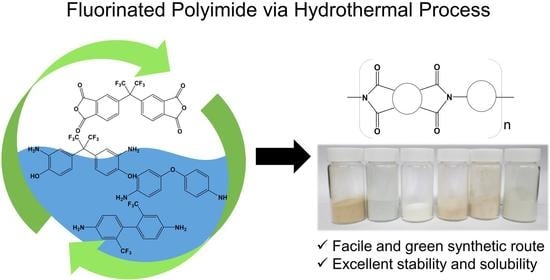
Highly Soluble Fluorinated Polyimides Synthesized with Hydrothermal Process towards Sustainable Green Technology
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Synthesis of Polyimides via Hydrothermal Process
2.3. Synthesis of Polyimides via Thermal Imidization
2.4. Synthesis of Polyimides via Chemical Imdization
2.5. Characterization
3. Results and Discussion
3.1. Polyimide Synthesis
3.2. Thermal Properties
3.3. Solubility and Processability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Lee, S.; Kim, G.; Kim, J.; Kim, D.; Lee, D.; Han, H.; Kim, J. Nitrogen-Doped Porous Carbon Structure from Melamine-Assisted Polyimide Sheets for Supercapacitor Electrodes. Adv. Sustain. Syst. 2018, 2, 1800007. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Jiao, Y.; Dong, J.; Gan, F.; Zhao, X.; Zhang, Q. Novel high-performance poly(benzoxazole-co-imide) resins with low dielectric constants and superior thermal stabilities derived from thermal rearrangement of ortho-hydroxy polyimide oligomers. Chem. Eng. J. 2019, 359, 641–651. [Google Scholar] [CrossRef]
- Agag, T.; Koga, T.; Takeichi, T. Studies on thermal and mechanical properties of polyimide-clay nanocomposites. Polymer 2001, 42, 3399–3408. [Google Scholar] [CrossRef]
- Lee, Y.J.; Huang, J.M.; Kuo, S.W.; Lu, J.S.; Chang, F.C. Polyimide and polyhedral oligomeric silsesquioxane nanocomposites for low-dielectric applications. Polymer 2005, 46, 173–181. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, J.; Kim, S.I.; Kim, M.; Lee, D.; Lee, S.; Kim, G.; Lee, J.; Han, H. One-step synthesis of nano-porous monolithic polyimide aerogel. Microporous Mesoporous Mater. 2016, 234, 35–42. [Google Scholar] [CrossRef]
- Kim, K.; Lee, J.; Lee, S.; Yoo, T.; Han, H. Synthesis of new flexible diamine for applications in transparent poly(amide-imide) thin films with low residual stress. Prog. Org. Coat. 2018, 117, 130–140. [Google Scholar] [CrossRef]
- Jiang, L.-Y.; Leu, C.-M.; Wei, K.-H. Layered Silicates/Fluorinated Polyimide Nanocomposites for Advanced Dielectric Materials Applications. Adv. Mater. 2002, 14, 426–429. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; He, J.; Li, Z.; Li, L. Synthesis of fluorinated polyimide towards a transparent triboelectric nanogenerator applied on screen surface. J. Mater. Chem. A 2021, 9, 6583–6590. [Google Scholar] [CrossRef]
- Kim, Y.; Nam, K.H.; Jung, Y.C.; Han, H. Interfacial adhesion and self-healing kinetics of multi-stimuli responsive colorless polymer bilayers. Compos. Part B Eng. 2020, 203, 108451. [Google Scholar] [CrossRef]
- Nam, K.H.; Jin, J.; Lee, D.H.; Han, H.; Goh, M.; Yu, J.; Ku, B.C.; You, N.H. Towards solution-processable, thermally robust, transparent polyimide-chain-end tethered organosilicate nanohybrids. Compos. Part B Eng. 2019, 163, 290–296. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Chung, M.; Jeong, H.; Choi, H.; Han, H. Flexible polymer thermoelectric device based on PEDOT:PSS and surface treated pyromellitic dianhydride-oxydianiline polyimide substrate. J. Appl. Polym. Sci. 2020, 137, 49156. [Google Scholar] [CrossRef]
- Unsal, E.; Cakmak, M. Real-time characterization of physical changes in polyimide film formation: From casting to imidization. Macromolecules 2013, 46, 8616–8627. [Google Scholar] [CrossRef]
- Baumgartner, B.; Puchberger, M.; Unterlass, M.M. Towards a general understanding of hydrothermal polymerization of polyimides. Polym. Chem. 2015, 6, 5773–5781. [Google Scholar] [CrossRef]
- Hariharan, R.; Sarojadevi, M. Synthesis and Characterization of Organo-Soluble Fluorinated Polyimides. Polym. Int. 2007, 56, 22–31. [Google Scholar] [CrossRef]
