Possibility Routes for Textile Recycling Technology
Abstract
:1. Introduction
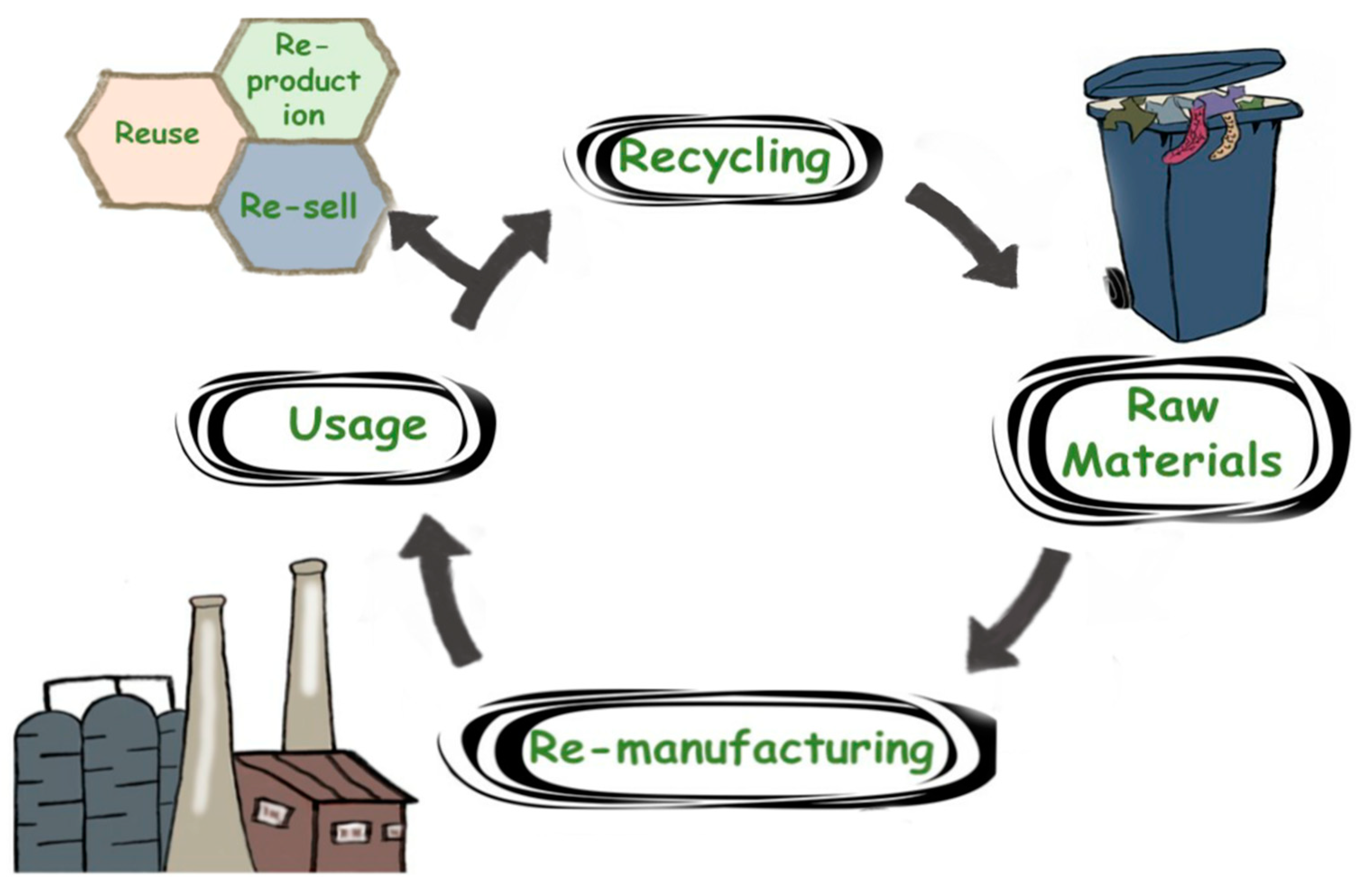
2. Circular Economy of Textile Recycling
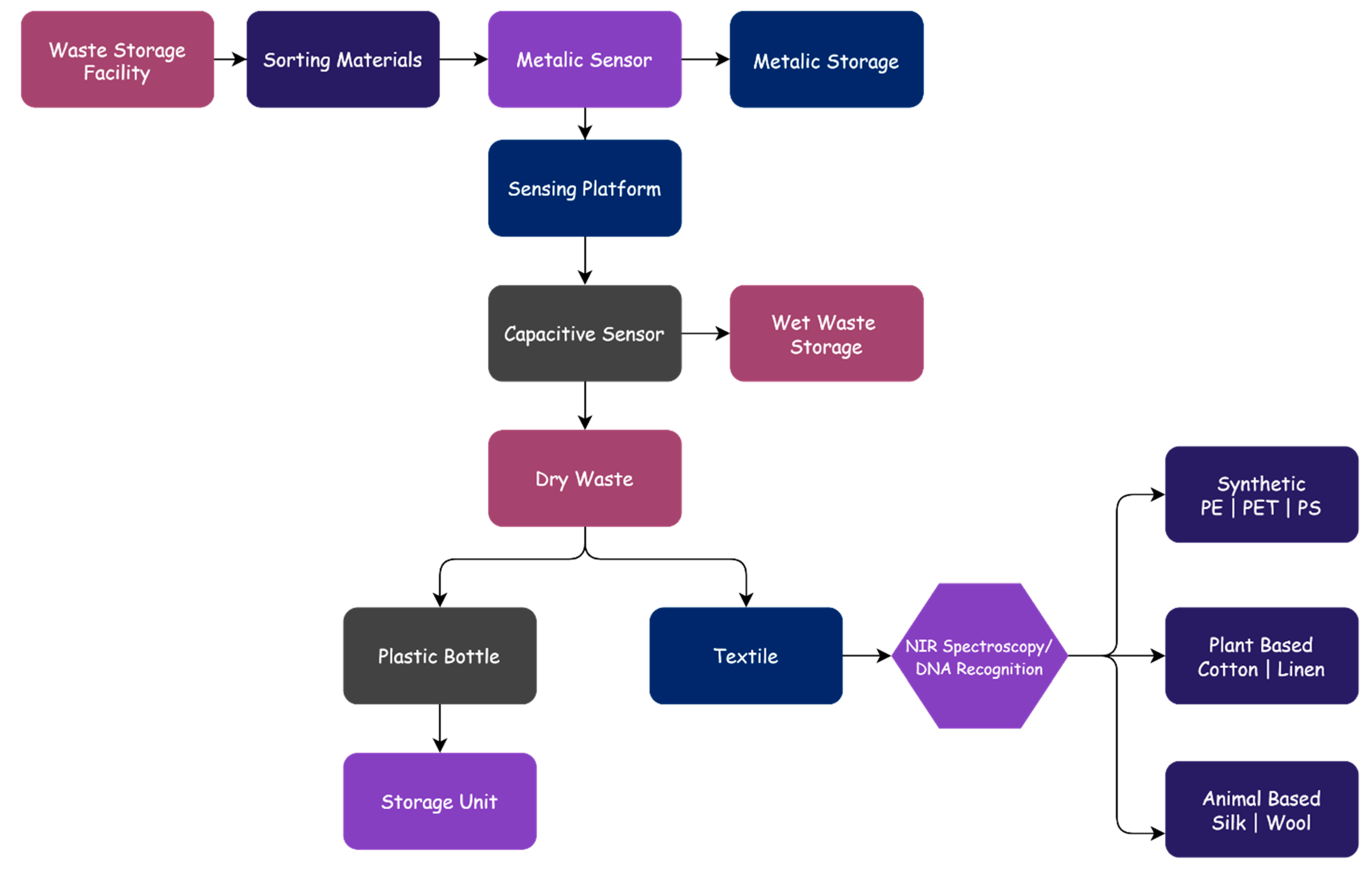
3. Starting with Municipal Waste to Textile Trash in Applied IoT
4. Sorting and Identification of Textile Waste
5. Mechanical Recycling of Textile
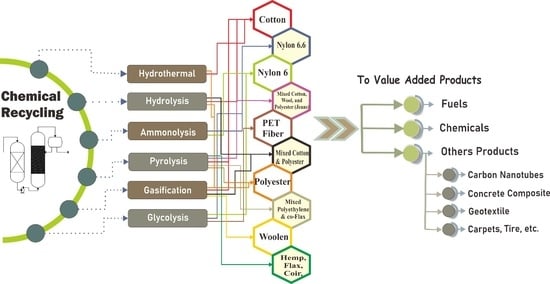
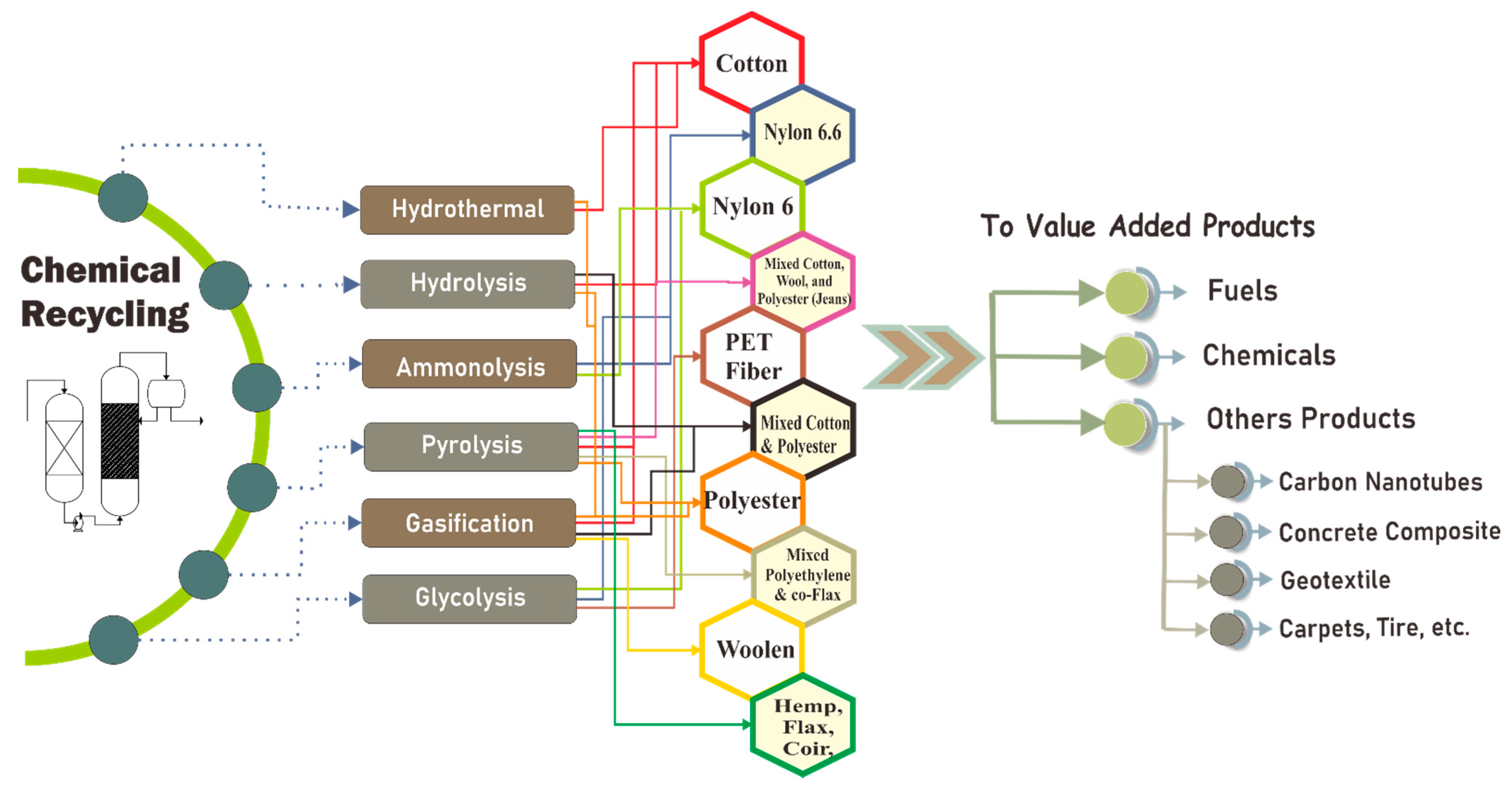
6. Chemical Recycling of Textile
6.1. Textile Recycling Using Pyrolysis
6.2. Textile Recycling Using Enzymatic Hydrolysis
6.3. Textile Recycling Using the Hydrothermal Method
6.4. Textile Recycling Using Ammonolysis
6.5. Textile Recycling Using Gasification
6.6. Textile Recycling Using Glycolysis
6.7. Decolorization Technology in Textile Recycling
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| BHET | Bis(2-hydroxyethyl) terephthalate |
| CBP | Consolidated bioprocessing |
| CE | Circular economy |
| CIE | C-Illuminant (light source C) |
| CNN | Convolutional neural network |
| CTW | Cotton textile waste |
| Ea | Activation energy |
| EG | Ethylene Glycol |
| FCM | Fuzzy C-means |
| HOG | Histogram of oriented gradients |
| ICT | Information and communication technology |
| IoT | Internet of Things |
| k-NN | k-nearest neighbors algorithm |
| LCA | Life cycle assessment |
| MLP | Multi-layer perceptron |
| NIR | Near-infrared spectroscopy |
| NMR | Nuclear magnetic resonance |
| PCA | Principal component analysis |
| PET | Polyethylene terephthalate |
| r-PET | Recycled polyethylene terephthalate |
| RMBs | Recycling business models |
| SAaaS | Software as a service |
| SHF | Separated hydrolysis fermentation |
| SIMCA | Soft independent modeling of class analogy |
| SMF | Submerged fermentation |
| SMOs | Smart manufacturing objects |
| SOM | Self-organizing map |
| SSF | Simultaneous saccharification and fermentation |
| SSnF | Semi-simultaneous saccharification and fermentation |
| SVM | Support vector machine |
| TILT | Transform invariant low-rank textures |
| WEEE | Waste electrical and electronic equipment |
References
- Stone, C.; Windsor, F.M.; Munday, M.; Durance, I. Natural or synthetic–how global trends in textile usage threaten freshwater environments. Sci. Total Environ. 2020, 718, 134689. [Google Scholar] [CrossRef] [PubMed]
- Gardetti, M.Á. Sustainability in the textile and fashion industries: Animal ethics and welfare. In Textiles and Clothing Sustainability; Muthu, S., Ed.; Springer: Singapore, 2017; pp. 47–73. [Google Scholar]
- Chapagain, A.K.; Hoekstra, A.Y.; Savenije, H.H.; Gautam, R. The water footprint of cotton consumption: An assessment of the impact of worldwide consumption of cotton products on the water resources in the cotton producing countries. Ecol. Econ. 2006, 60, 186–203. [Google Scholar] [CrossRef]
- Pfister, S.; Bayer, P.; Koehler, A.; Hellweg, S. Environmental impacts of water use in global crop production: Hotspots and trade-offs with land use. Environ. Sci. Technol. 2011, 45, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Navone, L.; Moffitt, K.; Hansen, K.-A.; Blinco, J.; Payne, A.; Speight, R. Closing the textile loop: Enzymatic fibre separation and recycling of wool/polyester fabric blends. J. Waste Manag. 2020, 102, 149–160. [Google Scholar] [CrossRef]
- Mäkelä, M.; Rissanen, M.; Sixta, H. Machine vision estimates the polyester content in recyclable waste textiles. Resour. Conserv. Recycl. 2020, 161, 105007. [Google Scholar] [CrossRef]
- MacArthur, E. A New Textiles Economy: Redesigning Fashion’s Future; Ellen MacArthur Foundation: Cowes, UK, 2017. [Google Scholar]
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef]
- Payne, A. Open-and closed-loop recycling of textile and apparel products. In Handbook of Life Cycle Assessment (LCA) of Textiles and Clothing; Elsevier: Amsterdam, The Netherlands, 2015; pp. 103–123. [Google Scholar]
- Heikkilä, P.; Cura, K.; Heikkilä, J.; Hinkka, V.; Ikonen, T.; Kamppuri, T.; Knuutila, H.; Kokko, M.; Lankiniemi, S.; Lehtinen, L. Telaketju: Towards Circularity of Textiles; VTT Research Report; No. VTT-R-00062-19; VTT Technical Research Centre of Finland: Espoo, Finland, 2019; 117p. [Google Scholar]
