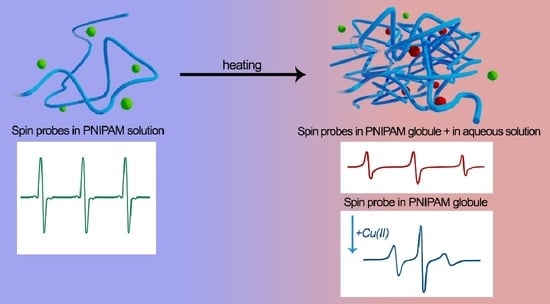
Inhomogeneities in PNIPAM Aqueous Solutions: The Inside View by Spin Probe EPR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substances
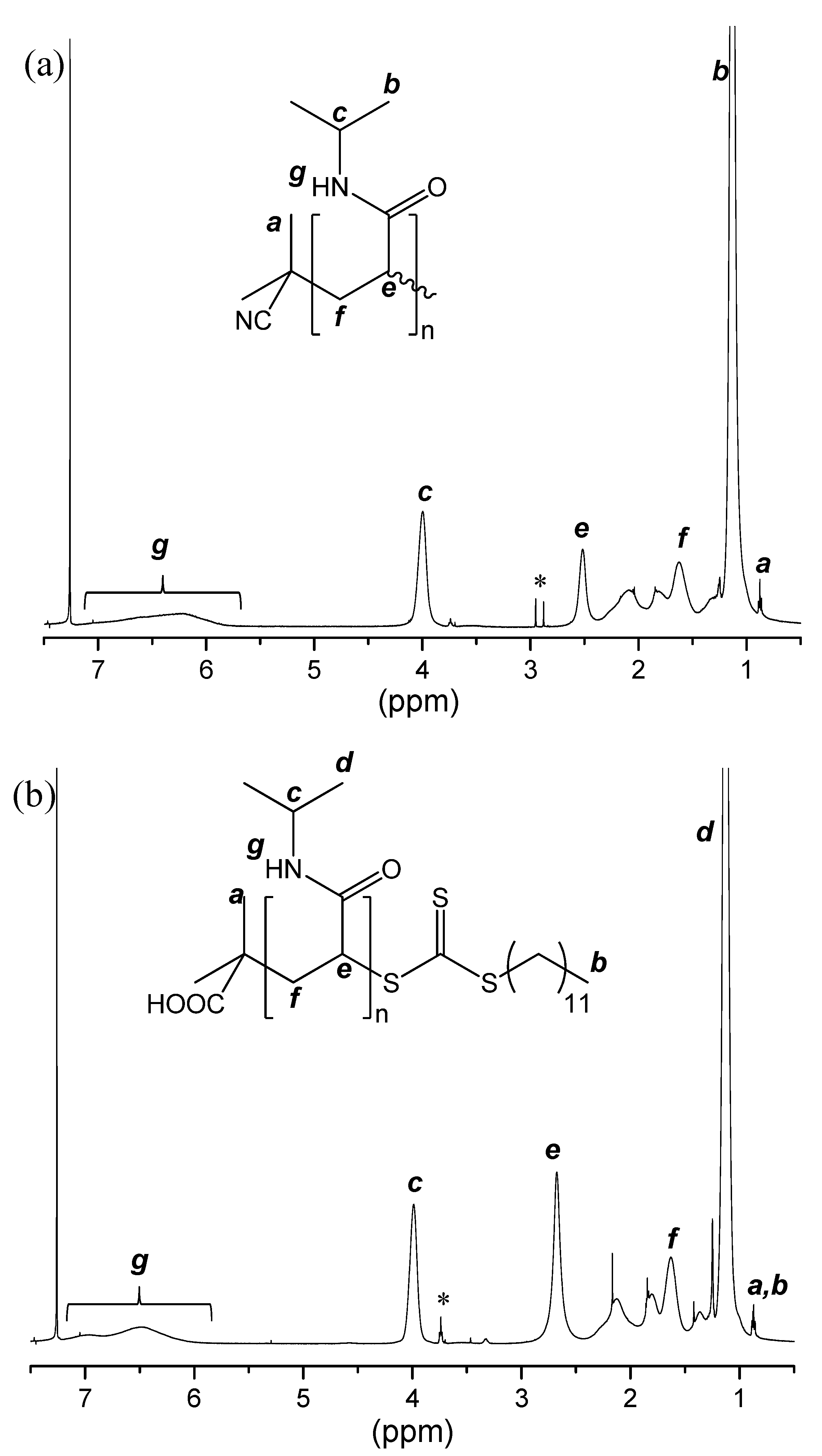
2.2. Polymer Synthesis
2.3. Polymers Characterization
2.3.1. H NMR Spectroscopy
2.3.2. Spectrophotometry
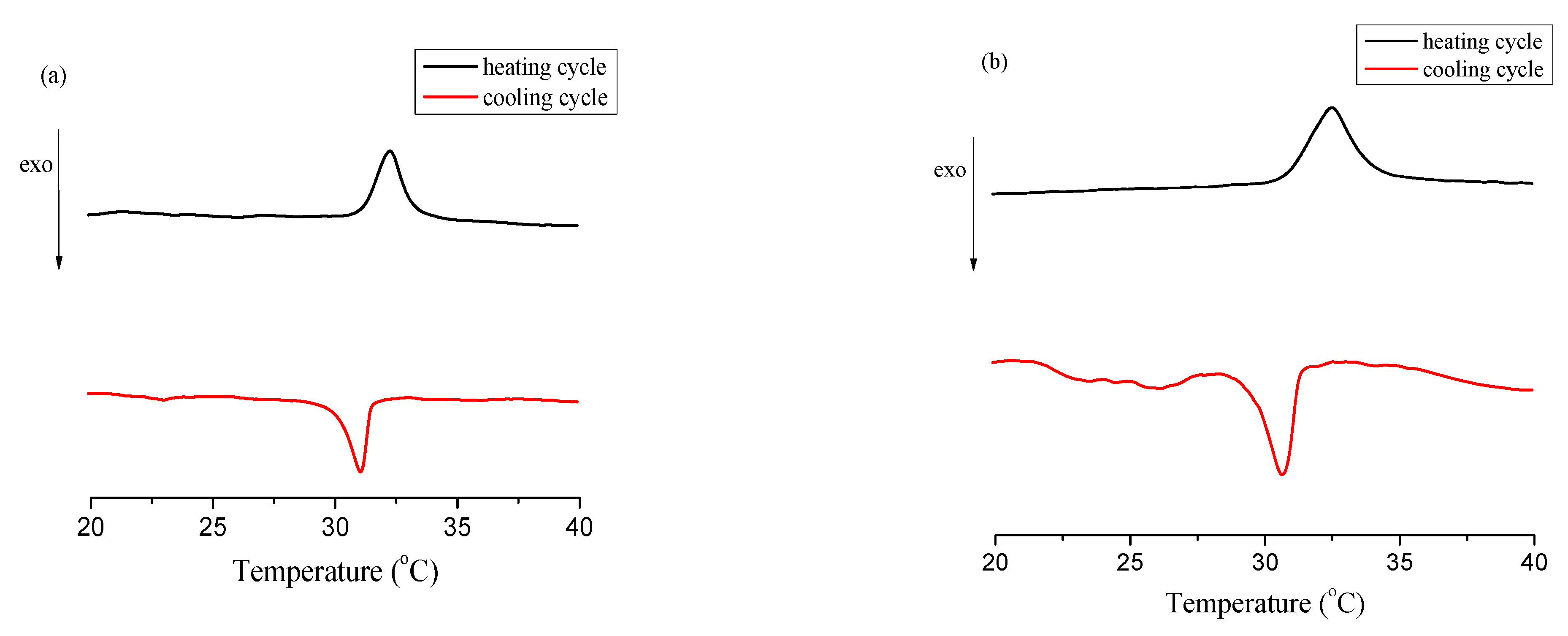
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Size Exclusion Chromatography
2.4. EPR Samples Preparation
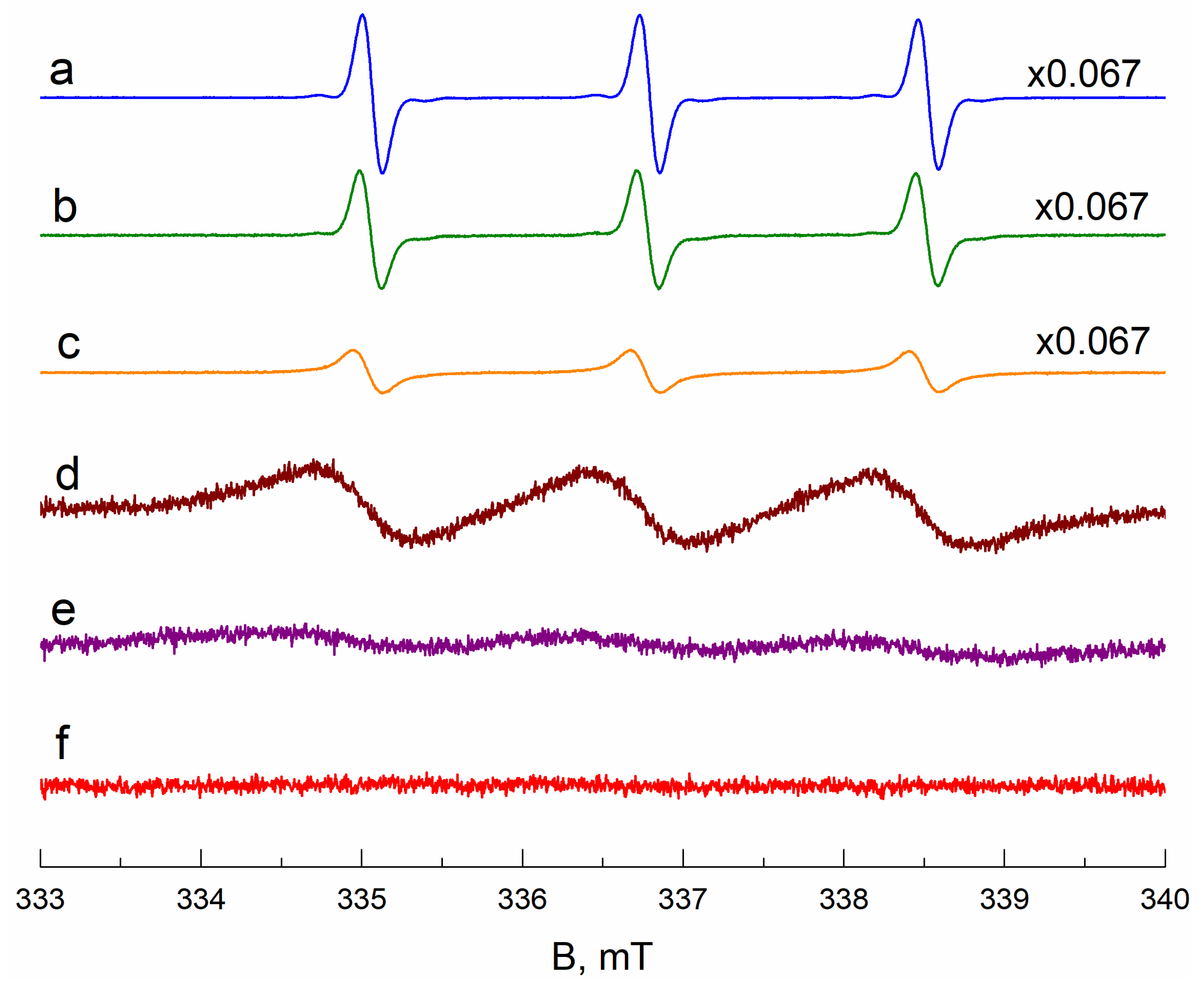
2.5. EPR Measurements
2.6. Data Analysis
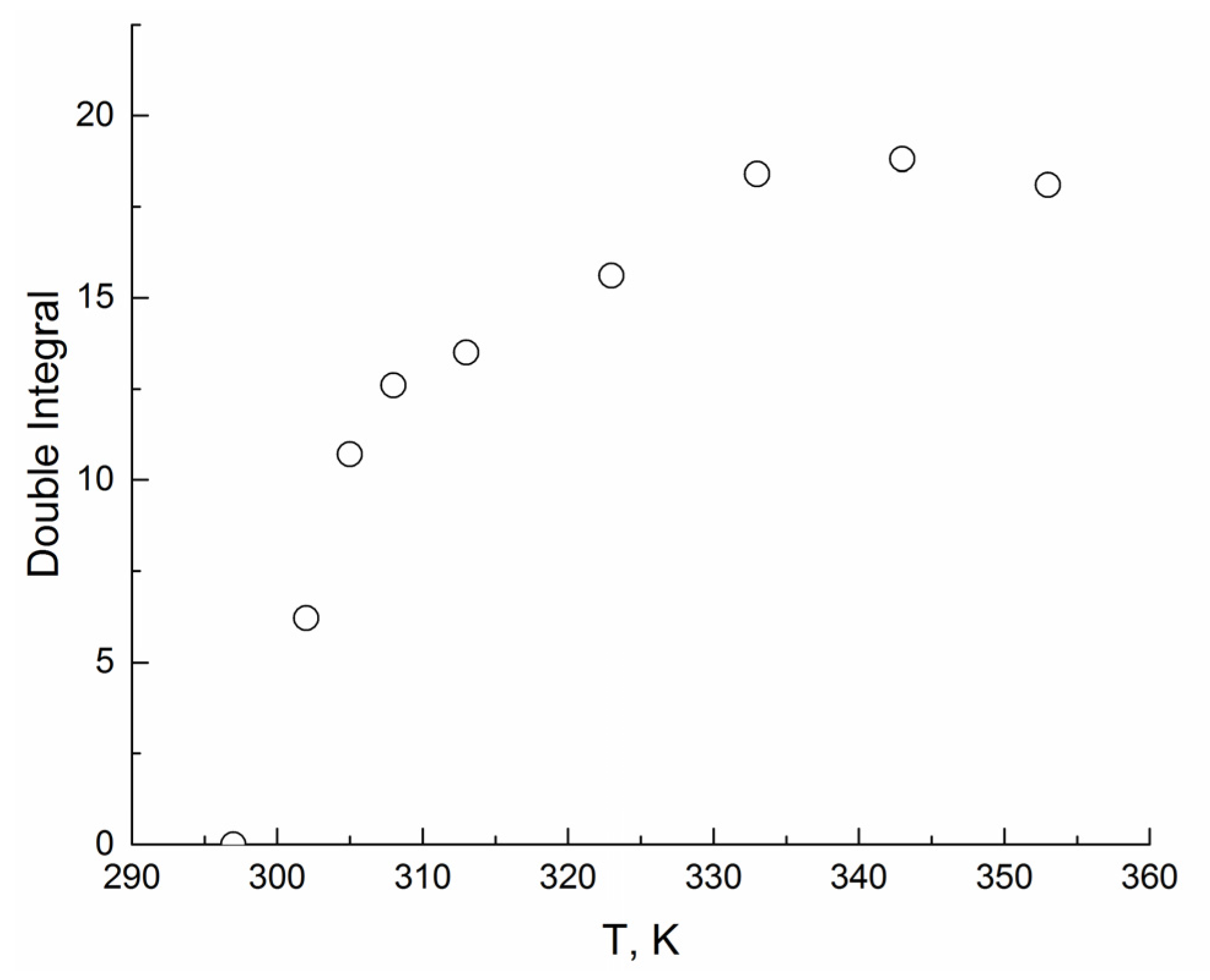
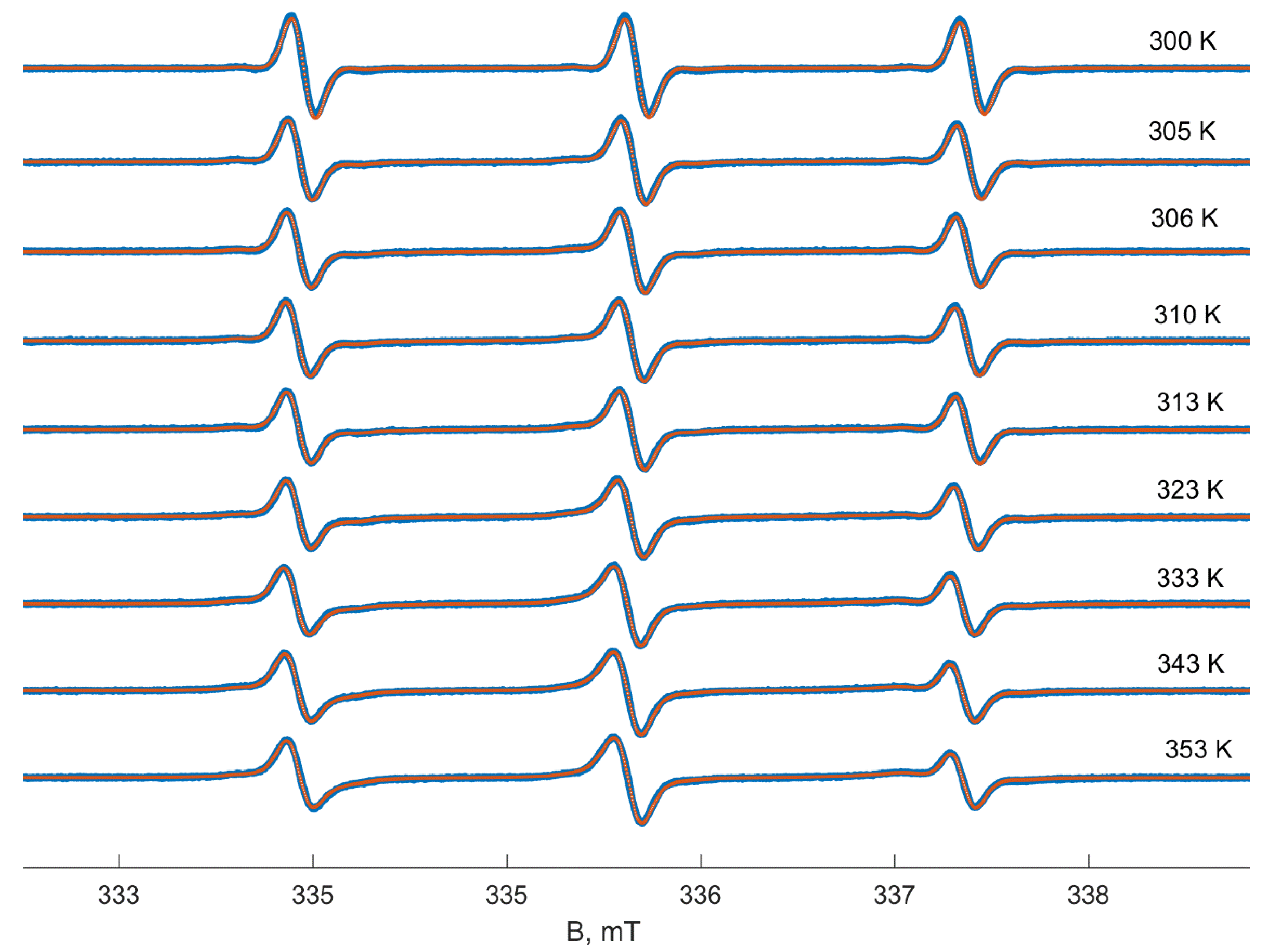
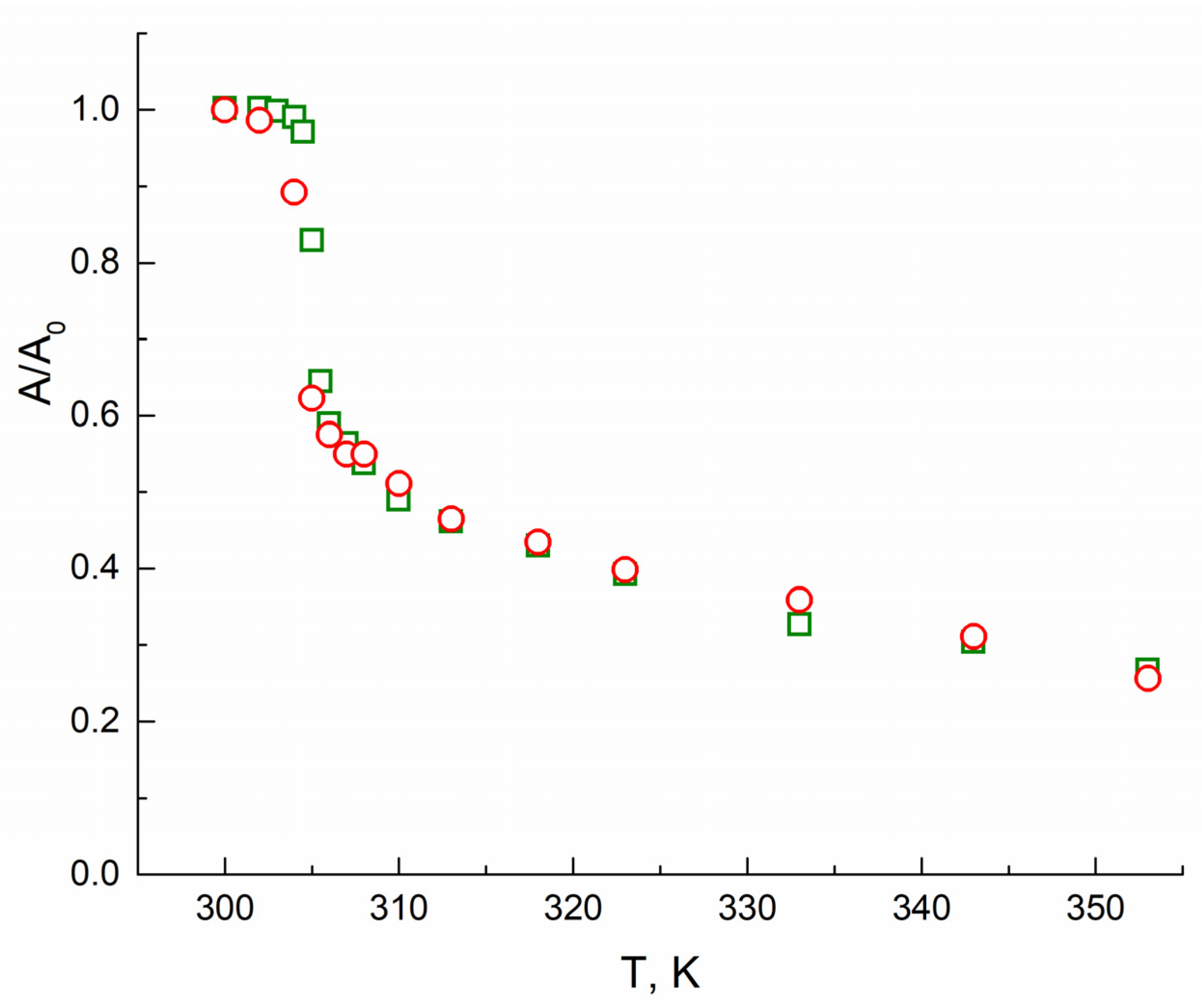
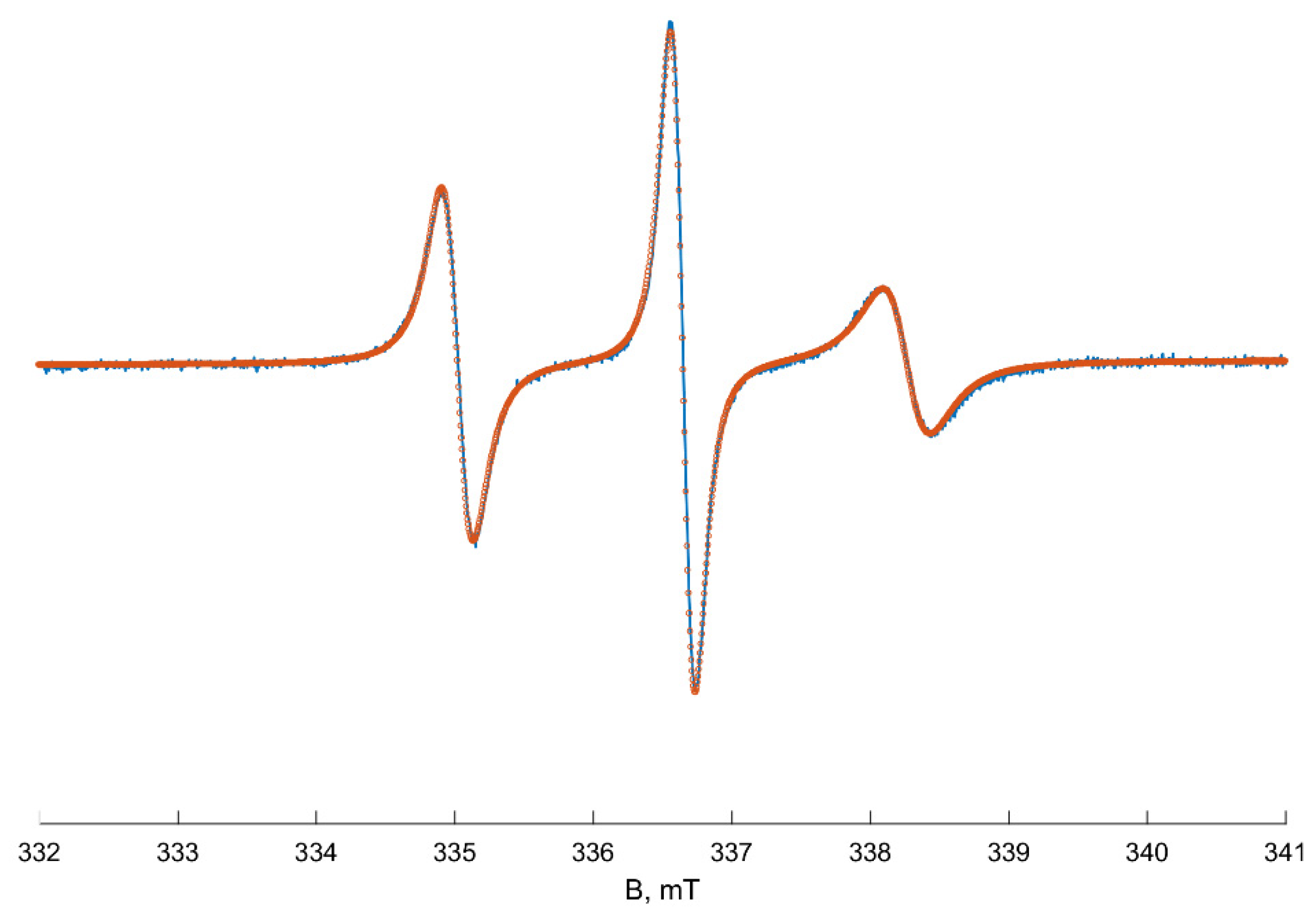
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

EPR Spectra Simulation Details

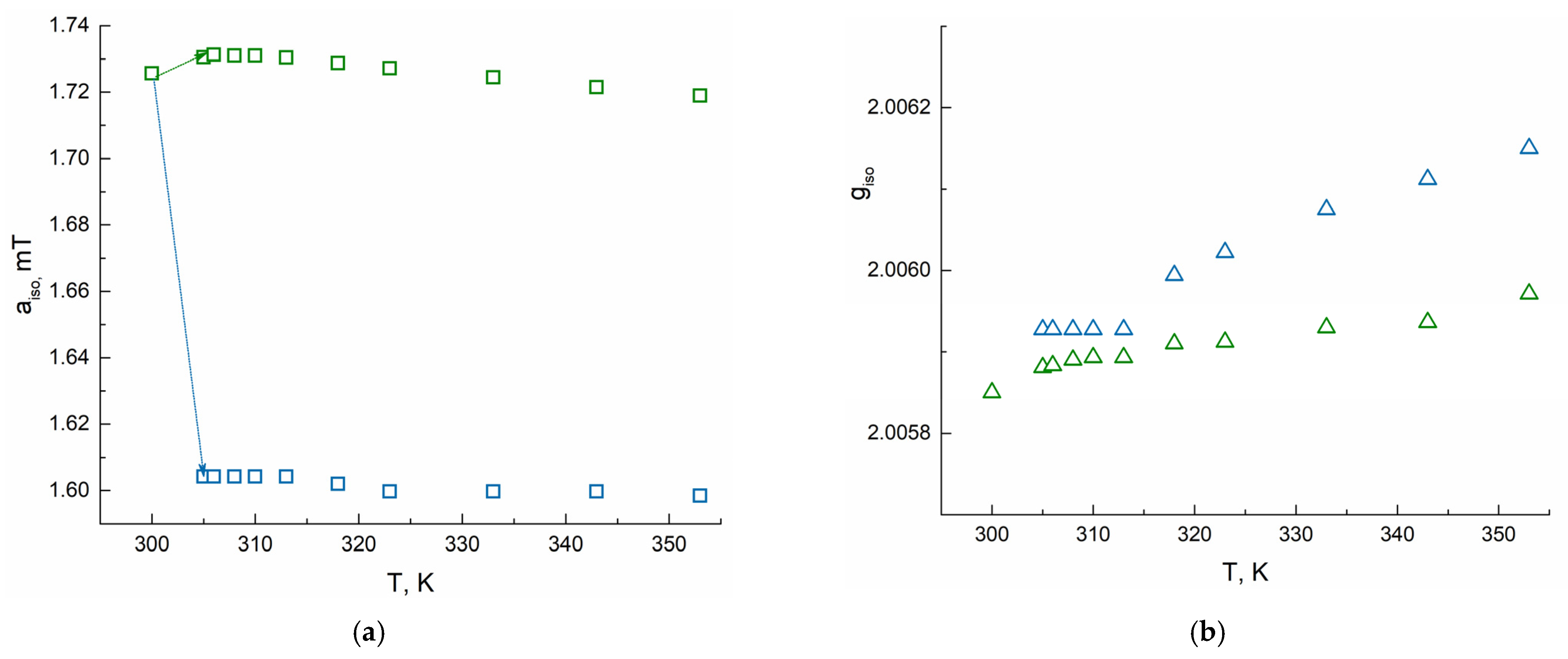
| T, K | Type A | Type B | ||||||
|---|---|---|---|---|---|---|---|---|
| giso | aiso, mT | τcorr, ps | giso | aiso, mT | τcorr, ns | χB, % (Polymer I) | χB, % (Polymer II) | |
| 300 | 2.00585 | 1.72 | 16 | - | - | - | 0 | 0 |
| 305 | 2.00588 | 1.73 | 10 | 2.00593 | 1.60 | 1.26 | 26.5 | 44.6 |
| 306 | 2.00588 | 1.73 | 8 | 2.00593 | 1.60 | 1.31 | 50.0 | 52.6 |