- Liu, H.; Zhai, L.; Bai, L.; He, M.; Wang, C.; Mo, S.; Fan, L. Synthesis and characterization of optically transparent semi-aromatic polyimide films with low fluorine content. Polymer 2019, 163, 106–114. [Google Scholar] [CrossRef]
- Chiefari, J.; Dao, B.; Groth, A.M.; Hodgkin, J.H. Water as Solvent in Polyimide Synthesis: Thermoset and Thermoplastic Examples. High Perform. Polym. 2003, 15, 269–279. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Basnet, P.; Chatterjee, S. Structure-directing property and growth mechanism induced by capping agents in nanostructured ZnO during hydrothermal synthesis—A systematic review. Nano-Struct. Nano-Objects 2020, 22, 100426. [Google Scholar] [CrossRef]
- Pan, L.; Pu, L.; Shi, Y.; Sun, T.; Zhang, R.; Zheng, Y. Hydrothermal synthesis of polyaniline mesostructures. Adv. Funct. Mater. 2006, 16, 1279–1288. [Google Scholar] [CrossRef]
- Brunel, R.; Marestin, C.; Martin, V.; Mercier, R. Water-borne polyimides via microwave-assisted polymerization. High Perform. Polym. 2010, 22, 82–94. [Google Scholar] [CrossRef]
- Baumgartner, B.; Bojdys, M.J.; Skrinjar, P.; Unterlass, M.M. Design strategies in hydrothermal polymerization of polyimides. Macromol. Chem. Phys. 2016, 217, 485–500. [Google Scholar] [CrossRef]
- Unterlass, M.M.; Kopetzki, D.; Antonietti, M.; Weber, J. Mechanistic study of hydrothermal synthesis of aromatic polyimides. Polym. Chem. 2011, 2, 1744–1753. [Google Scholar] [CrossRef]
- Takemori, M.T. Towards an Understanding of the Heat Distortion Temperature of Thernoplastics. Polym. Eng. Sci. 1979, 19, 1104–1109. [Google Scholar] [CrossRef]
- Kriechbaum, K.; Cerrón-Infantes, D.A.; Stöger, B.; Unterlass, M.M. Shape-Anisotropic Polyimide Particles by Solid-State Polycondensation of Monomer Salt Single Crystals. Macromolecules 2015, 48, 8773–8780. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.; Jeon, J.; An, J.; Khan, S.B.; Lee, S.; Seo, J.; Han, H. A photoinitiator-free photosensitive polyimide with low dielectric constant. J. Appl. Polym. Sci. 2010, 117, 2937–2945. [Google Scholar] [CrossRef]
- Imai, Y. Recent progress in synthesis of polyimides. J. Photopolym. Sci. Technol. 1994, 7, 251–256. [Google Scholar] [CrossRef]
- Ozawa, H.; Ishiguro, E.; Kyoya, Y.; Kikuchi, Y.; Matsumoto, T. Colorless Polyimides Derived from an Alicyclic Tetracarboxylic Dianhydride, CpODA. Polymers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Luo, X.; Lu, X.; Chen, X.; Chen, Y.; Song, C.; Yu, C.; Wang, N.; Su, D.; Wang, C.; Gao, X.; et al. A robust flame retardant fluorinated polyimide nanofiber separator for high-temperature lithium-sulfur batteries. J. Mater. Chem. A 2020, 8, 14788–14798. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, Z.; Zhang, Y.; Wang, X.; Zhou, F. Solvent-free and photocurable polyimide inks for 3D printing. J. Mater. Chem. A 2017, 5, 16307–16314. [Google Scholar] [CrossRef]
- Shao, Y.; Li, Y.F.; Zhao, X.; Wang, X.L.; Ma, T.; Yang, F.C. Synthesis and properties of fluorinated polyimides from a new unsymmetrical diamine: 1,4-(2′-trifluoromethyl-4′,4″-diaminodiphenoxy) benzene. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6836–6846. [Google Scholar] [CrossRef]
- Leblanc, N.; Le Cerf, D.; Chappey, C.; Langevin, D.; Métayer, M.; Muller, G. Influence of solvent and non-solvent on polyimide asymmetric membranes formation in relation to gas permeation. Sep. Purif. Technol. 2001, 22–23, 277–285. [Google Scholar] [CrossRef]





| Polyimide | Monomer Molar Ratio | Note a | |||
|---|---|---|---|---|---|