- Koszewska, M. Circular Economy—Challenges for the Textile and Clothing Industry. Autex Res. J. 2018, 18, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Kim, S.H. Poly (ethylene terephthalate) recycling for high value added textiles. Fash. Text. 2014, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Sandin, G.; Peters, G.M. Environmental impact of textile reuse and recycling–A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Dahlbo, H.; Aalto, K.; Eskelinen, H.; Salmenperä, H. Increasing textile circulation—consequences and requirements. Sustain. Prod. Consum. 2017, 9, 44–57. [Google Scholar] [CrossRef]
- Muthu, S.S.; Li, Y.; Hu, J.Y.; Ze, L. Carbon footprint reduction in the textile process chain: Recycling of textile materials. Fibers Polym. 2012, 13, 1065–1070. [Google Scholar] [CrossRef]
- Franco, M.A. Circular economy at the micro level: A dynamic view of incumbents’ struggles and challenges in the textile industry. J. Clean. Prod. 2017, 168, 833–845. [Google Scholar] [CrossRef]
- Galvão, G.D.A.; de Nadae, J.; Clemente, D.H.; Chinen, G.; de Carvalho, M.M. Circular economy: Overview of barriers. Procedia CIRP 2018, 73, 79–85. [Google Scholar] [CrossRef]
- Hussain, A.; Kamboj, N.; Podgurski, V.; Antonov, M.; Goliandin, D. Circular economy approach to recycling technologies of postconsumer textile waste in Estonia: A review. Proc. Est. Acad. Sci. 2021, 70, 82–92. [Google Scholar]
- Manglani, H.; Hodge, G.L.; Oxenham, W. Application of the internet of things in the textile industry. Text. Prog. 2019, 51, 225–297. [Google Scholar] [CrossRef]
- Das, S.K.; Chinnappan, A.; Jayathilaka, W.A.D.M.; Gosh, R.; Baskar, C.; Ramakrishna, S. Challenges and potential solutions for 100% recycling of medical textiles. Mater. Circ. Econ. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Pagoropoulos, A.; Pigosso, D.C.; McAloone, T.C. The emergent role of digital technologies in the circular economy: A review. Procedia CIRP 2017, 64, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Yao, X.; Zhang, J. Big data in wisdom manufacturing for industry 4.0. In Proceedings of the 2017 5th International Conference on Enterprise Systems (ES), Beijing, China, 22–24 September 2017; pp. 107–112. [Google Scholar]
- Fatimah, Y.A.; Govindan, K.; Murniningsih, R.; Setiawan, A. Industry 4.0 based sustainable circular economy approach for smart waste management system to achieve sustainable development goals: A case study of Indonesia. J. Clean. Prod. 2020, 269, 122263. [Google Scholar] [CrossRef]
- Haslinger, S.; Hietala, S.; Hummel, M.; Maunu, S.L.; Sixta, H. Solid-state NMR method for the quantification of cellulose and polyester in textile blends. Carbohydr. Polym. 2019, 207, 11–16. [Google Scholar] [CrossRef]
- Ruckebusch, C.; Orhan, F.; Durand, A.; Boubellouta, T.; Huvenne, J. Quantitative analysis of cotton–polyester textile blends from near-infrared spectra. Appl. Spectrosc. 2006, 60, 539–544. [Google Scholar] [CrossRef]
- Park, S.; Johnson, D.K.; Ishizawa, C.I.; Parilla, P.A.; Davis, M.F. Measuring the crystallinity index of cellulose by solid state 13 C nuclear magnetic resonance. Cellulose 2009, 16, 641–647. [Google Scholar] [CrossRef]
- Rodgers, J.; Beck, K. NIR characterization and measurement of the cotton content of dyed blend fabrics. Text. Res. J. 2009, 79, 675–686. [Google Scholar] [CrossRef]
- Lv, F.; Wang, C.; Zhu, P.; Zhang, C. Isolation and recovery of cellulose from waste nylon/cotton blended fabrics by 1-allyl-3-methylimidazolium chloride. Carbohydr. Polym. 2015, 123, 424–431. [Google Scholar] [CrossRef]
- Spathas, T. The Environmental Performance of High Value Recycling for the Fashion Industry. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2017. [Google Scholar]
- Rosa, P.; Sassanelli, C.; Terzi, S. Towards Circular Business Models: A systematic literature review on classification frameworks and archetypes. J. Clean. Prod. 2019, 236, 117696. [Google Scholar] [CrossRef]
