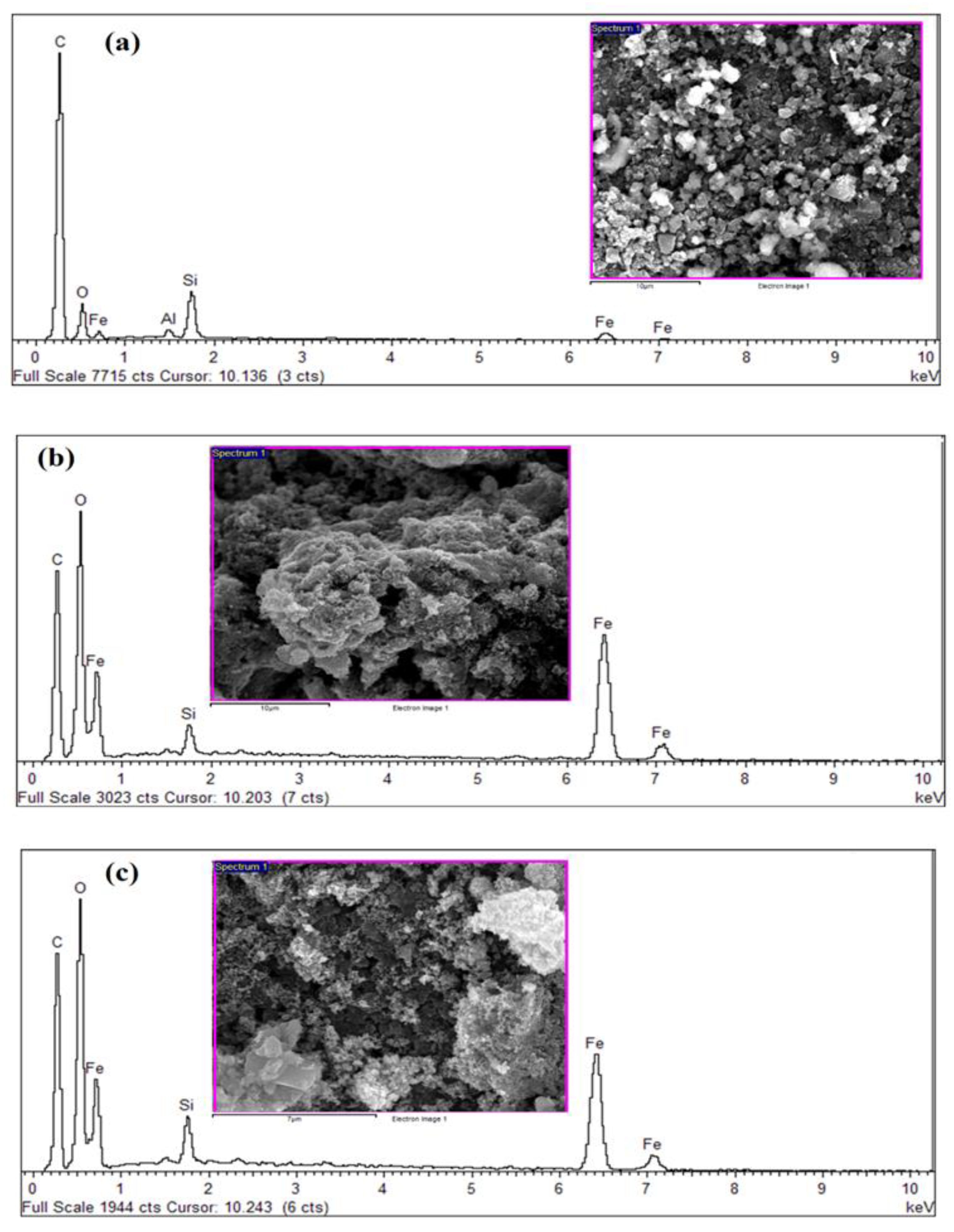
3.3. Elemental Analysis of Synthesized PKS Biochar
3.4. BET Surface Area Analysis of Biochar
3.5. Magnetic Properties of As-Synthesized Magnetic Biochar
3.6. XRD Analysis of Synthesized Biochar
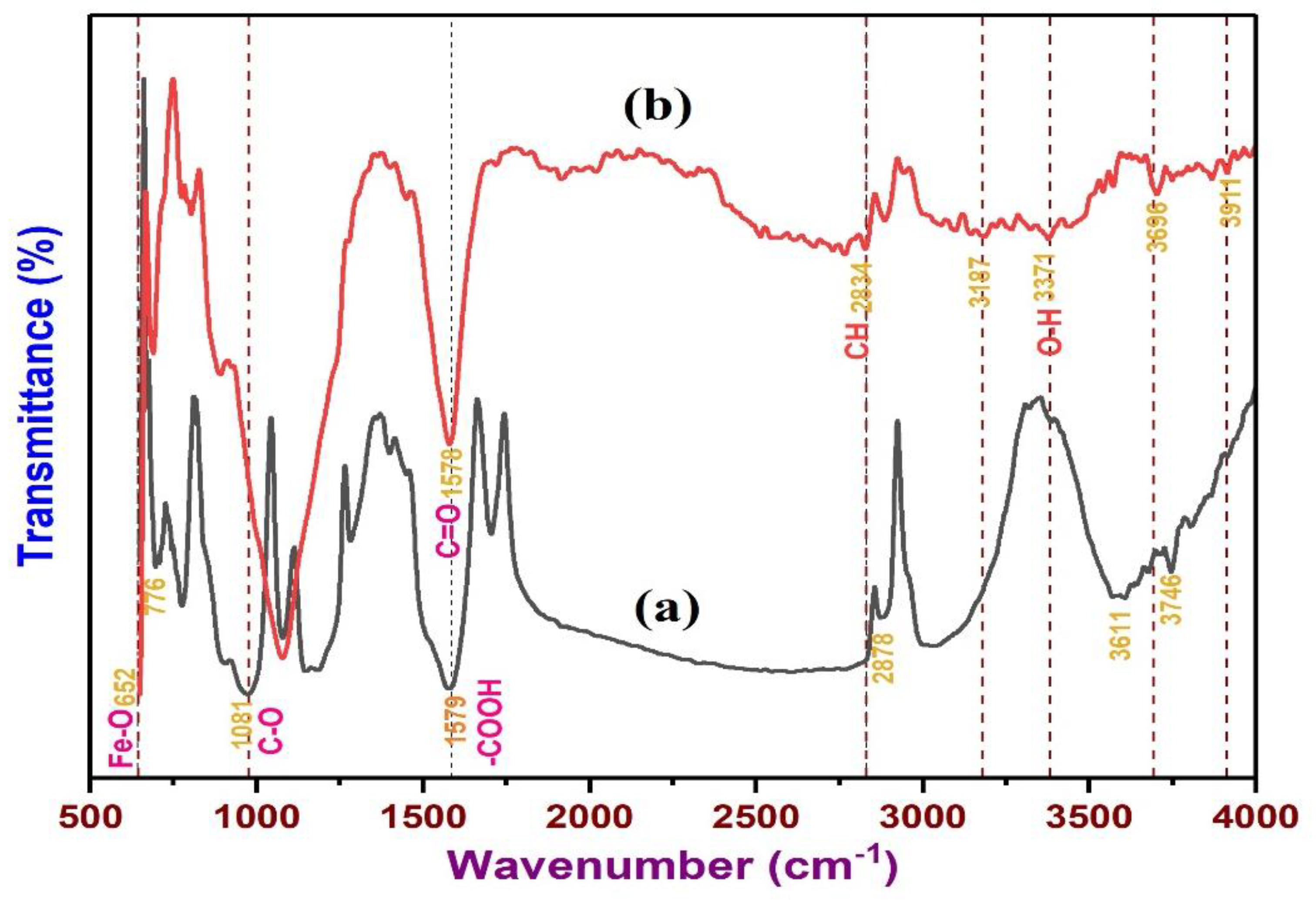
3.7. Analysis of Functional Group
3.8. Determination of Electrokinetic Charge (pHpzc) of Biochar
3.9. Influence of Working Conditions on the Adsorption of BPA
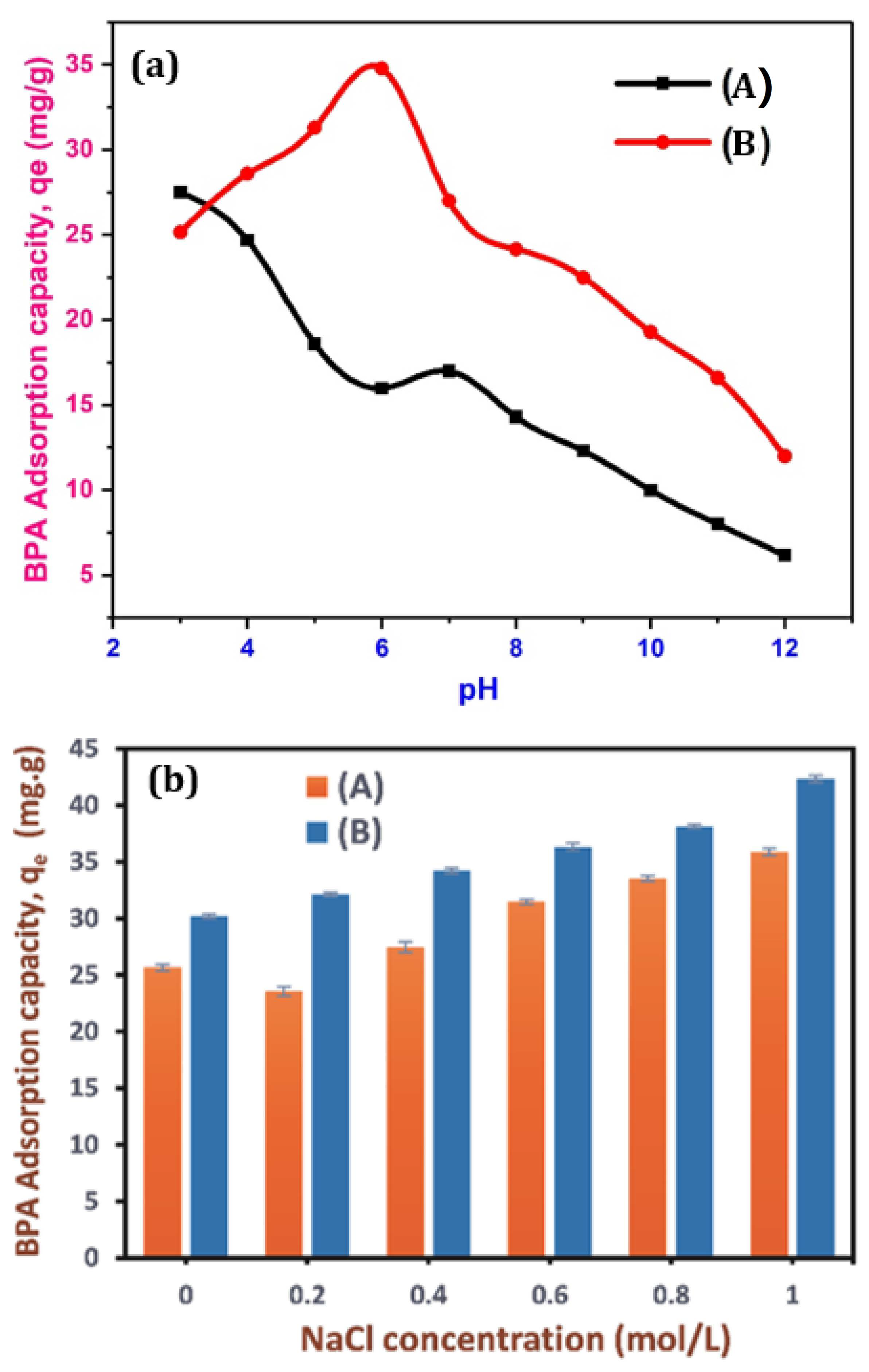
3.10. Effect of pH on BPA Adsorption
3.11. Effect of Ionic Strength on BPA Adsorption
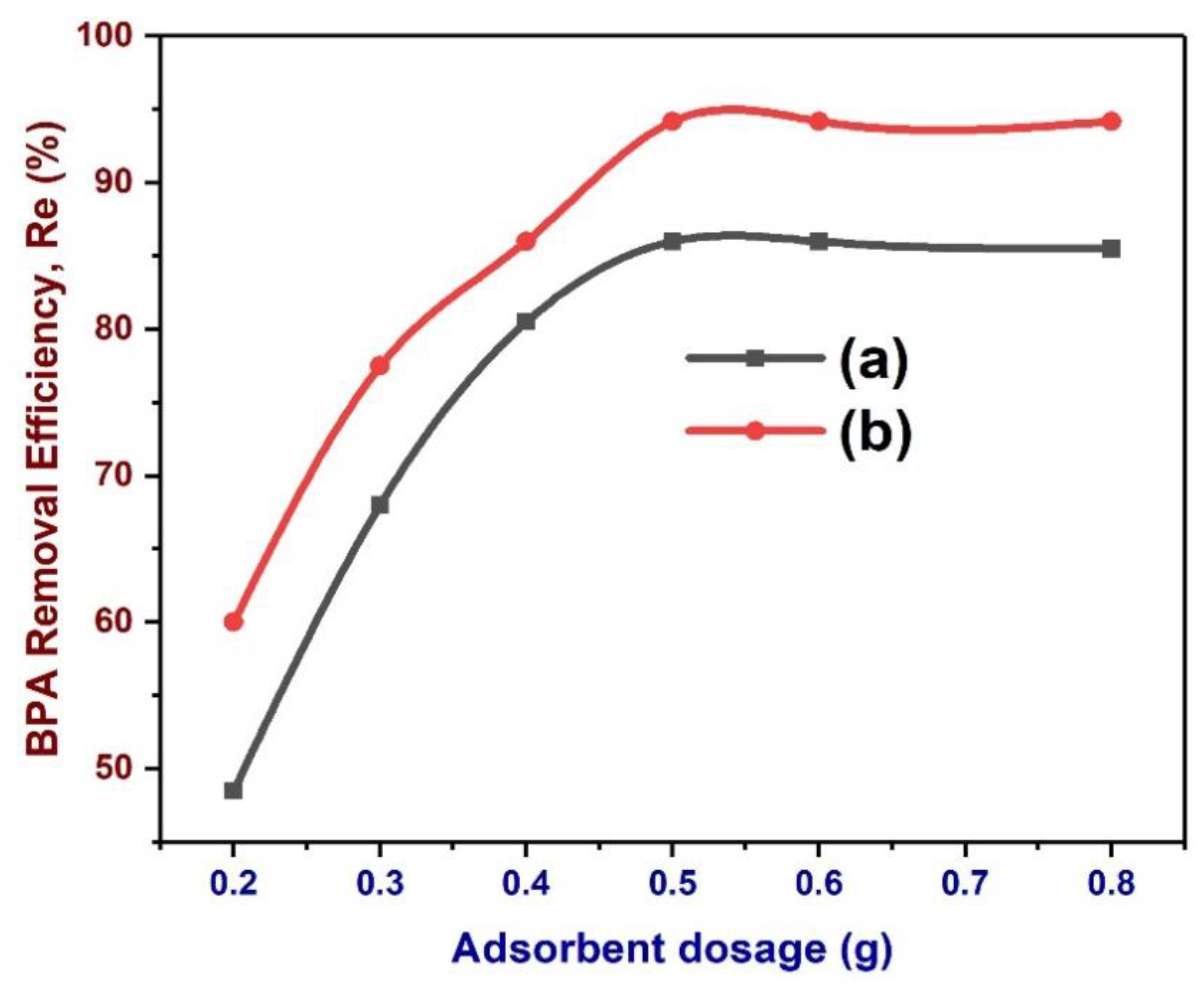
3.12. Effects of Biochar Dose on the Removal of BPA
3.13. Effects of Initial Concentration and Contact Time on the Removal of BPA
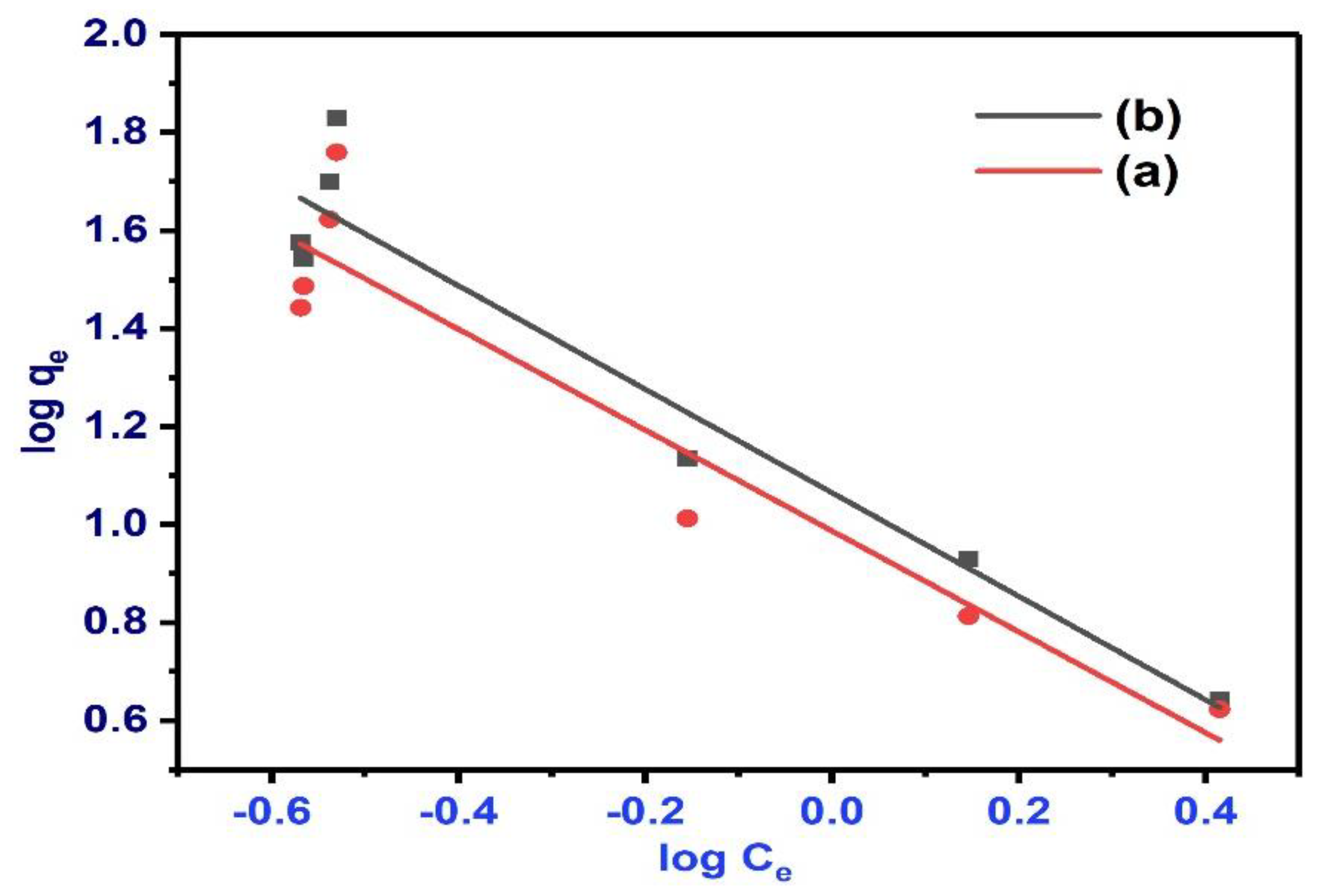
3.14. BPA Sorption Isotherm Study
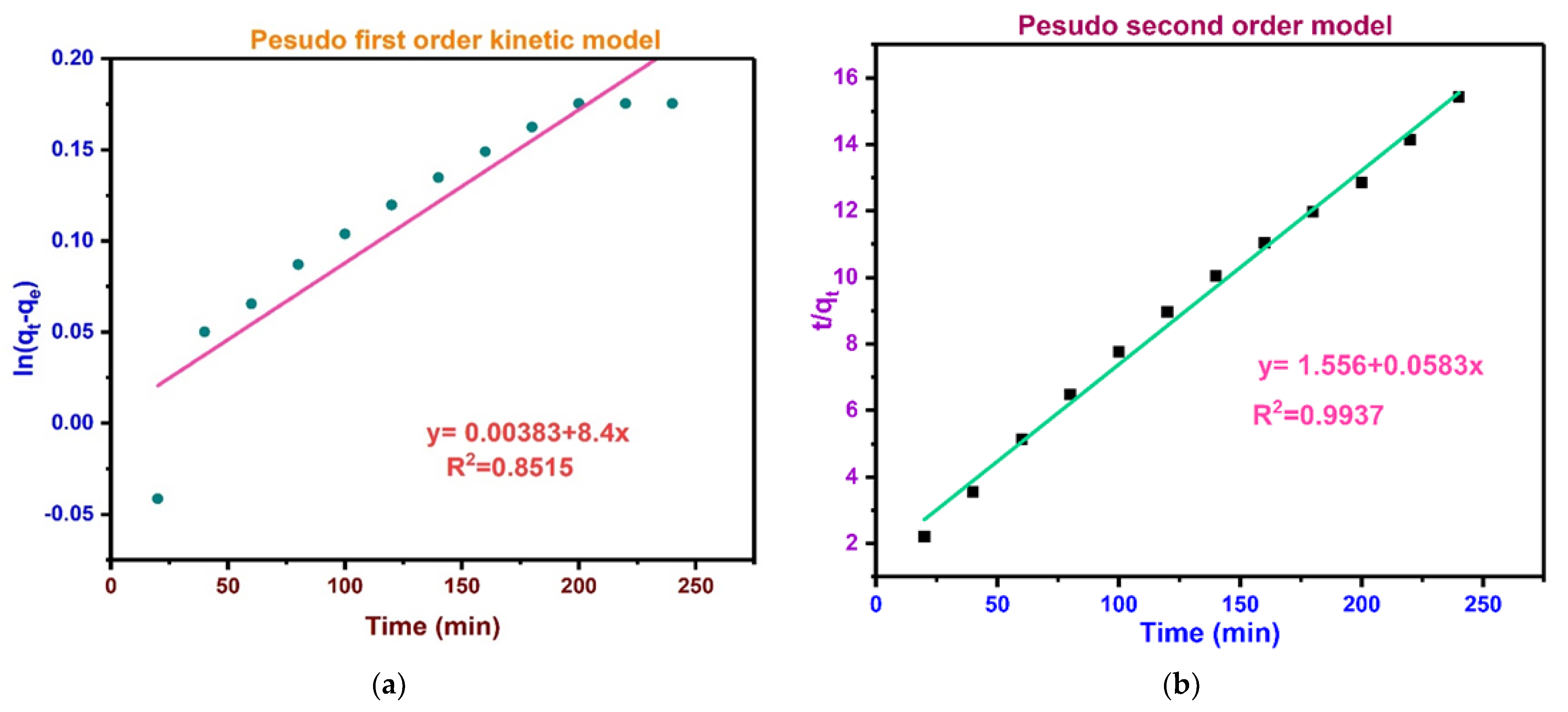
3.15. BPA Adsorption Kinetic Studies
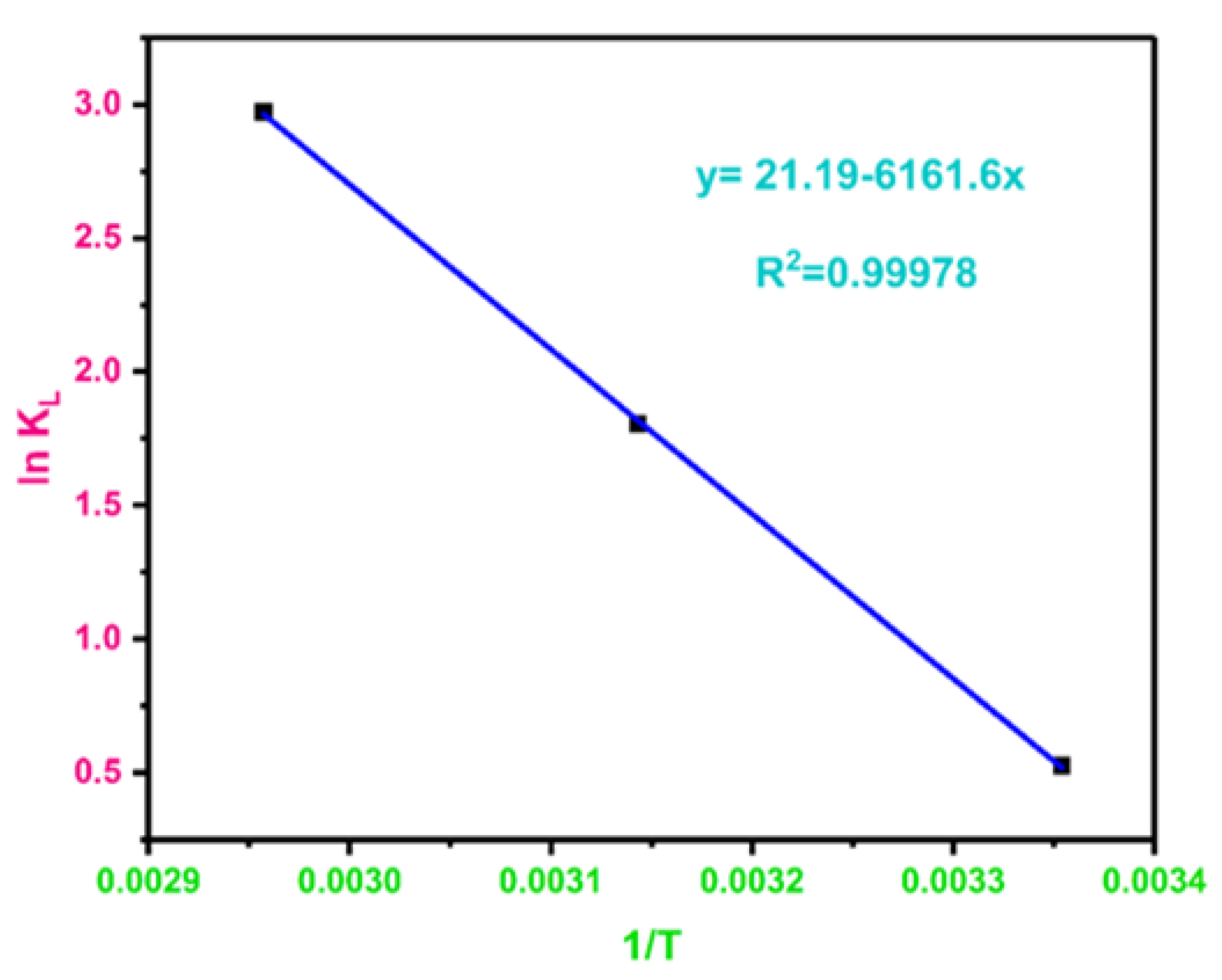
3.16. Adsorption Thermodynamics
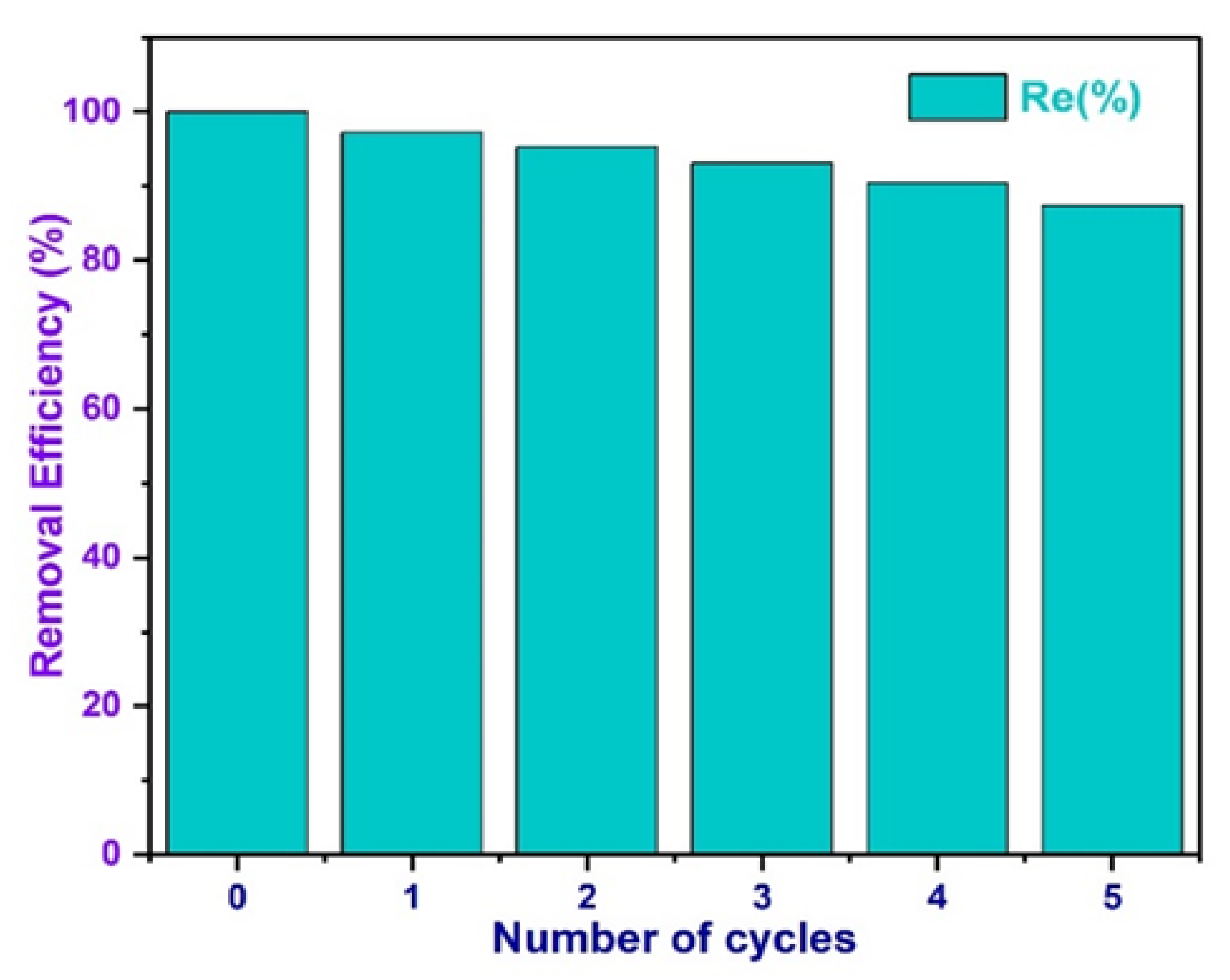
3.17. Investigation of Biochar Regeneration and Reusability
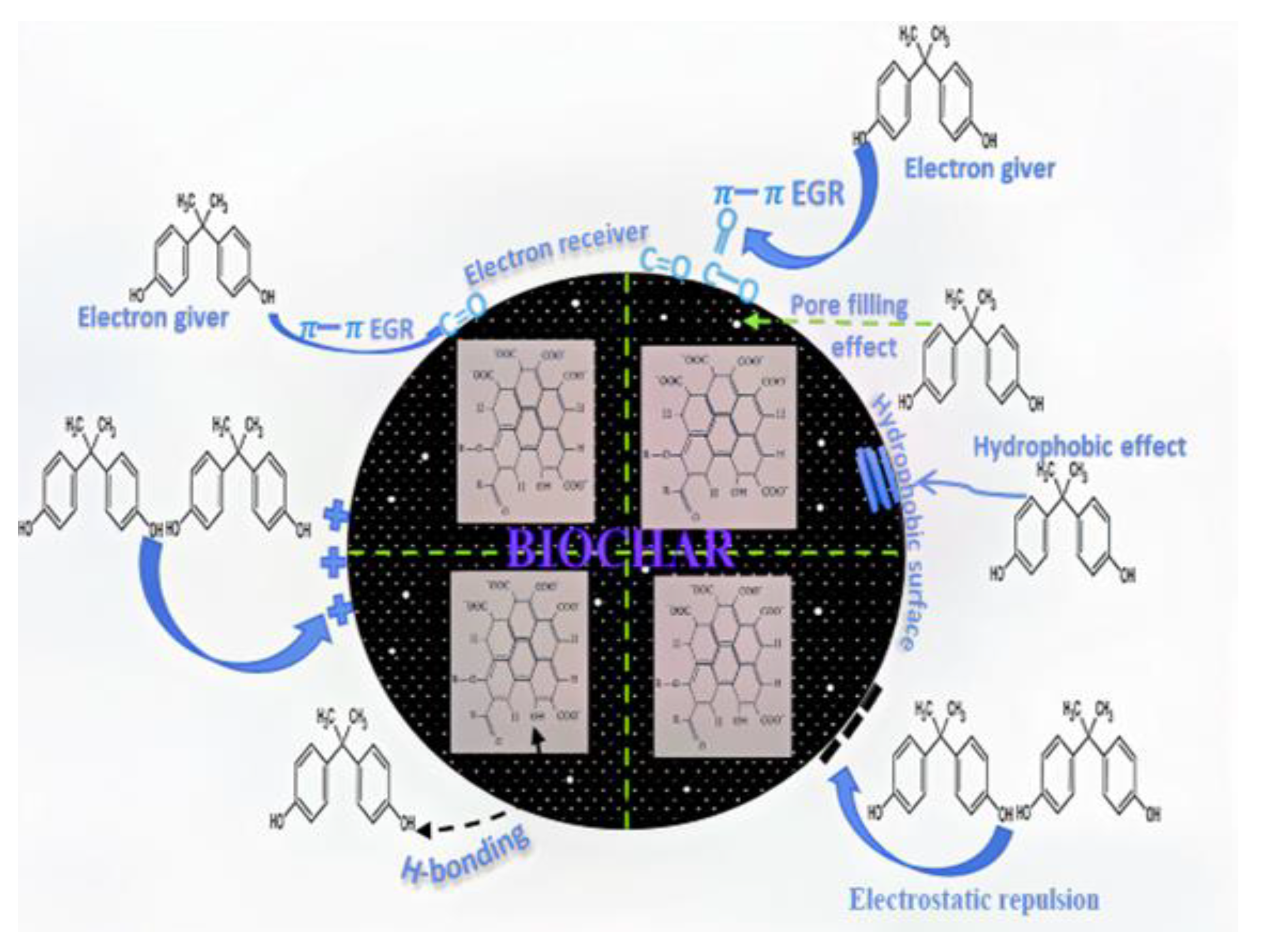
3.18. Controlling Mechanism for BPA Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahsan, M.A.; Islam, M.T.; Hernandez, C.; Kim, H.; Lin, Y.; Curry, M.L.; Gardea-Torresdey, J.; Noveron, J.C. Adsorptive Removal of Sulfamethoxazole and Bisphenol A from Contaminated Water using Functionalized Carbonaceous Material Derived from Tea Leaves. J. Environ. Chem. Eng. 2018, 6, 4215–4225. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Luthi, D.; Quinerly, D.A. Plastic bodies in a plastic world: Multi-disciplinary approaches to study endocrine disrupting chemicals: We dedicate this manuscript in memory of Dr. Theo Colborn: Mentor, colleague and friend. J. Clean. Prod. 2017, 140, 373–385. [Google Scholar] [CrossRef]
- Rathnayake, S.I.; Xi, Y.; Frost, R.L.; Ayoko, G.A. Environmental applications of inorganic-organic clays for recalcitrant organic pollutants removal: Bisphenol A. J. Colloid Interface Sci. 2016, 470, 183–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michałowicz, J. Bisphenol A–Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Hoekstra, E.J.; Simoneau, C. Release of Bisphenol A from Polycarbonate-A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 386–402. [Google Scholar] [CrossRef]
- Mileva, G.; Baker, S.L.; Konkle, A.T.M.; Bielajew, C. Bisphenol-A: Epigenetic reprogramming and effects on reproduction and behavior. Int. J. Environ. Res. Public Health 2014, 11, 7537–7561. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Nagel, S.C.; Coe, B.L.; Angle, B.M.; Taylor, J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 2012, 354, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Su-Hua, W.; Bing-zhi, D.; Yu, H. Adsorption of bisphenol A by polysulphone membrane. Desalination 2010, 253, 22–29. [Google Scholar] [CrossRef]
- Wintgens, T.; Gallenkemper, M.; Melin, T. Removal of endocrine disrupting compounds with membrane processes in wastewater treatment and reuse. Water Sci. Technol. 2004, 50, 1–8. [Google Scholar] [CrossRef]
- Rajasärkkä, J.; Pernica, M.; Kuta, J.; Lašňák, J.; Šimek, Z.; Bláha, L. Drinking water contaminants from epoxy resin-coated pipes: A field study. Water Res. 2016, 103, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M. Occurrence and risk assessment of multiclass endocrine disrupting compounds in an urban tropical river and a proposed risk management and monitoring framework. Sci. Total Environ. 2019, 671, 431–442. [Google Scholar] [CrossRef]
- Nazifa, T.H.; Kristanti, R.A.; Ike, M.; Kuroda, M.; Hadibarata, T. Occurrence and distribution of estrogenic chemicals in river waters of Malaysia. Toxicol. Environ. Health Sci. 2020, 12, 65–74. [Google Scholar] [CrossRef]
- Shekhar, S.; Sood, S.; Showkat, S.; Lite, C.; Chandrasekhar, A.; Vairamani, M.; Barathi, S.; Santosh, W. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen. Comp. Endocrinol. 2017, 241, 100–107. [Google Scholar] [CrossRef]
