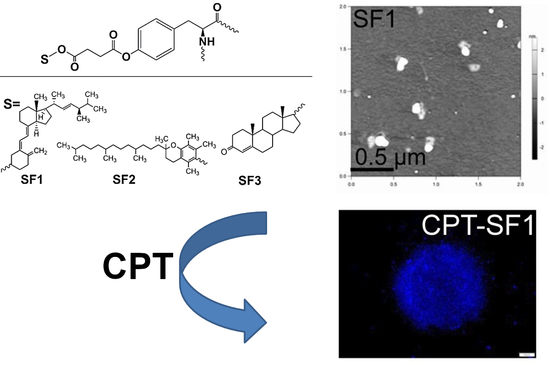
Self-Assembled Silk Fibroin-Based Aggregates for Delivery of Camptothecin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
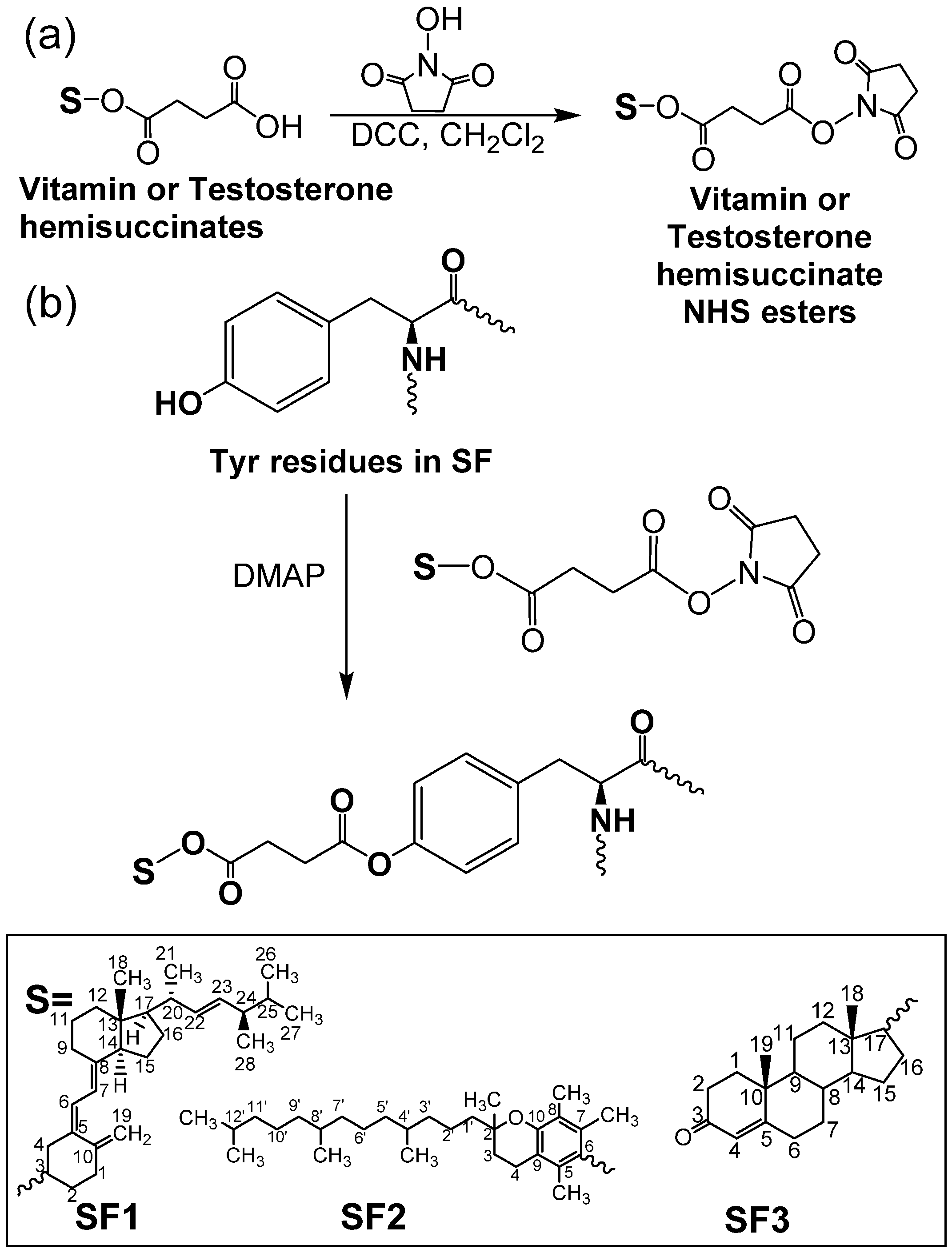
2.2. Preparation of Ergocalciferol-, Tocopherol-, and Testosterone-Grafted SF Aggregates
2.3. CPT Loading in SF Aggregates
2.4. CPT Content and Sustained Delivery
2.5. Characterization
2.6. Viability Assay
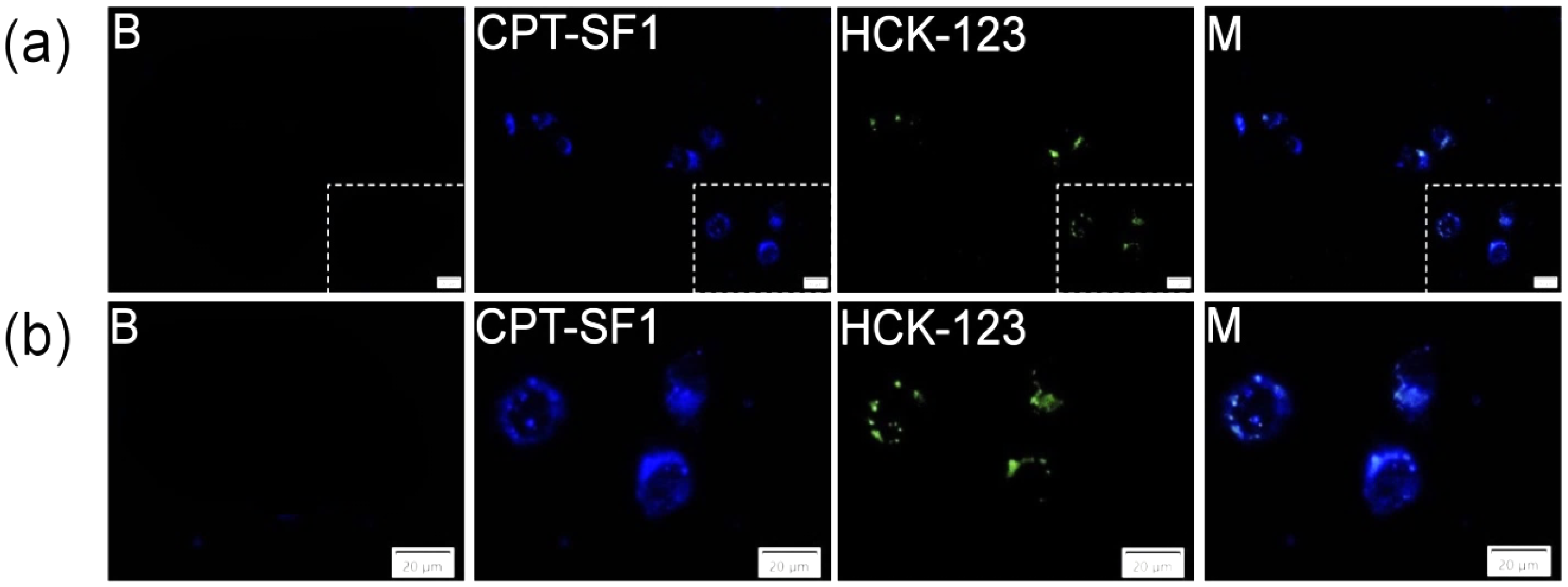
2.7. Cell Uptake
2.8. Annexin V/PI Assay
2.9. Cell Cycle Analysis
2.10. Spheroid Generation
2.11. Spheroid Uptake
2.12. Cell Viability Analysis of Spheroids
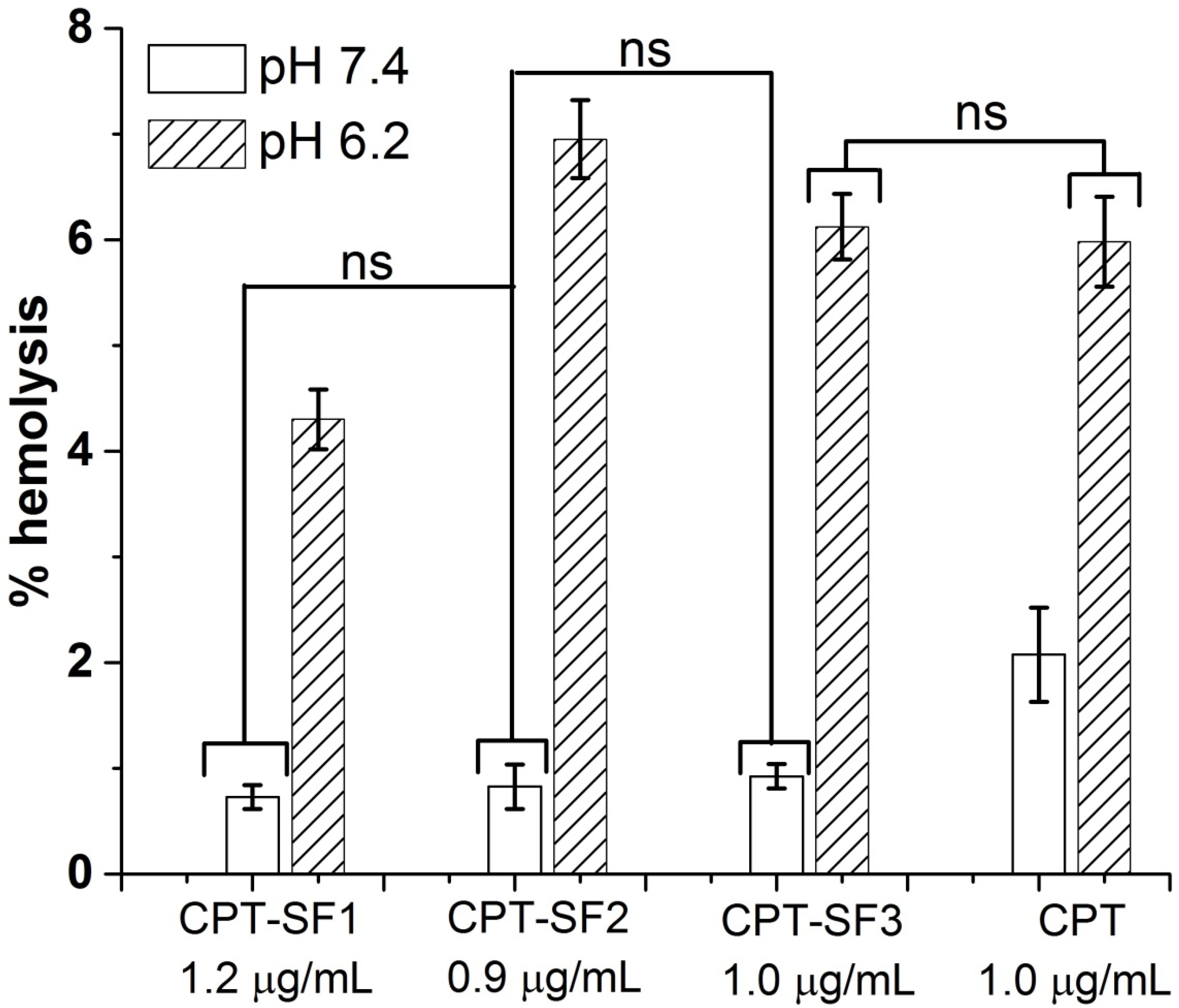
2.13. Hemolysis Assay
2.14. Statistical Analyses
3. Results
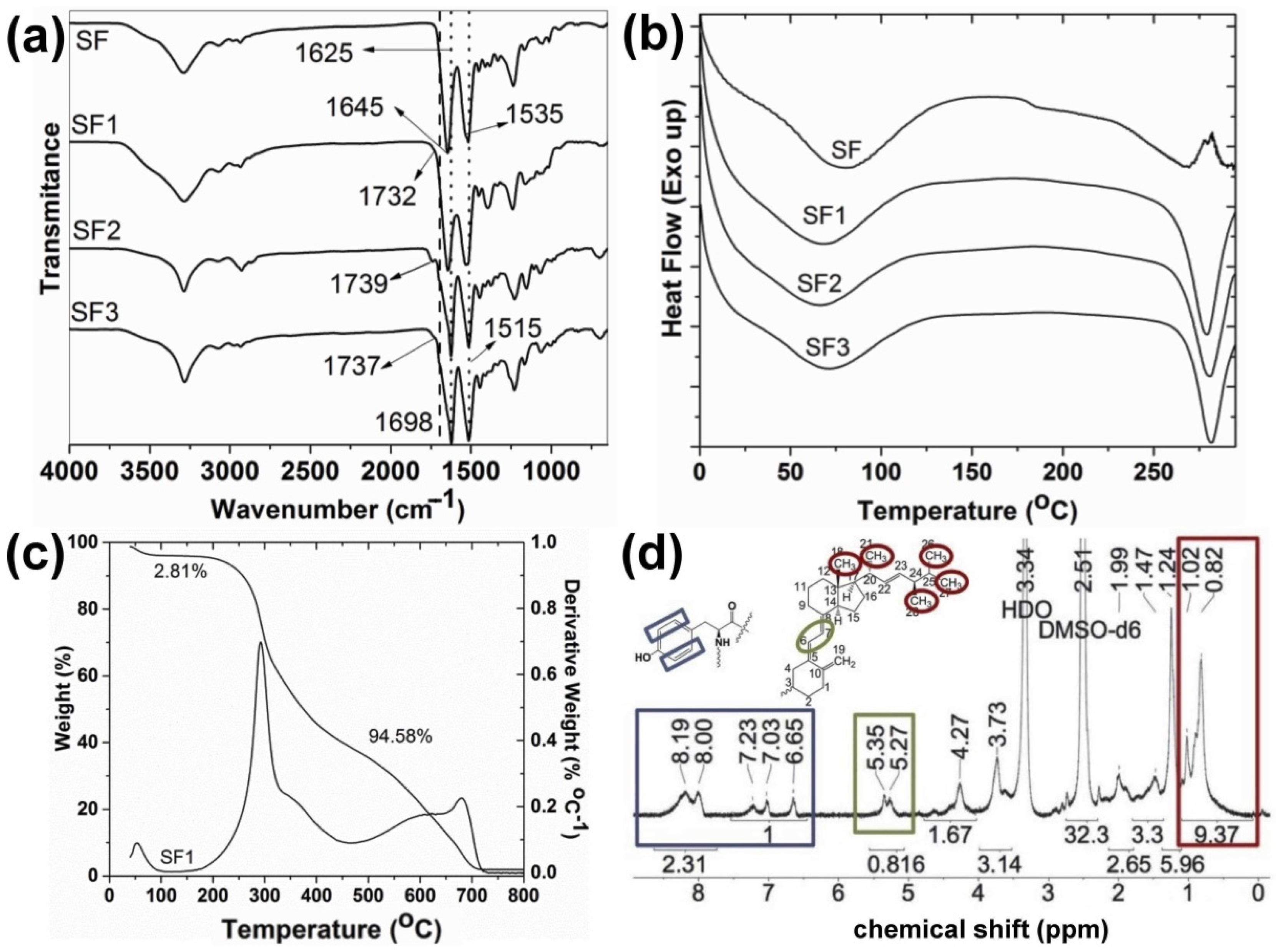
3.1. Synthesis and Characterization
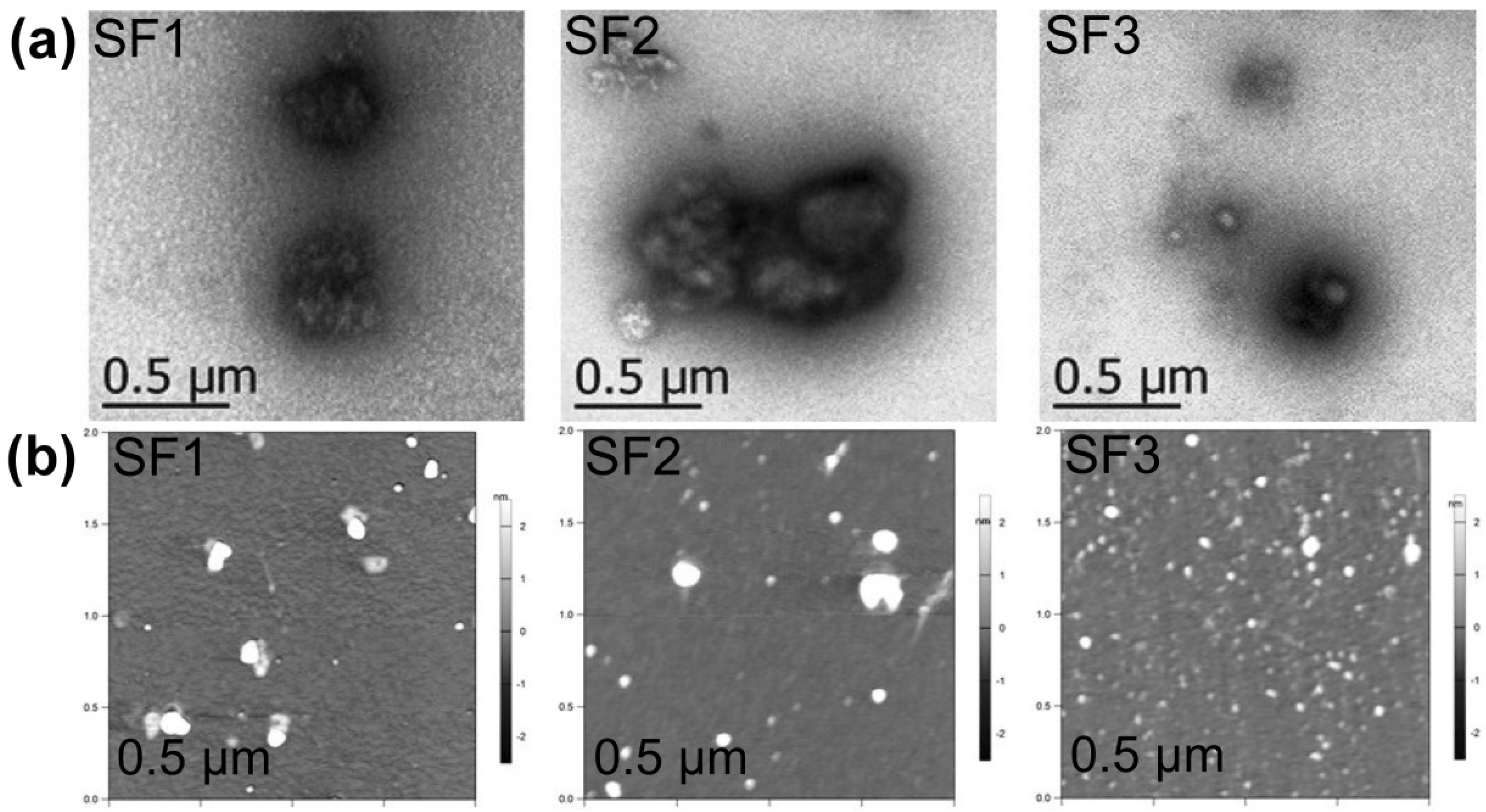
3.2. Structural Characterization and Morphological Studies
3.3. Drug Release
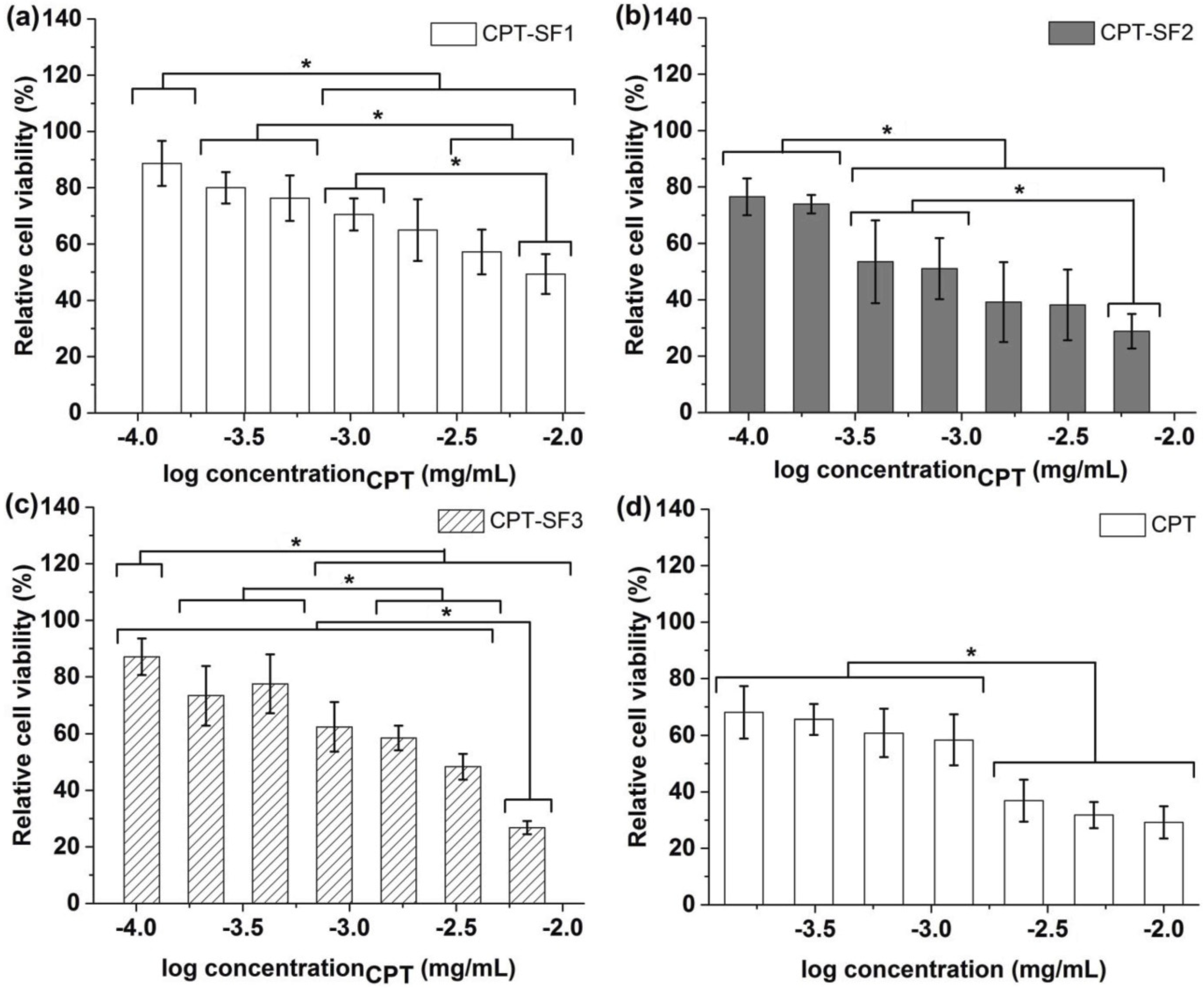
3.4. Cytotoxic Activity and Cell Uptake
3.5. Spheroid Uptake and Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, X.; Zhi, Z.; Jin, L.; Wang, F.; Wu, Y.; Wang, D.; Yan, K.; Shao, Y.; Meng, L. pH/redox dual-stimuli-responsive cross-linked polyphosphazene nanoparticles for multimodal imaging-guided chemo-photodynamic therapy. Nanoscale 2019, 11, 9457–9467. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Dai, X.-H.; Wu, C.; Pan, J.-M.; Wang, X.-H.; Yan, Y.-S.; Liu, D.-M.; Sun, L. Rational design of shear-thinning supramolecular hydrogels with porphyrin forcontrolled chemotherapeutics release and photodynamic therapy. Eur. Polym. J. 2015, 66, 149–159. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, X.; Qiu, L. Electroneutral composite polymersomes self-assembled by amphiphilic polyphosphazenes for effective miR-200c in vivo delivery to inhibit drug resistant lung cancer. Biomaterials 2016, 106, 1–12. [Google Scholar] [CrossRef]
- Kuehl, P.J.; Grimes, M.J.; Dubose, D.; Burke, M.; Revelli, D.A.; Gigliotti, A.P.; Belinsky, S.A.; Tessema, M. Inhalation delivery of topotecan is superior to intravenous exposure for suppressing lung cancer in a preclinical model. Drug Deliv. 2018, 25, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, H.; Saji, S.; Nomura, S.; Tanaka, S.; Ueno, T.; Onoue, M.; Iwata, H.; Yamanaka, T.; Sasaki, Y.; Toi, M. A phase I/II pharmacokinetics/pharmacodynamics study of irinotecan combined with S-1 for recurrent/metastatic breast cancer in patients with selected UGT 1A1 genotypes (the JBCRG-M01 study). Cancer Med. 2017, 6, 2909–2917. [Google Scholar] [CrossRef] [Green Version]
- Keyvani-Ghamsari, S.; Rabbani-Chadegani, A.; Sargolzaei, J.; Shahhoseini, M. Effect of irinotecan on HMGB1, MMP9 expression, cell cycle, and cell growth in breast cancer (MCF-7) cells. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. Onivyde for the therapy of multiple solid tumors. OncoTargets Ther. 2016, 9, 3001–3007. [Google Scholar] [CrossRef] [Green Version]
- Pommier, Y.; Cushman, M.; Doroshow, J.H. Novel clinical indenoisoquinoline topoisomerase I inhibitors: A twist around the camptothecins. Oncotarget 2018, 9, 37286–37288. [Google Scholar] [CrossRef]