- Winans, K.; Kendall, A.; Deng, H. The history and current applications of the circular economy concept. Renew. Sust. Energ. Rev. 2017, 68, 825–833. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.; Hultink, E.J. The Circular Economy–A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Kazancoglu, I.; Kazancoglu, Y.; Yarimoglu, E.; Kahraman, A. A conceptual framework for barriers of circular supply chains for sustainability in the textile industry. J. Sustain. Dev. 2020, 28, 1477–1492. [Google Scholar] [CrossRef]
- Allwood, J.M.; Laursen, S.E.; de Rodriguez, C.M.; Bocken, N.M. Well dressed?: The present and future sustainability of clothing and textiles in the United Kingdom. J. Home Econ. Inst. Aust. 2015, 22, 42. [Google Scholar]
- Manickam, P.; Duraisamy, G. 3Rs and circular economy. In Circular Economy in Textiles and Apparel; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–93. [Google Scholar]
- Chen, X.; Memon, H.A.; Wang, Y.; Marriam, I.; Tebyetekerwa, M. Circular Economy and sustainability of the clothing and textile Industry. Mater. Circ. Econ. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Balanay, R.; Halog, A. Tools for circular economy: Review and some potential applications for the Philippine textile industry. Circ. Econ. Text. Appar. 2019, 49–75. [Google Scholar] [CrossRef]
- Fortuna, L.M.; Diyamandoglu, V. Optimization of greenhouse gas emissions in second-hand consumer product recovery through reuse platforms. J. Waste Manag. 2017, 66, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Lüdeke-Freund, F.; Gold, S.; Bocken, N.M. A review and typology of circular economy business model patterns. J. Ind. Ecol. 2019, 23, 36–61. [Google Scholar] [CrossRef] [Green Version]
- Pal, R.; Samie, Y.; Chizaryfard, A. Demystifying process-level scalability challenges in fashion remanufacturing: An interdependence perspective. J. Clean. Prod. 2021, 286, 125498. [Google Scholar] [CrossRef]
- Dissanayake, G.; Sinha, P. An examination of the product development process for fashion remanufacturing. Resour. Conserv. Recycl. 2015, 104, 94–102. [Google Scholar] [CrossRef]
- Niinimäki, K.; Hassi, L. Emerging design strategies in sustainable production and consumption of textiles and clothing. J. Clean. Prod. 2011, 19, 1876–1883. [Google Scholar] [CrossRef]
- Gwilt, A.; Rissanen, T. Shaping Sustainable Fashion: Changing the Way We Make and Use Clothes; Routledge: England, UK, 2012. [Google Scholar]
- Wen-hui, X.; Dian-yan, J.; Yu-ying, H. The remanufacturing reverse logistics management based on closed-loop supply chain management processes. Procedia Environ. Sci. 2011, 11, 351–354. [Google Scholar] [CrossRef]
- Vogtlander, J.G.; Scheepens, A.E.; Bocken, N.M.; Peck, D. Combined analyses of costs, market value and eco-costs in circular business models: Eco-efficient value creation in remanufacturing. J. Remanufacturing 2017, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- De Silva, R.; Wang, X.; Byrne, N. Recycling textiles: The use of ionic liquids in the separation of cotton polyester blends. RSC Adv. 2014, 4, 29094–29098. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Du, C.; Lin, C.S.K. Recovery of glucose and polyester from textile waste by enzymatic hydrolysis. Waste Biomass Valorization 2019, 10, 3763–3772. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Yaashikaa, P. Recycled Fibres. In Sustainable Innovations in Recycled Textiles; Springer: Amsterdam, The Netherlands, 2018; pp. 1–7. [Google Scholar]
- Yin, Y.; Yao, D.; Wang, C.; Wang, Y. Removal of spandex from nylon/spandex blended fabrics by selective polymer degradation. Text. Res. J. 2014, 84, 16–27. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Azevedo, S.G.; Lin, T.-J.; Cheng, C.-S.; Lin, C.-T. Exploring the decisive barriers to achieve circular economy: Strategies for the textile innovation in Taiwan. Sustain. Prod. Consum. 2021, 27, 1406–1423. [Google Scholar] [CrossRef]
- Martina, R.A.; Oskam, I.F. Practical guidelines for designing recycling, collaborative, and scalable business models: A case study of reusing textile fibers into biocomposite products. J. Clean. Prod. 2021, 128542. [Google Scholar] [CrossRef]
- Christensen, T.B. Towards a circular economy in cities: Exploring local modes of governance in the transition towards a circular economy in construction and textile recycling. J. Clean. Prod. 2021, 305, 127058. [Google Scholar] [CrossRef]