- Colin, A.; Bach, C.; Rosin, C.; Munoz, J.F.; Dauchy, X. Is drinking water a major route of human exposure to alkylphenol and bisphenol contaminants in France? Arch. Environ. Contam. Toxicol. 2014, 66, 86–99. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M. Adsorption characteristics and mechanism of bisphenol a by magnetic biochar. Int. J. Environ. Res. Public Health 2020, 17, 1075. [Google Scholar] [CrossRef] [Green Version]
- Gadupudi, C.K.; Rice, L.; Xiao, L.; Kantamaneni, K. Endocrine Disrupting Compounds Removal Methods from Wastewater in the United Kingdom: A Review. Science 2019, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef]
- Zielińska, M.; Bułkowska, K.; Cydzik-Kwiatkowska, A.; Bernat, K.; Wojnowska-Baryła, I. Removal of bisphenol A (BPA) from biologically treated wastewater by microfiltration and nanofiltration. Int. J. Environ. Sci. Technol. 2016, 13, 2239–2248. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, L.; Adolfsson-Erici, M.; Björlenius, B.; Rutgersson, C.; Förlin, L.; Larsson, D.G.J. Comparison of six different sewage treatment processes-Reduction of estrogenic substances and effects on gene expression in exposed male fish. Sci. Total Environ. 2009, 407, 5235–5242. [Google Scholar] [CrossRef]
- Hu, Z.; Wen, X.; Si, X. Pre-ultrafiltration or pre-ozonation for EDCs removal in a combined ultrafiltration and ozonation process. J. Chem. Technol. Biotechnol. 2016, 91, 2929–2934. [Google Scholar] [CrossRef]
- Fan, X.; Tao, Y.; Wang, L.; Zhang, X.; Lei, Y.; Wang, Z.; Noguchi, H. Performance of an integrated process combining ozonation with ceramic membrane ultra-filtration for advanced treatment of drinking water. Desalination 2014, 335, 47–54. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Wang, P.; Zheng, H.; Zheng, Y. Microwave-enhanced Mn-Fenton process for the removal of BPA in water. Chem. Eng. J. 2016, 294, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Zahari, A.M.; Shuo, C.W.; Sathishkumar, P.; Yusoff, A.R.M.; Gu, F.L.; Buang, N.A.; Lau, W.J.; Gohari, R.J.; Yusop, Z. A reusable electrospun PVDF-PVP-MnO2 nanocomposite membrane for bisphenol A removal from drinking water. J. Environ. Chem. Eng. 2018, 6, 5801–5811. [Google Scholar] [CrossRef]
- Katibi, K.K.; Yunos, K.F.; Man, H.C.; Aris, A.Z.; Zuhair, M.; Syahidah, R. Recent Advances in the Rejection of Endocrine-Disrupting Compounds from Water Using Membrane and Membrane Bioreactor Technologies: A Review. Polymers 2021, 13, 329. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Singhania, T.; Singh, A.; Sharma, S.; Rani, S.; Neogy, A.; Yadav, S.R.; Sangal, V.K.; Garg, N. Photocatalytic Degradation of Bisphenol-A using N, Co Codoped TiO2 Catalyst under Solar Light. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Rodriguez-mozaz, S.; Insa, S.; Schoevaart, R.; Barcelo, D.; de Cazes, M.; Belleville, M.P.; Marcano, J.S.; Misovic, A.; Oehlmann, J.; et al. Removal of endocrine disrupting chemicals in wastewater by enzymatic treatment with fungal laccases. Org. Process. Res.Dev. 2017, 21, 480–491. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Enzymatic degradation of bisphenol-A with immobilized laccase on TiO2 sol-gel coated PVDF membrane. J. Memb. Sci. 2014, 469, 19–30. [Google Scholar] [CrossRef]
- Bastami, T.R.; Entezari, M.H. Activated carbon from carrot dross combined with magnetite nanoparticles for the efficient removal of p-nitrophenol from aqueous solution. Chem. Eng. J. 2012, 210, 510–519. [Google Scholar] [CrossRef]
- Grassi, M.; Kaykioglu, G.; Belgiorno, V.; Lofrano, G. Emerging Compounds Removal from Wastewater. Green Chem. Sustain. 2012, 15–38. [Google Scholar] [CrossRef]
- Saber, S.E.M.; Jamil, S.N.A.M.; Abdullah, L.C.; Choong, T.S.Y.; Ting, T.M. Insights into thep-nitrophenol adsorption by amidoxime-modified poly(acrylonitrile-co-acrylic acid): Characterization, kinetics, isotherm, thermodynamic, regeneration and mechanism study. RSC Adv. 2021, 11, 8150–8162. [Google Scholar] [CrossRef]
- Katibi, K.K.; Yunos, K.F.; Man, H.C.; Aris, A.Z.; Zuhair, M.; Nor, M.; Azis, R.S.; Umar, A.M. Contemporary Techniques for Remediating Endocrine-Disrupting Compounds in Various Water Sources: Advances in Treatment Methods and Their Limitations. Polymers 2021, 13, 3229. [Google Scholar] [CrossRef]