| 308 | 2.00589 | 1.73 | 8 | 2.00593 | 1.60 | 1.31 | 56.1 | 54.5 |
| 310 | 2.00589 | 1.73 | 5 | 2.00593 | 1.60 | 1.31 | 58.3 | 55.9 |
| 313 | 2.00589 | 1.73 | 5 | 2.00593 | 1.60 | 1.31 | 61.7 | 58.8 |
| 318 | 2.00591 | 1.73 | 2 | 2.00599 | 1.60 | 1.31 | 63.1 | 62.0 |
| 323 | 2.00591 | 1.73 | 2 | 2.00602 | 1.60 | 1.25 | 64.5 | 63.2 |
| 333 | 2.00593 | 1.72 | 2 | 2.00608 | 1.60 | 1.01 | 65.9 | 64.3 |
| 343 | 2.00594 | 1.72 | 2 | 2.00611 | 1.60 | 0.86 | 68.8 | 67.2 |
| 353 | 2.00597 | 1.72 | 2 | 2.00615 | 1.60 | 0.74 | 70.1 | 69.9 |
References
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-isopropylacrylamide). J. Macromol. Sci. Part A-Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-Ionic Thermoresponsive Polymers in Water. Adv. Polym. Sci. 2011, 242, 29–89. [Google Scholar] [CrossRef] [Green Version]
- Pasparakis, G.; Tsitsilianis, C. LCST Polymers: Thermoresponsive Nanostructured Assemblies towards Bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Nash, M.E.; Fan, X.; Carroll, W.M.; Gorelov, A.V.; Barry, F.P.; Shaw, G.; Rochev, Y.A. Thermoresponsive Substrates Used for the Expansion of Human Mesenchymal Stem Cells and the Preservation of Immunophenotype. Stem Cell Rev. Rep. 2013, 9, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Frolova, A.; Ksendzov, E.; Kostjuk, S.; Efremov, Y.; Solovieva, A.; Rochev, Y.; Timashev, P.; Kotova, S. Thin Thermoresponsive Polymer Films for Cell Culture: Elucidating an Unexpected Thermal Phase Behavior by Atomic Force Microscopy. Langmuir 2021, 37, 11386–11396. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible Thermoresponsive Peptide–PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive Polymers and Their Biomedical Application in Tissue Engineering—A Review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Lu, H.; Leng, J.; Du, S. A Phenomenological Approach for the Chemo-Responsive Shape Memory Effect in Amorphous Polymers. Soft Matter 2013, 9, 3851. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive Polymers with Lower Critical Solution Temperature: From Fundamental Aspects and Measuring Techniques to Recommended Turbidimetry Conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Ding, Y.; Ye, X.; Zhang, G. Microcalorimetric Investigation on Aggregation and Dissolution of Poly(N-Isopropylacrylamide) Chains in Water. Macromolecules 2005, 38, 904–908. [Google Scholar] [CrossRef]
- Antheunis, H.; van der Meer, J.-C.; de Geus, M.; Heise, A.; Koning, C.E. Autocatalytic Equation Describing the Change in Molecular Weight during Hydrolytic Degradation of Aliphatic Polyesters. Biomacromolecules 2010, 11, 1118–1124. [Google Scholar] [CrossRef]
- Kurzbach, D.; Junk, M.J.N.; Hinderberger, D. Nanoscale Inhomogeneities in Thermoresponsive Polymers. Macromol. Rapid Commun. 2013, 34, 119–134. [Google Scholar] [CrossRef]
- Winnik, F.M.; Ottaviani, M.F.; Bossmann, S.H.; Garcia-Garibay, M.; Turro, N.J. Consolvency of Poly(N-Isopropylacrylamide) in Mixed Water-Methanol Solutions: A Look at Spin-Labeled Polymers. Macromolecules 1992, 25, 6007–6017. [Google Scholar] [CrossRef]
- Kokorin, A. (Ed.) Nitroxides—Theory, Experiment and Applications; InTech: London, UK, 2012; ISBN 978-953-51-0722-4. [Google Scholar]
- Drescher, M.; Jeschke, G. (Eds.) EPR Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2012; Volume 321. [Google Scholar]
- Kurzbach, D.; Reh, M.N.; Hinderberger, D. Nanoscale Inhomogeneities in Thermoresponsive Triblock Copolymers. ChemPhysChem 2011, 12, 3566–3572. [Google Scholar] [CrossRef]
- Junk, M.J.N.; Jonas, U.; Hinderberger, D. EPR Spectroscopy Reveals Nanoinhomogeneities in the Structure and Reactivity of Thermoresponsive Hydrogels. Small 2008, 4, 1485–1493. [Google Scholar] [CrossRef]