- Seadon, J.K. Sustainable waste management systems. J. Clean. Prod. 2010, 18, 1639–1651. [Google Scholar] [CrossRef]
- Payne, A.; Nay, Z.; Maguire, R. Regulating a circular economy for textiles in Australia. In Proceedings of the 4th PLATE 2021 Virtual Conference, Limerick, Ireland, 26–28 May 2021. [Google Scholar]
- Ghoreishi, M.; Happonen, A. The case of fabric and textile industry: The emerging role of digitalization, internet-of-Things and industry 4.0 for circularity. In Proceedings of the Sixth International Congress on Information and Communication Technology, London, UK, 25–26 February 2022; pp. 189–200. [Google Scholar]
- Abdullah, N.; Alwesabi, O.A.; Abdullah, R. Iot-based smart waste management system in a smart city. In Proceedings of the International Conference of Reliable Information and Communication Technology, Kuala Lumpur, Malaysia, 23–24 June 2018; pp. 364–371. [Google Scholar]
- Zhong, R.Y.; Dai, Q.; Qu, T.; Hu, G.; Huang, G.Q. RFID-enabled real-time manufacturing execution system for mass-customization production. Robot. Comput. Integr. Manuf. 2013, 29, 283–292. [Google Scholar] [CrossRef]
- Lu, B.; Bateman, R.; Cheng, K. RFID enabled manufacturing: Fundamentals, methodology and applications. Int. J. Agil. Syst. Manag. 2006, 1, 73–92. [Google Scholar] [CrossRef]
- Zhong, R.Y.; Li, Z.; Pang, L.; Pan, Y.; Qu, T.; Huang, G.Q. RFID-enabled real-time advanced planning and scheduling shell for production decision making. Int. J. Comput. Integr. Manuf. 2013, 26, 649–662. [Google Scholar] [CrossRef]
- Mavropoulos, A.; Nilsen, A.W. Industry 4.0 and Circular Economy: Towards a Wasteless Future or a Wasteful Planet? John Wiley & Sons: New Jersey, NJ, USA, 2020. [Google Scholar]
- Zhong, R.Y.; Xu, X.; Klotz, E.; Newman, S.T. Intelligent manufacturing in the context of industry 4.0: A review. Engineering 2017, 3, 616–630. [Google Scholar] [CrossRef]
- Kumar, K.; Zindani, D.; Davim, J.P. Intelligent Manufacturing. In Industry 4.0; Springer: Amsterdam, The Netherlands, 2019; pp. 1–17. [Google Scholar]
- Wu, D.; Greer, M.J.; Rosen, D.W.; Schaefer, D. Cloud manufacturing: Strategic vision and state-of-the-art. J. Manuf. Syst. 2013, 32, 564–579. [Google Scholar] [CrossRef] [Green Version]
- Lopes, S.; Machado, S. IoT based automatic waste segregator. In Proceedings of the 2019 International Conference on Advances in Computing, Communication and Control (ICAC3), Mumbai, India, 20–21 December 2019; pp. 1–5. [Google Scholar]
- Yu, K.H.; Zhang, Y.; Li, D.; Montenegro-Marin, C.E.; Kumar, P.M. Environmental planning based on reduce, reuse, recycle and recover using artificial intelligence. Environ. Impact Assess. Rev. 2021, 86, 106492. [Google Scholar] [CrossRef]
- Giacobbe, M.; Puliafito, C.; Scarpa, M. The big bucket: An iot cloud solution for smart waste management in smart cities. In Proceedings of the European Conference on Service-Oriented and Cloud Computing, Vienna, Austria, 5–7 September 2016; pp. 43–58. [Google Scholar]
- Merlino, G.; Bruneo, D.; Distefano, S.; Longo, F.; Puliafito, A. Stack4things: Integrating iot with openstack in a smart city context. In Proceedings of the 2014 International Conference on Smart Computing Workshops, Hongkong, China, 5 November 2014; pp. 21–28. [Google Scholar]
- Marin Perez, M. Analysis of European Post-Consumer Textile Waste for Automated Sorting. Master’s Thesis, Uppsala University, Stockholm, Sweden, 2021. [Google Scholar]
- Bergfjord, C.; Holst, B. A procedure for identifying textile bast fibres using microscopy: Flax, nettle/ramie, hemp and jute. Ultramicroscopy 2010, 110, 1192–1197. [Google Scholar] [CrossRef]
- Nørup, N.; Pihl, K.; Damgaard, A.; Scheutz, C. Development and testing of a sorting and quality assessment method for textile waste. J. Waste Manag. 2018, 79, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, P.K.; Majumdar, P.K.; Sarkar, B. Thermal behaviour of textiles: A review. Man-Made Text. India. 2013, 41, 3. [Google Scholar]