- Haddad, M.; Oie, C.; Vo Duy, S.; Sauvé, S.; Barbeau, B. Adsorption of micropollutants present in surface waters onto polymeric resins: Impact of resin type and water matrix on performance. Sci. Total Environ. 2019, 660, 1449–1458. [Google Scholar] [CrossRef]
- Park, C.M.; Han, J.; Chu, K.H.; Al-Hamadani, Y.A.J.; Her, N.; Heo, J.; Yoon, Y. Influence of solution pH, ionic strength, and humic acid on cadmium adsorption onto activated biochar: Experiment and modeling. J. Ind. Eng. Chem. 2017, 48, 186–193. [Google Scholar] [CrossRef]
- Hanigan, D.; Zhang, J.; Herckes, P.; Krasner, S.W.; Chen, C.; Westerhoff, P. Adsorption of N-nitrosodimethylamine precursors by powdered and granular activated carbon. Environ. Sci. Technol. 2012, 46, 12630–12639. [Google Scholar] [CrossRef]
- Wirasnita, R.; Hadibarata, T.; Yusoff, A.R.M.; Yusop, Z. Removal of bisphenol a from aqueous solution by activated carbon derived from oil palm empty fruit bunch. Water. Air. Soil Pollut. 2014, 225, 2148. [Google Scholar] [CrossRef]
- Chang, K.L.; Hsieh, J.F.; Ou, B.M.; Chang, M.H.; Hseih, W.Y.; Lin, J.H.; Huang, P.J.; Wong, K.F.; Chen, S.T. Adsorption Studies on the Removal of an Endocrine-Disrupting Compound (Bisphenol A) using Activated Carbon from Rice Straw Agricultural Waste. Sep. Sci. Technol. 2012, 47, 1514–1521. [Google Scholar] [CrossRef]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Chen, W.; Parette, R.; Zou, J.; Cannon, F.S.; Dempsey, B.A. Arsenic removal by iron-modified activated carbon. Water Res. 2007, 41, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Insight into biochar properties and its cost analysis. Biomass Bioenergy 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent-A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Liao, T.; Li, T.; Su, X.; Yu, X.; Song, H.; Zhu, Y.; Zhang, Y. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal. Bioresour. Technol. 2018, 263, 207–213. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, C.; Wu, J.; Luo, Y. Adsorptive Removal of Bisphenol A Using N-Doped Biochar Made of Ulva prolifera. Water. Air Soil Pollut. 2017, 228, 1–9. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, Y.; Lee, G.; Kim, Y.; Han, J.; Park, C.M. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4–biochar composite. Bioresour. Technol. 2019, 281, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Yahayu, M.; Abas, F.Z.; Zulkifli, S.E.; Ani, F.N. Utilization of Oil Palm Fiber and Palm Kernel Shell in Various Applications. Sustain. Technol. Manag. Agric. Wastes 2018, 45–56. [Google Scholar] [CrossRef]
- Loh, S.K. The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers. Manag. 2017, 141, 285–298. [Google Scholar] [CrossRef]
- Umar, M.S.; Jennings, P.; Urmee, T. Generating renewable energy from oil palm biomass in Malaysia: The Feed-in Tariff policy framework. Biomass Bioenergy 2014, 62, 37–46. [Google Scholar] [CrossRef] [Green Version]
- AI, M. National Biomass Strategy 2020: New Wealth Creation for Malaysia’s Palm Oil Industry; Agensi Inovasi Malaysia: Kuala Lumpur, Malaysia, 2011. [Google Scholar]
- Kong, S.H.; Loh, S.K.; Bachmann, R.T.; Zainal, H.; Cheong, K.Y. Palm kernel shell biochar production, characteristics and carbon sequestration potential. J. Oil Palm Res. 2019, 31, 508–520. [Google Scholar] [CrossRef]
- Zahraee, S.M.; Golroudbary, S.R.; Shiwakoti, N.; Kraslawski, A.; Stasinopoulos, P. An investigation of the environmental sustainability of palm biomass supply chains via dynamic simulation modeling: A case of Malaysia. J. Clean. Prod. 2019, 237, 117740. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Rios, R.V.R.A.; Garg, V.K.; Lago, R.M. Clay–iron oxide magnetic composites for the adsorption of contaminants in water. Appl. Clay Sci. 2003, 22, 169–177. [Google Scholar] [CrossRef]