- Kang, D.; Liu, A.L.; Wang, J.H.; Du, G.-H. Camptothecin. In Natural Small Molecules Drugs from Plants; Du, G.-H., Ed.; Springer: Singapore, 2018; pp. 491–496. [Google Scholar]
- Zou, J.; Li, S.; Chen, Z.; Lu, Z.; Gao, J.; Zou, J.; Lin, X.; Li, Y.; Zhang, C.; Shen, L. A novel oral camptothecin analog, gimatecan, exhibits superior antitumor efficacy than irinotecan toward esophageal squamous cell carcinoma in vitro and in vivo. Cell Death Dis. 2018, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Mao, X.; Xiao, Y.; Yang, Z.; Zheng, X.; Jiang, Z.-X. Monodisperse oligoethylene glycols modified Camptothecin, 10-Hydroxycamptothecin and SN38 prodrugs. Bioorg. Med. Chem. Lett. 2019, 29, 581–584. [Google Scholar] [CrossRef]
- Li, F.; Jiang, T.; Li, Q.; Ling, X. Camptothecin (CPT) and its derivatives are known to target topoisomerase 1 (Top1) as their mechanism of action: Did we miss something in CPT analogue molecular targets for treating human disease such as cancer? Am. J. Cancer Res. 2017, 7, 2350–2394. [Google Scholar] [PubMed]
- Liu, L.; Ye, Q.; Lu, M.; Lo, Y.-C.; Hsu, Y.-H.; Wei, M.-C.; Chen, Y.-H.; Lo, S.-C.; Wang, S.-J.; Bain, D.J.; et al. A new approach to reduce toxicities and to improve bioavailabilities of platinum-containing anti-cancer nanodrugs. Sci. Rep. 2015, 5, 10881. [Google Scholar] [CrossRef] [Green Version]