- Sombatsompop, N.; Wood, A. Measurement of thermal conductivity of polymers using an improved Lee’s disc apparatus. Polym. Test. 1997, 16, 203–223. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Sarwar, Z.; Jonuškienė, I.; Kliucininkas, L. A new strategy for using textile waste as a sustainable source of recovered cotton. Resour. Conserv. Recycl. 2019, 145, 359–369. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Wei, Z. Qualitative classification of waste textiles based on near infrared spectroscopy and the convolutional network. Text. Res. J. 2020, 90, 1057–1066. [Google Scholar] [CrossRef]
- Zitting, J. Optical Sorting Technology for Textile Waste: Development of an Identification Method with NIR Spectroscopy. Bechelor’s Thesis, Lahti University, Lahti, Finland, 2017. [Google Scholar]
- Wilts, H.; Garcia, B.R.; Garlito, R.G.; Gómez, L.S.; Prieto, E.G. Artificial intelligence in the sorting of municipal waste as an enabler of the circular economy. Resources 2021, 10, 28. [Google Scholar] [CrossRef]
- Xiong, E. Autonomous sorting of plastic resin using near-infrared and machine learning. CSFJ. 2021, 3, 1–5. [Google Scholar]
- Berghmans, A.C.; Huys, M.J.G. Progress for Identifying Objects Using an Optical Spectrometer and a Transport System. U.S. Patent 7,071,469, 4 July 2006. [Google Scholar]
- Zhou, C.; Han, G.; Via, B.K.; Song, Y.; Gao, S.; Jiang, W. Rapid identification of fibers from different waste fabrics using the near-infrared spectroscopy technique. Text. Res. J. 2019, 89, 3610–3616. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, L.; Ding, Q.; Wang, R. Textile fiber identification using near-infrared spectroscopy and pattern recognition. Autex Res. J. 2019, 19, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Tan, C.; Lin, Z. Quantitative determination of wool in textile by near-infrared spectroscopy and multivariate models. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 201, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Iqbal Hussain, M.A.; Khan, B.; Wang, Z.; Ding, S. Woven fabric pattern recognition and classification based on deep convolutional neural networks. Electronics 2020, 9, 1048. [Google Scholar] [CrossRef]
- Xiao, Z.; Guo, Y.; Geng, L.; Wu, J.; Zhang, F.; Wang, W.; Liu, Y. Automatic recognition of woven fabric pattern based on TILT. Math. Probl. Eng. 2018, 2018, 9707104. [Google Scholar] [CrossRef]
- Kuo, C.-F.J.; Kao, C.-Y. Self-organizing map network for automatically recognizing color texture fabric nature. Fibers Polym. 2007, 8, 174–180. [Google Scholar] [CrossRef]
- Cura, K.; Rintala, N.; Kamppuri, T.; Saarimäki, E.; Heikkilä, P. Textile recognition and sorting for recycling at an automated line using near infrared spectroscopy. Recycling 2021, 6, 11. [Google Scholar] [CrossRef]
- Riba, J.-R.; Cantero, R.; Canals, T.; Puig, R. Circular economy of post-consumer textile waste: Classification through infrared spectroscopy. J. Clean. Prod. 2020, 272, 123011. [Google Scholar] [CrossRef]
- Peets, P.; Kaupmees, K.; Vahur, S.; Leito, I. Reflectance FT-IR spectroscopy as a viable option for textile fiber identification. Herit. Sci. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Morgado, V.; Gomes, L.; Bettencourt da Silva, R.J.N.; Palma, C. Validated spreadsheet for the identification of PE, PET, PP and PS microplastics by micro-ATR-FTIR spectra with known uncertainty. Talanta 2021, 234, 122624. [Google Scholar] [CrossRef]
- Gulich, B. Development of products made of reclaimed fibres. Recycl. Text. Camb. 2006, 1, 117–136. [Google Scholar]
- Valerio, O.; Muthuraj, R.; Codou, A. Strategies for polymer to polymer recycling from waste: Current trends and opportunities for improving the circular economy of polymers in South America. Curr. Opin. Green Sustain. Chem. 2020, 25, 100381. [Google Scholar] [CrossRef]
- Michaud, J.-C.; Farrant, L.; Jan, O.; Kjær, B.; Bakas, I. Environmental Benefits of Recycling—2010 Update. Material Change for a Better Environment. 2010. Available online: http://localhost:8080/xmlui/handle/123456789/174 (accessed on 22 August 2021).
- Euratex. Circular Textiles, Prospering in the Circular Economy. Available online: https://euratex.eu/wp-content/uploads/EURATEX-Prospering-in-the-Circular-Economy-2020.pdf (accessed on 22 August 2021).