- Han, Z.; Sani, B.; Akkanen, J.; Abel, S.; Nybom, I.; Karapanagioti, H.K.; Werner, D. A critical evaluation of magnetic activated carbon’s potential for the remediation of sediment impacted by polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2015, 286, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Mohan, D.; Sarswat, A.; Singh, V.K.; Alexandre-Franco, M.; Pittman, C.U. Development of magnetic activated carbon from almond shells for trinitrophenol removal from water. Chem. Eng. J. 2011, 172, 1111–1125. [Google Scholar] [CrossRef]
- Petrova, B.; Budinova, T.; Tsyntsarski, B.; Kochkodan, V. Removal of aromatic hydrocarbons from water by activated carbon from apricot stones. Chem. Eng. J. 2010, 165, 258–264. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Wu, D.-Y.; Li, C.-J. [Adsorption of phenol chemicals by surfactant-modified zeolites]. Huan Jing Ke Xue Huanjing Kexue 2012, 33, 4361–4366. [Google Scholar]
- Cheng, M.; Jiang, J.; Wang, J.; Fan, J. Highly Salt Resistant Polymer Supported Ionic Liquid Adsorbent for Ultrahigh Capacity Removal of p-Nitrophenol from Water. ACS Sustain. Chem. Eng. 2019, 7, 8195–8205. [Google Scholar] [CrossRef]
- Abdelnaeim, M.Y.; El Sherif, I.Y.; Attia, A.A.; Fathy, N.A.; El-Shahat, M.F. Impact of chemical activation on the adsorption performance of common reed towards Cu(II) and Cd(II). Int. J. Miner. Process. 2016, 157, 80–88. [Google Scholar] [CrossRef]
- Vassileva, P.S.; Radoykova, T.H.; Detcheva, A.K.; Avramova, I.A.; Aleksieva, K.I.; Nenkova, S.K.; Valchev, I.V.; Mehandjiev, D.R. Adsorption of Ag+ ions on hydrolyzed lignocellulosic materials based on willow, paulownia, wheat straw and maize stalks. Int. J. Environ. Sci. Technol. 2016, 13, 1319–1328. [Google Scholar] [CrossRef] [Green Version]
- Rangabhashiyam, S.; Anu, N.; Giri Nandagopal, M.S.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Muhammad, M.; Choong, T.S.Y.; Chuah, T.G.; Yunus, R.; Yap, Y.H.T. Adsorption of β-carotene onto mesoporous carbon coated monolith in isopropyl alcohol and n-hexane solution: Equilibrium and thermodynamic study. Chem. Eng. J. 2010, 164, 178–182. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Bt Ahmad Khairudin, N.B.; Bt Mohamad, S.E.; Hara, H.; Bt Mad Nordin, M.F.; Lee, K.X. An eco-friendly means of biosynthesis of superparamagnetic magnetite nanoparticles via marine polymer. IEEE Trans. Nanotechnol. 2017, 16, 1047–1052. [Google Scholar] [CrossRef]
- Liu, C.; Tang, Z.; Chen, Y.; Su, S.; Jiang, W. Characterization of mesoporous activated carbons prepared by pyrolysis of sewage sludge with pyrolusite. Bioresour. Technol. 2010, 101, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Ghani, Z.A.; Yusoff, M.S.; Zaman, N.Q.; Zamri, M.F.M.A.; Andas, J. Optimization of preparation conditions for activated carbon from banana pseudo-stem using response surface methodology on removal of color and COD from landfill leachate. Waste Manag. 2017, 62, 177–187. [Google Scholar] [CrossRef]
- Sulaiman, S.; Azis, R.S.; Ismail, I.; Man, H.C.; Rosdi, N. Rapid Adsorption of Magnetite Nanoparticles from Recycled Mill Scale Waste as Potential Adsorbent for Removal of Cu(II) Ions. Solid State Phenom. 2021, 317, 270–275. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, C.; Hou, K.; Cheng, Y.; Deng, J.; Jiang, Z.; Tang, L.; Zeng, G. Removal of trichloroethylene by biochar supported nanoscale zero-valent iron in aqueous solution. Sep. Purif. Technol. 2017, 188, 188–196. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Zhou, J.; Guo, J.; Ren, J.; Zhou, F. Efficient removal of lead from aqueous solution by urea-functionalized magnetic biochar: Preparation, characterization and mechanism study. J. Taiwan Inst. Chem. Eng. 2018, 91, 457–467. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef]
- Hamedi, A.; Trotta, F.; Zarandi, M.B.; Zanetti, M.; Caldera, F.; Anceschi, A.; Nateghi, M.R. In situ synthesis of MIL-100(Fe) at the surface of fe3o4@ac as highly efficient dye adsorbing nanocomposite. Int. J. Mol. Sci. 2019, 20, 5612. [Google Scholar] [CrossRef] [Green Version]