- Rochev, Y.; O’Halloran, D.; Gorelova, T.; Gilcreest, V.; Selezneva, I.; Gavrilyuk, B.; Gorelov, A. Rationalising the Design of Polymeric Thermoresponsive Biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Caragheorgheopol, A.; Schlick, S. Hydration in the Various Phases of the Triblock Copolymers EO13PO30EO13 (Pluronic L64) and EO6PO34EO6 (Pluronic L62), Based on Electron Spin Resonance Spectra of Cationic Spin Probes. Macromolecules 1998, 31, 7736–7745. [Google Scholar] [CrossRef]
- Eaton, G.R.; Eaton, S.S.; Barr, D.P.; Weber, R.T. Quantitative EPR; Springer: Vienna, Austria, 2010; ISBN 978-3-211-92947-6. [Google Scholar]
- Stoll, S.; Schweiger, A. EasySpin, a Comprehensive Software Package for Spectral Simulation and Analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.J.; Freed, J.H. Calculating Slow Motional Magnetic Resonance Spectra. In Spin Labeling: Theory and Applications; Berliner, L.J., Reuben, J., Eds.; Springer: Boston, MA, USA, 1989; pp. 1–76. ISBN 978-1-4613-0743-3. [Google Scholar]
- Yanase, K.; Buchner, R.; Sato, T. Microscopic Insights into the Phase Transition of Poly(N-Isopropylacrylamide) in Aqueous Media: Effects of Molecular Weight and Polymer Concentration. J. Mol. Liq. 2020, 302, 112025. [Google Scholar] [CrossRef]
- Hunold, J.; Eisermann, J.; Brehm, M.; Hinderberger, D. Characterization of Aqueous Lower-Polarity Solvation Shells Around Amphiphilic 2,2,6,6-Tetramethylpiperidine-1-Oxyl Radicals in Water. J. Phys. Chem. B 2020, 124, 8601–8609. [Google Scholar] [CrossRef]
- Salikhov, K.M. Current State of the Spin Exchange Theory in Dilute Solutions of Paramagnetic Particles. New Paradigm of Spin Exchange and Its Manifestations in EPR Spectroscopy. Physics-Uspekhi 2019, 62, 951–975. [Google Scholar] [CrossRef]
- Salikhov, K.M. New Paradigm of Spin Exchange and Its Manifestations in EPR Spectroscopy. Appl. Magn. Reson. 2020, 51, 297–325. [Google Scholar] [CrossRef]
- Salikhov, K.M. Consistent Paradigm of the Spectra Decomposition into Independent Resonance Lines. Appl. Magn. Reson. 2016, 47, 1207–1227. [Google Scholar] [CrossRef]
- Kecki, Z.; Lyczkowski, Z. KW Critical Comparison of Empirical Systems Used to Describe Solvent Properties. J. Solution Chem. 1986, 15, 413–422. [Google Scholar] [CrossRef]