- Pensupa, N.; Leu, S.-Y.; Hu, Y.; Du, C.; Liu, H.; Jing, H.; Wang, H.; Lin, C.S.K. Recent trends in sustainable textile waste recycling methods: Current situation and future prospects. Chem. Chem. Technol. Waste Valoriz. 2017, 189–228. [Google Scholar] [CrossRef]
- Bartlett, C.; McGill, I.; Willis, P. Textiles Flow and Market Development Opportunities in the UK; Waste & Resources Action Programme: Banbury, UK, 2013. [Google Scholar]
- Korhonen, M.-R.; Dahlbo, H. Reducing Greenhouse Gas Emissions by Recycling Plastics and Textiles into Products; Finnish Environment Institute: Helsinki, Finland, 2007. [Google Scholar]
- Johansson, L. On the Mechanical Recycling of Woven Fabrics: Improving the Reusable Fibre Yield of Mechanical Methods. Master’s Thesis, Uppala University, Stockholm, Sweden, 2020. [Google Scholar]
- Hutten, I.M. Handbook of Nonwoven Filter Media; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Jin, K.; Banerji, A.; Kitto, D.; Bates, F.S.; Ellison, C.J. Mechanically robust and recyclable cross-linked fibers from melt blown anthracene-functionalized commodity polymers. ACS Appl. Mater. Interfaces 2019, 11, 12863–12870. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.P.-Y. Methods of Saturating Nonwoven Fabrics with Liquid and the Making of Electret Therof. U.S. Patent 17/056,276, 26 August 2021. [Google Scholar]
- Mondragon, G.; Kortaberria, G.; Mendiburu, E.; González, N.; Arbelaiz, A.; Peña-Rodriguez, C. Thermomechanical recycling of polyamide 6 from fishing nets waste. J. Appl. Polym. Sci. 2020, 137, 48442. [Google Scholar] [CrossRef]
- Russell, S.; Swan, P.; Trebowicz, M.; Ireland, A. Review of wool recycling and reuse. In Natural Fibres: Advances in Science and Technology towards Industrial Applications; Springer: Dordrecht, The Netherlands, 2016; pp. 415–428. [Google Scholar] [CrossRef]
- Alexander, C.; Reno, J. Economies of Recycling: The Global Transformation of Materials, Values and Social Relations; Zed Books Ltd.: London, UK, 2012. [Google Scholar]
- Klepp, I.G.; Tobiasson, T.S.; Laitala, K. Wool as an Heirloom: How Natural Fibres Can Reinvent Value in Terms of Money, Life-Span and Love. In Natural Fibres: Advances in Science and Technology towards Industrial Applications; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Harmsen, P.; Scheffer, M.; Bos, H. Textiles for circular fashion: The logic behind recycling options. Sustainability 2021, 13, 9714. [Google Scholar] [CrossRef]
- Klaus-nietrost, C.; Richard, H.; Weilach, C. Method of Reusing a Mixed Textile Comprising Cellulose and Synthetic Plastic. U.S. Patent 16/962,498, 18 March 2021. [Google Scholar]
- Piribauer, B.; Bartl, A. Textile recycling processes, state of the art and current developments: A mini review. Waste Manag. Res. 2019, 37, 112–119. [Google Scholar] [CrossRef]
- Liu, R.-G.; Shen, Y.-Y.; Shao, H.-L.; Wu, C.-X.; Hu, X.-C. An analysis of Lyocell fiber formation as a melt–spinning process. Cellulose 2001, 8, 13–21. [Google Scholar] [CrossRef]
- Kaminsky, W.; Scheirs, J. Feedstock Recycling and Pyrolysis of Waste Plastics: Converting Waste Plastics into Diesel and Other Fuels; J. Wiley & Sons: New Jersey, NJ, USA, 2006. [Google Scholar]
- Damayanti, D.; Wu, H.-S. Strategic possibility routes of recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Ambercycle. Creating a Circular System. Available online: www.ambercycle.com (accessed on 29 July 2021).
- BlockTexx. Textile Recovery Technology. Available online: https://www.blocktexx.com (accessed on 29 July 2021).
- FENC. New Process Recycles any PET Waste. Available online: http://news.fenc.com/news_detail.aspx?lang=en&id=4557 (accessed on 4 March 2021).
- Fiber, I. Infinited Fiber Company Brochure. Available online: https://infinitedfiber.com/wp-content/uploads/2019/06/IFC_Brochure_5-2019.pdf (accessed on 29 July 2021).
- Asikainen, S.; Määttänen, M.; Harlin, A.; Valta, K.; Sivonen, E. Method of Producing Dissolving Pulp, Dissolving Pulp and Use of Method. U.S. Patent 14/428,377, 13 August 2015. [Google Scholar]
- Ioncell. Ioncell® Process. Available online: https://ioncell.fi/research/ (accessed on 30 July 2021).
- Lenzing. Closing the Loops. Available online: https://www.lenzing.com/sustainability/production/resources/chemicals (accessed on 4 September 2021).
- BioSciences, T. Tyton BioSciences. Available online: www.tytonbio.com (accessed on 4 September 2021).
- Technologies, W.A. Available online: https://wornagain.co.uk/ (accessed on 22 May 2021).
- Sherwood, J. Closed-loop recycling of polymers using solvents. Johnson Matthey Technol. Rev. 2020, 4–15. [Google Scholar] [CrossRef]
- Ribul, M.; Lanot, A.; Tommencioni-Pisapia, C.; Purnell, P.; McQueen-Mason, S.J.; Baurley, S. Mechanical, chemical, biological: Moving towards closed-loop bio-based recycling in a circular economy of sustainable textiles. J. Clean. Prod. 2021, 129325. [Google Scholar] [CrossRef]
- Saha, S. Textile Recycling: The Chemical Recycling Process of Textiles. Available online: https://www.onlineclothingstudy.com/2020/08/textile-recycling-chemical-recycling.html (accessed on 24 October 2021).
- Coates, G.W.; Getzler, Y.D. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020, 5, 501–516. [Google Scholar] [CrossRef]
- Bruce, M.; Miller, W.D. Nylon Hydrolisis. U.S. Patent 2,840,606, 24 June 1958. [Google Scholar]
- Bodrero, S.; Canivenc, E.; Cansell, F. Chemical Recycling of Polyamide 6.6 and Polyamide 6 through a Two Step Ami-/Ammonolysis Process; Georgia Institute of Technology: Atlanta, GA, USA, 1999. [Google Scholar]
- Polk, M.B.; Leboeuf, L.L.; Shah, M.; Won, C.-Y.; Hu, X.; Ding, W. Nylon 66, nylon 46, and PET phase-transfer-catalyzed alkaline depolymerization at atmospheric pressure. Polym. Plast. Technol. Eng. 1999, 38, 459–470. [Google Scholar] [CrossRef]
- Rickson, R. Controlling sediment at source: An evaluation of erosion control geotextiles. Earth Surf. Process.Land. BGRG 2006, 31, 550–560. [Google Scholar] [CrossRef]
- Broda, J.; Przybyło, S.; Gawłowski, A.; Grzybowska-Pietras, J.; Sarna, E.; Rom, M.; Laszczak, R. Utilisation of textile wastes for the production of geotextiles designed for erosion protection. J. Text. Inst. 2019, 110, 435–444. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Si, J.; Cui, Z.; Peng, K. Morphological, mechanical and thermal properties of poly (lactic acid)(PLA)/cellulose nanofibrils (CNF) composites nanofiber for tissue engineering. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 207–215. [Google Scholar] [CrossRef]
- Rizal, S.; Olaiya, F.G.; Saharudin, N.; Abdullah, C.; Ng, O.; Mohamad Haafiz, M.; Yahya, E.B.; Sabaruddin, F.; Khalil, H. Isolation of textile waste cellulose nanofibrillated fibre reinforced in polylactic acid-chitin biodegradable composite for green packaging application. Polymers 2021, 13, 325. [Google Scholar] [CrossRef]