- Quiñones, J.P.; Jokinen, J.; Keinänen, S.; Covas, C.P.; Brüggemann, O.; Ossipov, D. Self-assembled hyaluronic acid-testosterone nanocarriers for delivery of anticancer drugs. Eur. Polym. J. 2018, 99, 384–393. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Mardare, C.C.; Hassel, A.W.; Brüggemann, O. Testosterone- and vitamin-grafted cellulose ethers for sustained release of camptothecin. Carbohydr. Polym. 2019, 206, 641–652. [Google Scholar] [CrossRef]
- Atoum, M.; Alzoughool, F. Vitamin D and breast cancer: Latest evidence and future steps. Breast Cancer Basic Clin. Res. 2017, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, R.L.; York, A.E.; Dimitrakis, C. Subcutaneous testosterone-letrozole therapy before and concurrent with neoadjuvant breast chemotherapy: Clinical response and therapeutic implications. Menopause 2017, 24, 859–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Lu, S.; Zhu, L.; Tang, Z.; Wang, Q.; Qin, G.; Yang, J.; Sun, G.; Zhang, Q.; Chen, Q. Conductive regenerated silk-fibroin-based hydrogels with integrated high mechanical performances. J. Mater. Chem. B 2019, 7, 1708–1715. [Google Scholar] [CrossRef]
- Kasoju, N.; Hawkins, N.; Pop-Georgievski, O.; Kubies, D.; Vollrath, F. Silk fibroin gelation via non-solvent induced phase separation. Biomater. Sci. 2016, 4, 460–473. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Meinel, A.J.; Hilbe, M.; Meinel, L.; Merkle, H.P. Silk fibroin/hyaluronan scaffolds for human mesenchymal stem cell culture in tissue engineering. Biomaterials 2009, 30, 5068–5076. [Google Scholar] [CrossRef] [Green Version]