- Kalderis, D.; Kayan, B.; Akay, S.; Kulaksiz, E.; Gözmen, B. Adsorption of 2,4-dichlorophenol on paper sludge/wheat husk biochar: Process optimization and comparison with biochars prepared from wood chips, sewage sludge and HOG fuel/demolition waste. J. Environ. Chem. Eng. 2017, 5, 2222–2231. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, L.; Qin, Y.; Xu, M.; Jia, X.; Chen, Z. Removal of aqueous Cr(VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour. Technol. 2018, 256, 1–10. [Google Scholar] [CrossRef]
- Rong, X.; Xie, M.; Kong, L.; Natarajan, V.; Ma, L.; Zhan, J. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation. Chem. Eng. J. 2019, 372, 294–303. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, X.; Cai, H.; Ma, Z.; Liang, H. study on the adsorption of CuFe2O4-loaded corncob biochar for Pb(II). Molecules 2020, 25, 3456. [Google Scholar] [CrossRef]
- Fe, O.; Lin, Q. RSC Advances Removal of Cu (II) from aqueous solution using. RSC Adv. 2017, 7, 53135–53144. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Gan, K.; Lu, D.; Zhang, J. Hydrophilic Fe3O4@C for High-Capacity Adsorption of 2,4-Dichlorophenol. Eur. J. Inorg. Chem. 2016, 2016, 890–896. [Google Scholar] [CrossRef]
- Jun, B.M.; Kim, Y.; Han, J.; Yoon, Y.; Kim, J.; Park, C.M. Preparation of activated biochar-supported magnetite composite for adsorption of polychlorinated phenols from aqueous solutions. Water 2019, 11, 1899. [Google Scholar] [CrossRef] [Green Version]
- Saleh, S.; Kamarudin, K.B.; Ghani, W.A.W.A.K.; Kheang, L.S. Removal of Organic Contaminant from Aqueous Solution Using Magnetic Biochar. Procedia Eng. 2016, 148, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Lyu, T.; Yi, H.; Bi, L.; Pan, G. Superior arsenate adsorption and comprehensive investigation of adsorption mechanism on novel Mn-doped La2O2CO3 composites. Chem. Eng. J. 2020, 391, 123623. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total Environ. 2020, 711, 134847. [Google Scholar] [CrossRef]
- Fan, H.; Ma, X.; Zhou, S.; Huang, J.; Liu, Y.; Liu, Y. Highly efficient removal of heavy metal ions by carboxymethyl cellulose-immobilized Fe3O4 nanoparticles prepared via high-gravity technology. Carbohydr. Polym. 2019, 213, 39–49. [Google Scholar] [CrossRef]
- Kim, E.; Jung, C.; Han, J.; Her, N.; Park, C.M.; Jang, M.; Son, A.; Yoon, Y. Sorptive removal of selected emerging contaminants using biochar in aqueous solution. J. Ind. Eng. Chem. 2016, 36, 364–371. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, J.; Pan, B. Fabrication of Novel Magnetic Nanoparticles of Multifunctionality for Water Decontamination. Environ. Sci. Technol. 2016, 50, 881–889. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Guo, L.; Zhang, Y.; Lou, Z.; Wang, Y.; Qian, G. Cu(II) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresour. Technol. 2013, 141, 83–88. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ. Sci. Technol. 2014, 48, 279–288. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Eng. J. 2012, 180, 81–90. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Cao, X.H.; Liang, P.; Liu, Y.H. Adsorption of uranium from aqueous solution using biochar produced by hydrothermal carbonization. J. Radioanal. Nucl. Chem. 2013, 295, 1201–1208. [Google Scholar] [CrossRef]
- Regmi, P.; Garcia Moscoso, J.L.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K.; Belhaj, D.; Kallel, M. Nano-Fe0 immobilized onto functionalized biochar gaining excellent stability during sorption and reduction of chloramphenicol via transforming to reusable magnetic composite. Chem. Eng. J. 2017, 322, 571–581. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Belhaj, D. Competitive sorption affinity of sulfonamides and chloramphenicol antibiotics toward functionalized biochar for water and wastewater treatment. Bioresour. Technol. 2017, 238, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, M.A.H.; Sornalingam, K. Sorptive removal of phenolic endocrine disruptors by functionalized biochar: Competitive interaction mechanism, removal efficacy and application in wastewater. Chem. Eng. J. 2018, 335, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Liu, X.; Wang, Y.; Shao, D.; Alharbi, N.S.; Alsaedi, A.; Li, J. Highly efficient entrapment of U(VI) by using porous magnetic Ni0.6Fe2.4O4 micro-particles as the adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 65, 367–377. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, R.; Ding, C.; Wang, X.; Cheng, W.; Chen, C.; Wang, X. Adsorption of U(VI) on sericite in the presence of Bacillus subtilis: A combined batch, EXAFS and modeling techniques. Geochim. Cosmochim. Acta 2016, 180, 51–65. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, P.; Lu, J. Application of natural biosorbent and modified peat for bisphenol a removal from aqueous solutions. Carbohydr. Polym. 2012, 88, 502–508. [Google Scholar] [CrossRef]