- Barisci, S.; Oncel, M.S. The disposal of combed cotton wastes by pyrolysis. Int. J. Green Energy 2014, 11, 255–266. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Godina, R.; Matias, J.C.O.; Catalão, J.P.S. Economic and environmental benefits of using textile waste for the production of thermal energy. J. Clean. Prod. 2018, 171, 1353–1360. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Nannou, T.; Anguilano, L.; Krzyżyńska, R.; Ghazal, H.; Spencer, N.; Jouhara, H. Potentials of pyrolysis processes in the waste management sector. Energy Procedia 2017, 123, 387–394. [Google Scholar] [CrossRef]
- Qi, R.; Xu, Z.; Zhou, Y.; Zhang, D.; Sun, Z.; Chen, W.; Xiong, M. Clean solid fuel produced from cotton textiles waste through hydrothermal carbonization with FeCl3: Upgrading the fuel quality and combustion characteristics. Energy 2021, 214, 118926. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, R.; Xiong, M.; Zhang, D.; Gu, H.; Chen, W. Conversion of cotton textile waste to clean solid fuel via surfactant-assisted hydrothermal carbonization: Mechanisms and combustion behaviors. Bioresour. Technol. 2021, 321, 124450. [Google Scholar] [CrossRef]
- Duman, G. Preparation of novel porous carbon from hydrothermal pretreated textile wastes: Effects of textile type and activation agent on structural and adsorptive properties. J. Water Process. Eng. 2021, 43, 102286. [Google Scholar] [CrossRef]
- Yasin, S.; Massimo, C.; Rovero, G.; Behary, N.; Perwuelz, A.; Giraud, S.; Migliavacca, G.; Chen, G.; Guan, J. An alternative for the end-of-life phase of flame retardant textile products: Degradation of flame retardant and preliminary settings of energy valorization by gasification. BioResources 2017, 12, 5196–5211. Available online: https://ojs.cnr.ncsu.edu/index.php/BioRes/article/viewFile/BioRes_12_3_5196_Yasin_Alternative_Flame_Retardant_Textile/5343 (accessed on 29 July 2021). [CrossRef]
- Sert, E.; Yılmaz, E.; Atalay, F.S. Chemical recycling of polyethlylene terephthalate by glycolysis using deep eutectic solvents. J. Polym. Environ. 2019, 27, 2956–2962. [Google Scholar] [CrossRef]
- Kalfas, G.A. Mathematical modeling of the depolymerization of polyamide mixtures-Part I: Kinetic mechanism and parametric studies in batch reactors. Polym. React. Eng. 1998, 6, 41–67. [Google Scholar] [CrossRef]
- Kwon, E.E.; Lee, T.; Ok, Y.S.; Tsang, D.C.; Park, C.; Lee, J. Effects of calcium carbonate on pyrolysis of sewage sludge. Energy 2018, 153, 726–731. [Google Scholar] [CrossRef]
- Lee, T.; Nam, I.-H.; Jung, S.; Park, Y.-K.; Kwon, E.E. Synthesis of nickel/biochar composite from pyrolysis of Microcystis aeruginosa and its practical use for syngas production. Bioresour. Technol. 2020, 300, 122712. [Google Scholar] [CrossRef]
- Damayanti, D.; Wu, H.S. Pyrolysis kinetic of alkaline and dealkaline lignin using catalyst. J. Polym. Res. 2018, 25, 7. [Google Scholar] [CrossRef]
- Miranda, R.; Sosa_Blanco, C.; Bustos-Martinez, D.; Vasile, C. Pyrolysis of textile wastes: I. Kinetics and yields. J. Anal. Appl. Pyrolysis 2007, 80, 489–495. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a catalyst. Renew. Sust. Energ. Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.; Kwon, E.E.; Lee, J.; Wang, C.-H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Kwon, D.; Yi, S.; Jung, S.; Kwon, E.E. Valorization of synthetic textile waste using CO2 as a raw material in the catalytic pyrolysis process. Environ. Pollut. 2021, 268, 115916. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Trofimov, E.; Hamdy, M.; Abdelnaby, M.A. Conversion of end-of-life cotton banknotes into liquid fuel using mini-pyrolysis plant. J. Clean. Prod. 2020, 267, 121612. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Damayanti, D.; Wulandari, Y.R.; Wu, H.-S. Product Distribution of Chemical Product Using Catalytic Depolymerization of Lignin. Bull. Chem. React. Eng. Catal. 2020, 15, 432–453. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenerg. 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Rittfors, J. Thermochemical Textile Recycling: Investigation of Pyrolysis and Gasification of Cotton and Polyester; Chalmers tekniska högskola: Göteborg, Sweden, 2020. [Google Scholar]
- Yousef, S.; Kalpokaitė-Dičkuvienė, R.; Baltušnikas, A.; Pitak, I.; Lukošiūtė, S.-I. A new strategy for functionalization of char derived from pyrolysis of textile waste and its application as hybrid fillers (CNTs/char and graphene/char) in cement industry. J. Clean. Prod. 2021, 128058. [Google Scholar] [CrossRef]
- Jagdale, P.; Nair, J.R.; Khan, A.; Armandi, M.; Meligrana, G.; Hernandez, F.R.; Rusakova, I.; Piatti, E.; Rovere, M.; Tagliaferro, A. Waste to life: Low-cost, self-standing, 2D carbon fiber green Li-ion battery anode made from end-of-life cotton textile. Electrochim. Acta 2021, 368, 137644. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A.R. Pre-formed activated carbon matting derived from the pyrolysis of biomass natural fibre textile waste. J. Anal. Appl. Pyrolysis 2003, 70, 563–577. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A.R. High grade activated carbon matting derived from the chemical activation and pyrolysis of natural fibre textile waste. J. Anal. Appl. Pyrolysis 2004, 71, 971–986. [Google Scholar] [CrossRef]
- Reed, A.R.; Williams, P.T. Thermal processing of biomass natural fibre wastes by pyrolysis. Int. J. Energy Res. 2004, 28, 131–145. [Google Scholar] [CrossRef]
- Xu, Z.; Gu, S.; Sun, Z.; Zhang, D.; Zhou, Y.; Gao, Y.; Qi, R.; Chen, W. Synthesis of char-based adsorbents from cotton textile waste assisted by iron salts at low pyrolysis temperature for Cr (VI) removal. Environ. Sci. Pollut. Res. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Eimontas, J.; Yousef, S.; Striūgas, N.; Abdelnaby, M.A. Catalytic pyrolysis kinetic behaviour and TG-FTIR-GC–MS analysis of waste fishing nets over ZSM-5 zeolite catalyst for caprolactam recovery. Renew. Energy 2021, 179, 1385–1403. [Google Scholar] [CrossRef]
- da Silva, J.E.; Calixto, G.Q.; de Araújo Medeiros, R.L.B.; de Freitas Melo, M.A.; de Araújo Melo, D.M.; de Carvalho, L.P.; Braga, R.M. Colored cotton wastes valuation through thermal and catalytic reforming of pyrolysis vapors (Py-GC/MS). Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, J.; Ding, J.; Wang, X.; Sun, Y.; Ruan, R.; Ragauskas, A.J.; Ok, Y.S.; Tsang, D.C. Promoting Diels-Alder reactions to produce bio-BTX: Co-aromatization of textile waste and plastic waste over USY zeolite. J. Clean. Prod. 2021, 127966. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Mohamed, A.; Abdelnaby, M.A. Morphology, compositions, thermal behavior and kinetics of pyrolysis of lint-microfibers generated from clothes dryer. J. Anal. Appl. Pyrolysis 2021, 155, 105037. [Google Scholar] [CrossRef]
- Gholamzad, E.; Karimi, K.; Masoomi, M. Effective conversion of waste polyester–cotton textile to ethanol and recovery of polyester by alkaline pretreatment. Chem. Eng. Sci. 2014, 253, 40–45. [Google Scholar] [CrossRef]