- Meinel, A.J.; Kubow, K.E.; Klotzsch, E.; Garcia-Fuentes, M.; Smith, M.L.; Vogel, V.; Merkle, H.P.; Meinel, L. Optimization strategies for electrospun silk fibroin tissue engineering scaffolds. Biomaterials 2009, 30, 3058–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ageitos, J.M.; Pulgar, A.; Csaba, N.; Garcia-Fuentes, M. Study of nanostructured fibroin/dextran matrixes for controlled protein release. Eur. Polym. J. 2019, 114, 197–205. [Google Scholar] [CrossRef]
- Elsner, M.B.; Herold, H.M.; Müller-Herrmann, S.; Bargel, H.; Scheibel, T. Enhanced cellular uptake of engineered spider silk particles. Biomater. Sci. 2015, 3, 543–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiñones, J.P.; Roschger, C.; Zierer, A.; Covas, C.P.; Brüggemann, O. Steroid-grafted silk fibroin conjugates for drug and agrochemical delivery. Eur. Polym. J. 2019, 119, 169–175. [Google Scholar] [CrossRef]
- Wang, M.; Du, Y.; Huang, H.; Zhu, Z.; Du, S.; Chen, S.; Zhao, H.; Yan, Z. Silk fibroin peptide suppresses proliferation and induces apoptosis and cell cycle arrest in human lung cancer cells. Acta Pharmacol. Sin. 2019, 40, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, Y.; Niimi, S.; Hayakawa, T.; Miyashita, T. Preparation of lactose-silk fibroin conjugates and their application as a scaffold for hepatocyte attachment. Biomaterials 2004, 25, 1131–1140. [Google Scholar] [CrossRef]
- Yeo, J.-H.; Lee, K.-G.; Kweon, H.-Y.; Woo, S.-O.; Han, S.-M.; Kim, S.-S.; Demura, M. Fractionation of a silk fibroin hydrolysate and its protective function of hydrogen peroxide toxicity. J. Appl. Polym. Sci. 2006, 102, 772–776. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Hasunuma, K.; Kurokawa, M. Vitamin E Orotate and a Method of Producing the Same. U.S. Patent 3,944,550 A, 16 March 1976. [Google Scholar]