| 6FDA | ODA | AHHFP | TFDB | ||
| 1 | 10 | 10 | - | - | 1H, 1T, 1C |
| 2 | 10 | - | 10 | - | 2H, 2T, 2C |
| 3 | 10 | - | - | 10 | 3H, 3T, 3C |
| 4 | 10 | 5 | 5 | - | 4H, 4T, 4C |
| 5 | 10 | 5 | - | 5 | 5H, 5T, 5C |
| 6 | 10 | - | 5 | 5 | 6H, 6T, 6C |
| Polyimide | Synthetic Methods a | Mn b | Mw c | PDI d |
|---|---|---|---|---|
| 1 | H | 9738 | 20,781 | 2.13 |
| T | 9880 | 47,136 | 4.77 | |
| C | 14,159 | 41,530 | 2.93 | |
| 2 | H | 6177 | 12,931 | 2.09 |
| T | 15,830 | 130,180 | 8.22 | |
| C | 42,151 | 99,051 | 2.35 | |
| 3 | H | 7293 | 10,005 | 1.37 |
| T | 9381 | 32,900 | 3.51 | |
| C | 16,442 | 47,192 | 2.87 | |
| 4 | H | 7397 | 28,337 | 3.83 |
| T | 13,953 | 90,184 | 6.46 | |
| C | 28,339 | 65,128 | 2.30 | |
| 5 | H | 10,484 | 21,370 | 2.04 |
| T | 13,599 | 48,542 | 3.57 | |
| C | 21,349 | 62,517 | 2.93 | |
| 6 | H | 7507 | 14,332 | 1.91 |
| T | 28,514 | 78,155 | 2.74 | |
| C | 21,171 | 71,648 | 3.38 |
| Polyimide | Synthetic Methods a | Td5% b (°C) | Td10% b (°C) | Tg c (°C) |
|---|---|---|---|---|
| 1 | H | 523.49 | 541.84 | 273.63 |
| T | 524.22 | 540.56 | 299.98 | |
| C | 523.94 | 539.49 | 288.21 | |
| 2 | H | 432.22 | 488.97 | 284.91 |
| T | 431.51 | 484.58 | 316.69 | |
| C | 400.26 | 466.32 | 249.89 | |
| 3 | H | 500.22 | 534.08 | 322.93 |
| T | 521.25 | 542.45 | 324.43 | |
| C | 520.49 | 542.38 | 312.53 | |
| 4 | H | 449.84 | 508.81 | 310.93 |
| T | 464.86 | 516.24 | 312.82 | |
| C | 421.26 | 500.06 | 257.17 | |
| 5 | H | 517.03 | 543.74 | 300.83 |
| T | 515.57 | 536.94 | 304.35 | |
| C | 526.22 | 544.41 | 297.63 | |
| 6 | H | 473.07 | 521.97 | 301.36 |
| T | 486.07 | 517.79 | 317.67 | |
| C | 422.13 | 500.06 | 256.82 |
| Polyimide | Synthetic Method | Solvent | ||||||
|---|---|---|---|---|---|---|---|---|
| DMAc a | NMP b | THF c | CHCl3 d | Toluene | Acetone | Ethanol | ||
| 1 | H | ++ | ++ | ++ | + | ++ | ++ | - |
| T | ++ | ++ | ++ | + | + | ++ | - | |
| C | ++ | ++ | ++ | + | ++ | ++ | - | |
| 2 | H | ++ | ++ | ++ | ++ | ++ | ++ | + |
| T | ++ | ++ | ++ | ++ | ++ | ++ | - | |
| C | ++ | ++ | ++ | ++ | ++ | ++ | - | |
| 3 | H | ++ | ++ | ++ | ++ | ++ | ++ | + |
| T | ++ | ++ | ++ | ++ | ++ | ++ | - | |
| C | ++ | ++ | ++ | ++ | ++ | ++ | - | |
| 4 | H | ++ | ++ | ++ | + | ++ | ++ | + |
| T | ++ | ++ | ++ | + | ++ | ++ | - | |
| C | ++ | ++ | ++ | + | ++ | ++ | - | |
| 5 | H | ++ | ++ | ++ | + | ++ | ++ | - |
| T | ++ | ++ | ++ | + | + | ++ | - | |
| C | ++ | ++ | ++ | + | ++ | ++ | - | |
| 6 | H | ++ | ++ | ++ | ++ | ++ | ++ | + |
| T | ++ | ++ | ++ | ++ | ++ | ++ | - | |
| C | ++ | ++ | ++ | ++ | ++ | ++ | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Baek, S.; Kim, J.; Lee, S.; Kim, J.; Han, H. Highly Soluble Fluorinated Polyimides Synthesized with Hydrothermal Process towards Sustainable Green Technology. Polymers 2021, 13, 3824. https://doi.org/10.3390/polym13213824
Lee J, Baek S, Kim J, Lee S, Kim J, Han H. Highly Soluble Fluorinated Polyimides Synthesized with Hydrothermal Process towards Sustainable Green Technology. Polymers. 2021; 13(21):3824. https://doi.org/10.3390/polym13213824
Chicago/Turabian StyleLee, Juheon, Seungho Baek, Jinsu Kim, Sangrae Lee, Jinyoung Kim, and Haksoo Han. 2021. "Highly Soluble Fluorinated Polyimides Synthesized with Hydrothermal Process towards Sustainable Green Technology" Polymers 13, no. 21: 3824. https://doi.org/10.3390/polym13213824
APA StyleLee, J., Baek, S., Kim, J., Lee, S., Kim, J., & Han, H. (2021). Highly Soluble Fluorinated Polyimides Synthesized with Hydrothermal Process towards Sustainable Green Technology. Polymers, 13(21), 3824. https://doi.org/10.3390/polym13213824