- Ellison, W.J. Permittivity of Pure Water, at Standard Atmospheric Pressure, over the Frequency Range 0–25THz and the Temperature Range 0–100 °C. J. Phys. Chem. Ref. Data 2007, 36, 1–18. [Google Scholar] [CrossRef]
- Korson, L.; Drost-Hansen, W.; Millero, F.J. Viscosity of Water at Various Temperatures. J. Phys. Chem. 1969, 73, 34–39. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly( N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chemie Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Franchi, P.; Lucarini, M.; Pedrielli, P.; Pedulli, G.F. Nitroxide Radicals as Hydrogen Bonding Acceptors. An Infrared and EPR Study. ChemPhysChem 2002, 3, 789–793. [Google Scholar] [CrossRef]
- Rinkevicius, Z.; Murugan, N.A.; Kongsted, J.; Aidas, K.; Steindal, A.H.; Ågren, H. Density Functional Theory/Molecular Mechanics Approach for Electronic g-Tensors of Solvated Molecules. J. Phys. Chem. B 2011, 115, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, X. Globule-to-Coil Transition of a Single Homopolymer Chain in Solution. Phys. Rev. Lett. 1998, 80, 4092–4094. [Google Scholar] [CrossRef] [Green Version]
- Lebedev, Y.S.; Grinberg, O.Y.; Dubinsky, A.A.; Poluektov, O.G. Investigation of Spin Labels and Probes by Millimeter Band EPR. In Bioactive Spin Labels; Springer: Berlin/Heidelberg, Germany, 1992; pp. 227–278. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubanova, E.M.; Kostjuk, S.V.; Timashev, P.S.; Rochev, Y.A.; Kokorin, A.I.; Melnikov, M.Y.; Golubeva, E.N. Inhomogeneities in PNIPAM Aqueous Solutions: The Inside View by Spin Probe EPR Spectroscopy. Polymers 2021, 13, 3829. https://doi.org/10.3390/polym13213829
Zubanova EM, Kostjuk SV, Timashev PS, Rochev YA, Kokorin AI, Melnikov MY, Golubeva EN. Inhomogeneities in PNIPAM Aqueous Solutions: The Inside View by Spin Probe EPR Spectroscopy. Polymers. 2021; 13(21):3829. https://doi.org/10.3390/polym13213829
Chicago/Turabian StyleZubanova, Ekaterina M., Sergei V. Kostjuk, Peter S. Timashev, Yury A. Rochev, Alexander I. Kokorin, Mikhail Ya. Melnikov, and Elena N. Golubeva. 2021. "Inhomogeneities in PNIPAM Aqueous Solutions: The Inside View by Spin Probe EPR Spectroscopy" Polymers 13, no. 21: 3829. https://doi.org/10.3390/polym13213829
APA StyleZubanova, E. M., Kostjuk, S. V., Timashev, P. S., Rochev, Y. A., Kokorin, A. I., Melnikov, M. Y., & Golubeva, E. N. (2021). Inhomogeneities in PNIPAM Aqueous Solutions: The Inside View by Spin Probe EPR Spectroscopy. Polymers, 13(21), 3829. https://doi.org/10.3390/polym13213829