- Quiñones, J.P.; Brüggemann, O.; Covas, C.P.; Ossipov, D.A. Self-assembled hyaluronic acid nanoparticles for controlled release of agrochemicals and diosgenin. Carbohydr. Polym. 2017, 173, 157–169. [Google Scholar] [CrossRef]
- Gotlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemicals shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [Green Version]
- Kupchan, S.M.; Casy, A.F.; Swintosky, J.V. Drug latentiation: Synthesis and preliminary evaluation of testosterone derivatives. J. Pharm. Sci. 1965, 54, 514–524. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, S.; Liu, W.; Yuan, Z.; Yin, P.; Gao, F. Vitamin E succinate-grafted-chitosan oligosaccharide/RGD-conjugated TPGS mixed micelles loaded with paclitaxel for U87MG tumor therapy. Mol. Pharm. 2017, 14, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kaplan, D.; Cebe, P. Effect of water on the thermal properties of silk fibroin. Thermochim. Acta 2007, 461, 137–144. [Google Scholar]
- Jaramillo-Quiceno, N.; Álvarez-López, C.; Restrepo-Osorio, A. Structural and thermal properties of silk fibroin films obtained from cocoon and waste silk fibers as raw materials. Procedia Eng. 2017, 200, 384–388. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nanodrug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdury, P. Kinetic modelling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Papadopolou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of the drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kondaveeti, S.; Damato, T.C.; Carmona-Ribeiro, A.M.; Sierakowski, M.R.; Petri, D.F.S. Sustainable hydroxypropyl methylcellulose/xyloglucan/gentamicin films with antimicrobial properties. Carbohydr. Polym. 2017, 165, 285–293. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Zak, J.K. Biomaterial-based regional chemotherapy: Local anticancer drug delivery to enhance chemotherapy and minimize its side-effects. Mater. Sci. Eng. C 2016, 62, 927–942. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokzhad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Cao, Z.; Li, Y.; Ella-Menye, J.-R.; Bai, T.; Jiang, S. Softer zwitterionic nanogels for longer circulation and lower splenic accumulation. ACS Nano 2012, 6, 6681–6686. [Google Scholar] [CrossRef] [PubMed]
- Totten, J.D.; Wongpinyochit, T.; Seib, F.P. Silk nanoparticles: Proof of lysosomotropic anticancer drug delivery at single-cell resolution. J. Drug Target. 2017, 25, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sample | C/N a | DS b (%) | DS c (%) | Y (%) | Mn d (kg/mol) | Mw/Mn d |
|---|---|---|---|---|---|---|
| SF | 2.6049 | - | - | 27.2 | 1.22 | |
| SF1 | 3.6218 | 3.2 | 3.0 | 71 | - | - |
| SF2 | 3.8355 | 3.8 | 3.6 | 64 | - | - |
| SF3 | 2.7061 | 0.4 | - | 58 | - | - |
| Sample | dh a (nm)/(PDI) | dh b (nm)/(PDI) | ζ a (mV) | dAFM (nm) | wt % | EE % |
|---|---|---|---|---|---|---|
| SF | 216 ± 7 (0.5) | 260 ± 4 (0.45) | −14.8 ± 0.8 | - | - | - |
| SF1 | 554 ± 3 (0.32) | 471 ± 7 (0.37) | −32.2 ± 0.4 | 75 ± 10 | - | - |