- Fontecha-Cámara, M.A.; López-Ramón, M.V.; Álvarez-Merino, M.A.; Moreno-Castilla, C. Effect of surface chemistry, solution pH, and ionic strength on the removal of herbicides diuron and amitrole from water by an activated carbon fiber. Langmuir 2007, 23, 1242–1247. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.-G.; Liu, S.-B.; Liu, H.-Y.; Zeng, G.-M.; Tan, X.-F.; Yang, C.-P.; Ding, Y.; Yan, Z.-L.; Cai, X.-X. Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem. Eng. J. 2017, 314, 223–231. [Google Scholar] [CrossRef]
- Li, M.-F.; Liu, Y.-G.; Zeng, G.-M.; Liu, S.-B.; Hu, X.-J.; Shu, D.; Jiang, L.-H.; Tan, X.-F.; Cai, X.-X.; Yan, Z.-L. Tetracycline absorbed onto nitrilotriacetic acid-functionalized magnetic graphene oxide: Influencing factors and uptake mechanism. J. Colloid Interface Sci. 2017, 485, 269–279. [Google Scholar] [CrossRef]
- Liu, S.; Li, M.; Liu, Y.; Liu, N.; Tan, X.; Jiang, L. Supported activated magnetic biochar: Adsorption behavior and mechanism. J. Taiwan Inst. Chem. Eng. 2019, 102, 330–339. [Google Scholar] [CrossRef]
- Garg, V.K.; Gupta, R.; Yadav, A.B.; Kumar, R. Dye removal from aqueous solution by adsorption on treated sawdust. Bioresour. Technol. 2003, 89, 121–124. [Google Scholar] [CrossRef]
- Tuzen, M.; Sari, A. Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: Equilibrium, thermodynamic and kinetic studies. Chem. Eng. J. 2010, 158, 200–206. [Google Scholar] [CrossRef]
- Lee, C.G.; Park, J.A.; Choi, J.W.; Ko, S.O.; Lee, S.H. Removal and Recovery of Cr(VI) from Industrial Plating Wastewater Using Fibrous Anion Exchanger. Water. Air Soil Pollut. 2016, 227, 287. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Kalsoom, T.; Yasmeen, S.; Kalsoom, A.; Raina, S.; Zhuang, Q.; Kong, J. Animal manure-derived biochars produced via fast pyrolysis for the removal of divalent copper from aqueous media. J. Environ. Manag. 2018, 213, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Haghdoost, G.; Aghaie, H.; Monajjemi, M. Investigation of Langmuir and Freundlich Adsorption Isotherm of Co2+ Ion by Micro Powder of Cedar Leaf. Orient. J. Chem. 2017, 33, 1569–1574. [Google Scholar] [CrossRef] [Green Version]
- Boparai, H.K.; Joseph, M.; Carroll, D.M.O. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.H.; Gao, M.; Qiu, W.; Islam, M.S.; Song, Z. Mechanisms for cadmium adsorption by magnetic biochar composites in an aqueous solution. Chemosphere 2020, 246, 125701. [Google Scholar] [CrossRef] [PubMed]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Effects of humic acid and suspended solids on the removal of heavy metals from water by adsorption onto granular activated carbon. Int. J. Environ. Res. Public Health 2015, 12, 10475–10489. [Google Scholar] [CrossRef] [Green Version]
- Silas, K.; Ghani, W.A.W.A.K.; Choong, T.S.Y.; Rashid, U. Breakthrough studies of Co3O4 supported activated carbon monolith for simultaneous SO2/NOx removal from flue gas. Fuel Process. Technol. 2018, 180, 155–165. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, Isotherms, and Thermodynamic Modeling of the Adsorption of Phosphates from Model Wastewater Using Recycled Brick Waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Radnia, H. Isotherm and Kinetics of Fe(II) Adsorption onto Chitosan in a Batch Process. Iran. J. Energy Environ. 2011, 2, 250–257. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Alicia, R.F.; Abdullah, E.C.; Sahu, J.N.; Haslija, A.B.A.; Tan, J. Statistical optimization and kinetic studies on removal of Zn2+ using functionalized carbon nanotubes and magnetic biochar. J. Environ. Chem. Eng. 2013, 1, 486–495. [Google Scholar] [CrossRef]