| SF2 | 653 ± 1 (0.45) | 727 ± 4 (0.15) | −28.3 ± 0.5 | 53 ± 9 ns | - | - |
| SF3 | 620 ± 3 (0.31) | 824 ± 6 (0.28) | −30.8 ± 0.3 | 45 ± 7 ns | - | - |
| CPT–SF1 | - | 420 ± 5 (0.5) | - | - | 8.3 | 84 |
| CPT–SF2 | - | 370 ± 7 (0.51) | - | - | 6.3 | 60 |
| CPT–SF3 | - | 340 ± 5 (0.34) | - | - | 6.8 | 67 |
| Sample | G0, G1 (%) | S (%) | G2, M (%) |
|---|---|---|---|
| Control | 69 ± 7 | 7 ± 1 | 23 ± 6 |
| CPT–SF1 | 54.4 ± 0.5 | 7.3 ± 0.6 | 38.6 ± 0.7 |
| CPT–SF2 | 38 ± 2 | 3 ± 2 | 51 ± 13 |
| CPT–SF3 | 60 ± 3 | 5.51 ± 0.07 | 34 ± 3 |
| CPT | 40 ± 2 | 4 ± 2 | 55 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Quiñones, J.; Roschger, C.; Zierer, A.; Peniche-Covas, C.; Brüggemann, O. Self-Assembled Silk Fibroin-Based Aggregates for Delivery of Camptothecin. Polymers 2021, 13, 3804. https://doi.org/10.3390/polym13213804
Pérez Quiñones J, Roschger C, Zierer A, Peniche-Covas C, Brüggemann O. Self-Assembled Silk Fibroin-Based Aggregates for Delivery of Camptothecin. Polymers. 2021; 13(21):3804. https://doi.org/10.3390/polym13213804
Chicago/Turabian StylePérez Quiñones, Javier, Cornelia Roschger, Andreas Zierer, Carlos Peniche-Covas, and Oliver Brüggemann. 2021. "Self-Assembled Silk Fibroin-Based Aggregates for Delivery of Camptothecin" Polymers 13, no. 21: 3804. https://doi.org/10.3390/polym13213804
APA StylePérez Quiñones, J., Roschger, C., Zierer, A., Peniche-Covas, C., & Brüggemann, O. (2021). Self-Assembled Silk Fibroin-Based Aggregates for Delivery of Camptothecin. Polymers, 13(21), 3804. https://doi.org/10.3390/polym13213804