- Doumer, M.E.; Rigol, A.; Vidal, M.; Mangrich, A.S. Removal of Cd, Cu, Pb, and Zn from aqueous solutions by biochars. Environ. Sci. Pollut. Res. 2016, 23, 2684–2692. [Google Scholar] [CrossRef]
- Van Hien, N.; Valsami-Jones, E.; Vinh, N.C.; Phu, T.T.; Tam, N.T.T.; Lynch, I. Effectiveness of different biochar in aqueous zinc removal: Correlation with physicochemical characteristics. Bioresour. Technol. Reports 2020, 11, 100466. [Google Scholar] [CrossRef]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Doǧan, M.; Alkan, M.; Demirbaş, Ö.; Özdemir, Y.; Özmetin, C. Adsorption kinetics of maxilon blue GRL onto sepiolite from aqueous solutions. Chem. Eng. J. 2006, 124, 89–101. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Park, Y.; Sun, Z.; Ayoko, G.A.; Frost, R.L. Bisphenol A sorption by organo-montmorillonite: Implications for the removal of organic contaminants from water. Chemosphere 2014, 107, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, M.; Luo, Z.; Yang, S. Enhanced removal of bisphenol A from aqueous solution by organo-montmorillonites modified with novel Gemini pyridinium surfactants containing long alkyl chain. Chem. Eng. J. 2016, 285, 27–38. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Bilad, M.R.; Zakaria, Z.Y.; Jagaba, A.H.; Ghaleb, A.A.S.; Mohammed, H.G. Pristine and Magnetic Kenaf Fiber Biochar for Cd2+ Adsorption from Aqueous Solution. Int. J. Environ. Res. Public Health 2021, 18, 7949. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Sharma, Y.C.; Sillanpää, M. Response surface methodological approach for the optimization of adsorption process in the removal of Cr(VI) ions by Cu2(OH)2CO3 nanoparticles. Appl. Surf. Sci. 2015, 326, 257–270. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Zhao, G.; Zhang, Z.; Xu, C.; Liu, Y.; Chen, J.; Zhuang, L.; Haya, T.; Wang, X. Enhanced adsorption of U(VI) and 241Am(III) from wastewater using Ca/Al layered double hydroxide@carbon nanotube composites. J. Hazard. Mater. 2018, 347, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, A.L.; Pezoti, O.; Bedin, K.C.; Silva, T.L.; Paesano Junior, A.; Asefa, T.; Almeida, V.C. Magnetic Activated Carbon Derived from Biomass Waste by Concurrent Synthesis: Efficient Adsorbent for Toxic Dyes. ACS Sustain. Chem. Eng. 2016, 4, 1058–1068. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, Y.L.; Kim, I.S.; Yang, J.K.; Koduru, J.R.; Chang, Y.Y. Preparation and characterization of porous reduced graphene oxide based inverse spinel nickel ferrite nanocomposite for adsorption removal of radionuclides. J. Hazard. Mater. 2017, 326, 145–156. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Dong, C.; Sun, Y. Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption. Sci. Total Environ. 2019, 651, 1020–1028. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Van, H.T.; Nguyen, V.Q.; Dam, X.V.; Hoang, L.P.; Ha, L.T.; Ha, L.T. Magnetic Fe3O4 Nanoparticle Biochar Derived from Pomelo Peel for Reactive Red 21 Adsorption from Aqueous Solution. J. Chem. 2020, 2020. [Google Scholar] [CrossRef]
- Yang, X.J.; Zhan, J.Y.; Yu, J.; Liu, G.; Huang, R.X. Adsorption features and kinetics of bisphenol A onto magnetic composite organic sepiolite. Chin. J. Environ. Eng. 2016, 10, 3597–3602. [Google Scholar]
- Zhao, Y.; Zhang, R.; Liu, H.; Li, M.; Chen, T.; Chen, D.; Zou, X.; Frost, R.L. Green preparation of magnetic biochar for the effective accumulation of Pb(II): Performance and mechanism. Chem. Eng. J. 2019, 375, 122011. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Cao, N.; Sun, D.; Zhang, Z.; Wang, Y.; Wen, Y.; Yang, Y.; Lyu, T. Sustainable chromium (VI) removal from contaminated groundwater using nano-magnetite-modified biochar via rapid microwave synthesis. Molecules 2021, 26, 103. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Z.; Ifthikar, J.; Shi, L.; Chen, Z.; Chen, Z. One-step preparation and application of magnetic sludge-derived biochar on acid orange 7 removal via both adsorption and persulfate based oxidation. RSC Adv. 2017, 7, 18696–18706. [Google Scholar] [CrossRef] [Green Version]
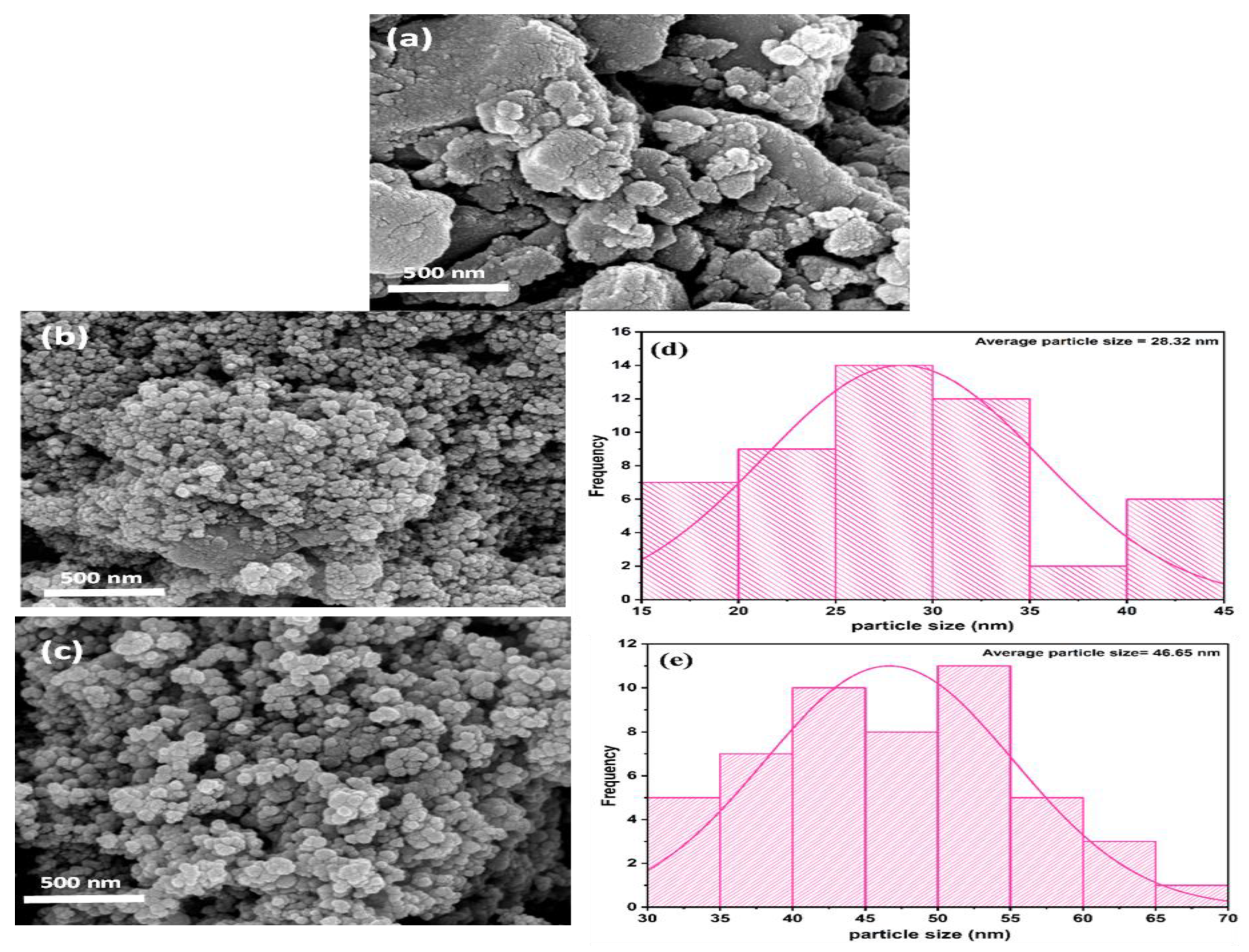







| Compound Name | Lipid-Water Partition Coefficient (Log Kow) | Molecular Mass (g/mol.) | Chemical Structure | Molecular Formula | pKa |
|---|---|---|---|---|---|
| Bisphenol A | 3.32 | 228 | 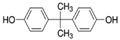 | C15H16O2 | 10.1 |
| Elements | C | O | Si | Fe | Total |
|---|---|---|---|---|---|
| Mass ratio (%) | 32.52 | 27.23 | 2.11 | 38.14 | 100.00 |
| Atom ratio (%) | 52.40 | 32.93 | 1.45 | 13.22 | 100.00 |
| Materials | Pore Diameter (Å) | Pore Volume (cm3/g) | BET Specific Surface Area (m2/g) | pHpzc |
|---|---|---|---|---|
| NBC | 15.516 | 0.416260 | 536.5398 | 4.829 |
| MBC | 24.427 | 0.442203 | 362.0673 | 5.612 |
| Adsorbents | Freundlich Model | Langmuir Model | |||||
|---|---|---|---|---|---|---|---|
| 1/n | KF(L·mg−1) | R2* | qmax(mg/g) | KL(L·mg−1) | RL | R2** | |
| NBC | 1.02974 | 9.718964 | 0.88964 | 5.438033607 | 3.95207 | 0.00509 | 0.7535 |
| MBC | 1.05679 | 11.62947 | 0.9195 | 4.72701489 | 4.01652 | 0.005 | 0.85608 |
| Kinetic Models | Parameters | Value | Linear Regression |
|---|---|---|---|
| PSO where K2 is rate constant (g·mg−1·min−1) | K2 (g·mg−1·min−1) | 0.005289 | |
| Comp. qe (mg/g) | 47.15266 | ||
| Exp. qe (mg/g) | 37.75 | ||
| R2 | 0.99376 | ||
| PFO
ln(qe − qt) = ln(qe) − K1t
| K1 (min−1) | 3.50 × 10−6 | |
| Comp. qe (mg/g) | 12.003837 | ||
| Exp. qe (mg/g) | 37.75 | ||
| R2 | 0.8515 |
| Adsorbate | Temperature (K) | lnKL | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (J·mol·K−1) |
|---|---|---|---|---|---|
| BPA | 298.15 | 0.526093278 | −1.304090065 | 51.22768066 | 176.1349999 |
| 318.15 | 1.803593997 | −4.770684859 | |||
| 338.15 | 2.971634746 | −8.354391819 |
| Adsorbents | Surface Area (m2/g) | Magnetic Strength (emu/g) | Regeneration (%) | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|---|
| ulva prolifera (marine macroalgae) | 25.43 | ND | ND | 84.19 | [48] |
| pomelo peel | 889.8 | ND | ND | 26.25 | [133] |
| magnetic composite sepiolite | NA | 14.1 | NA | 36.30 | [134] |
| bamboo | 61.5 | 37.6 and 32.6 | 7.6 and 8.2 | 263.2 | [125] |
| sewage sludge | |||||
| wheat straw | 65.03 | ND | 196.91 | [135] | |
| dried pineapple | 84.89 | 12.83 | 34.93 | 101.16 | [47] |
| corn straw | 313.88 | 14.5 | ND | 46.90 | [111] |
| local reed biomass | 154.79 | ND | ND | 9.92 | [136] |
| grapefruit peel | 20.732 | 30.60 | 20 | 229.19 | [16] |
| magnetic biochar palm kernel shell | 362.0673 | 6.4882 | 12.85 | 37.64 | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katibi, K.K.; Yunos, K.F.; Man, H.C.; Aris, A.Z.; Mohd Nor, M.Z.; Azis, R.S. An Insight into a Sustainable Removal of Bisphenol A from Aqueous Solution by Novel Palm Kernel Shell Magnetically Induced Biochar: Synthesis, Characterization, Kinetic, and Thermodynamic Studies. Polymers 2021, 13, 3781. https://doi.org/10.3390/polym13213781
Katibi KK, Yunos KF, Man HC, Aris AZ, Mohd Nor MZ, Azis RS. An Insight into a Sustainable Removal of Bisphenol A from Aqueous Solution by Novel Palm Kernel Shell Magnetically Induced Biochar: Synthesis, Characterization, Kinetic, and Thermodynamic Studies. Polymers. 2021; 13(21):3781. https://doi.org/10.3390/polym13213781
Chicago/Turabian StyleKatibi, Kamil Kayode, Khairul Faezah Yunos, Hasfalina Che Man, Ahmad Zaharin Aris, Mohd Zuhair Mohd Nor, and Rabaah Syahidah Azis. 2021. "An Insight into a Sustainable Removal of Bisphenol A from Aqueous Solution by Novel Palm Kernel Shell Magnetically Induced Biochar: Synthesis, Characterization, Kinetic, and Thermodynamic Studies" Polymers 13, no. 21: 3781. https://doi.org/10.3390/polym13213781
APA StyleKatibi, K. K., Yunos, K. F., Man, H. C., Aris, A. Z., Mohd Nor, M. Z., & Azis, R. S. (2021). An Insight into a Sustainable Removal of Bisphenol A from Aqueous Solution by Novel Palm Kernel Shell Magnetically Induced Biochar: Synthesis, Characterization, Kinetic, and Thermodynamic Studies. Polymers, 13(21), 3781. https://doi.org/10.3390/polym13213781