- Subramanian, K.; Sarkar, M.K.; Wang, H.; Qin, Z.-H.; Chopra, S.S.; Jin, M.; Kumar, V.; Chen, C.; Tsang, C.-W.; Lin, C.S.K. An overview of cotton and polyester, and their blended waste textile valorisation to value-added products: A circular economy approach–research trends, opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2021, 1–22. [Google Scholar] [CrossRef]
- Nikolić, S.; Lazić, V.; Veljović, Đ.; Mojović, L. Production of bioethanol from pre-treated cotton fabrics and waste cotton materials. Carbohydr. Polym. 2017, 164, 136–144. [Google Scholar] [CrossRef]
- Yang, T.C.; Kumaran, J.; Amartey, S.; Maki, M.; Li, X.; Lu, F.; Qin, W. Biofuels and bioproducts produced through microbial conversion of biomass. Bioenergy Res. Adv. Appl. 2014, 71–93. [Google Scholar] [CrossRef]
- Elsayed, S.; Hellsten, S.; Guizani, C.; Witos, J.; Rissanen, M.; Rantamäki, A.H.; Varis, P.; Wiedmer, S.K.; Sixta, H. Recycling of superbase-based ionic liquid solvents for the production of textile-grade regenerated cellulose fibers in the lyocell process. ACS Sustain. Chem. Eng. 2020, 8, 14217–14227. [Google Scholar] [CrossRef]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.-f.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, C.; Nakagawa, T.; Asada, C.; Nakamura, Y. Microwave-assisted hydrolysis of cotton waste to glucose in combination with the concentrated sulfuric acid impregnation method. Waste Biomass Valoriz. 2020, 11, 4279–4287. [Google Scholar] [CrossRef]
- Hasanzadeh, E.; Mirmohamadsadeghi, S.; Karimi, K. Enhancing energy production from waste textile by hydrolysis of synthetic parts. Fuel 2018, 218, 41–48. [Google Scholar] [CrossRef]
- Keskin, T.; Abubackar, H.N.; Arslan, K.; Azbar, N. Biohydrogen production from solid wastes. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2019; pp. 321–346. [Google Scholar]
- Dimos, K.; Paschos, T.; Louloudi, A.; Kalogiannis, K.G.; Lappas, A.A.; Papayannakos, N.; Kekos, D.; Mamma, D. Effect of various pretreatment methods on bioethanol production from cotton stalks. Fermentation 2019, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekhar, B.; Mishra, M.S.; Sharma, K.; Dubey, S. Bio-ethanol production from textile cotton waste via dilute acid hydrolysis and fermentation by Saccharomyces cerevisiae. J. Ecobiotechnol. 2011, 3, 6–9. [Google Scholar]
- Binczarski, M.J.; Malinowska, J.; Stanishevsky, A.; Severino, C.J.; Yager, R.; Cieslak, M.; Witonska, I.A. A Model Procedure for Catalytic Conversion of Waste Cotton into Useful Chemicals. Materials 2021, 14, 1981. [Google Scholar] [CrossRef] [PubMed]
- Quartinello, F.; Vecchiato, S.; Weinberger, S.; Kremenser, K.; Skopek, L.; Pellis, A.; Guebitz, G.M. Highly selective enzymatic recovery of building blocks from wool-cotton-polyester textile waste blends. Polymers 2018, 10, 1107. [Google Scholar] [CrossRef] [Green Version]
- Shen, F.; Xiao, W.; Lin, L.; Yang, G.; Zhang, Y.; Deng, S. Enzymatic saccharification coupling with polyester recovery from cotton-based waste textiles by phosphoric acid pretreatment. Bioresour. Technol. 2013, 130, 248–255. [Google Scholar] [CrossRef]
- Kuo, C.H.; Lin, P.J.; Lee, C.K. Enzymatic saccharification of dissolution pretreated waste cellulosic fabrics for bacterial cellulose production by Gluconacetobacter xylinus. J. Chem. Technol. Biotechnol. 2010, 85, 1346–1352. [Google Scholar] [CrossRef]
- Yousef, S.; Kuliešienė, N.; Sakalauskaitė, S.; Nenartavičius, T.; Daugelavičius, R. Sustainable green strategy for recovery of glucose from end-of-life euro banknotes. Waste Manag. 2021, 123, 23–32. [Google Scholar] [CrossRef]
- Piribauer, B.; Bartl, A.; Ipsmiller, W. Enzymatic textile recycling–best practices and outlook. Waste Manag. Res. 2021, 39, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Pelaz, B.; Chakraborty, I.; Parak, W.J. Investigating possible enzymatic degradation on polymer shells around inorganic nanoparticles. Int. J. Mol. Sci. 2019, 20, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feliu, N.; Docter, D.; Heine, M.; Del Pino, P.; Ashraf, S.; Kolosnjaj-Tabi, J.; Macchiarini, P.; Nielsen, P.; Alloyeau, D.; Gazeau, F. In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 2016, 45, 2440–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soenen, S.J.; Himmelreich, U.; Nuytten, N.; Pisanic, T.R.; Ferrari, A.; De Cuyper, M. Intracellular nanoparticle coating stability determines nanoparticle diagnostics efficacy and cell functionality. Small 2010, 6, 2136–2145. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.B. Covalent enzyme-substrate intermediates in transferase reactions. Bioorg. Chem. 1973, 2, 311–321. [Google Scholar] [CrossRef]
- Chanana, M.; Rivera_Gil, P.; Correa-Duarte, M.A.; Liz-Marzán, L.M.; Parak, W.J. Physicochemical properties of protein-coated gold nanoparticles in biological fluids and cells before and after proteolytic digestion. Angew. Chem. Int. Ed. 2013, 52, 4179–4183. [Google Scholar] [CrossRef]
- Duff, S.J.; Murray, W.D. Bioconversion of forest products industry waste cellulosics to fuel ethanol: A review. Bioresour. Technol. 1996, 55, 1–33. [Google Scholar] [CrossRef]
- Olguin-Maciel, E.; Singh, A.; Chable-Villacis, R.; Tapia-Tussell, R.; Ruiz, H.A. Consolidated bioprocessing, an innovative strategy towards sustainability for biofuels production from crop residues: An overview. Agronomy 2020, 10, 1834. [Google Scholar] [CrossRef]
- Damayanti, D.; Supriyadi, D.; Amelia, D.; Saputri, D.R.; Devi, Y.L.L.; Auriyani, W.A.; Wu, H.S. Conversion of Lignocellulose for Bioethanol Production, Applied in Bio-Polyethylene Terephthalate. Polymers 2021, 13, 2886. [Google Scholar] [CrossRef]
- Dahnum, D.; Tasum, S.O.; Triwahyuni, E.; Nurdin, M.; Abimanyu, H. Comparison of SHF and SSF processes using enzyme and dry yeast for optimization of bioethanol production from empty fruit bunch. Energy Procedia 2015, 68, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Leong, Y.K.; Chew, K.W.; Chen, W.-H.; Chang, J.-S.; Show, P.L. Reuniting the Biogeochemistry of Algae for a Low-Carbon Circular Bioeconomy. Trends Plant Sci. 2021. [Google Scholar] [CrossRef]
- Parisutham, V.; Kim, T.H.; Lee, S.K. Feasibilities of consolidated bioprocessing microbes: From pretreatment to biofuel production. Bioresour. Technol. 2014, 161, 431–440. [Google Scholar] [CrossRef]
- Hasunuma, T.; Kondo, A. Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocellulose to ethanol with thermotolerant yeast strains. Process. Biochem. 2012, 47, 1287–1294. [Google Scholar] [CrossRef]
- Okamoto, K.; Uchii, A.; Kanawaku, R.; Yanase, H. Bioconversion of xylose, hexoses and biomass to ethanol by a new isolate of the white rot basidiomycete Trametes versicolor. Springerplus 2014, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynd, L.R.; Van Zyl, W.H.; McBride, J.E.; Laser, M. Consolidated bioprocessing of cellulosic biomass: An update. Curr. Opin. Biotechnol. 2005, 16, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Jeihanipour, A.; Taherzadeh, M.J. Ethanol production from cotton-based waste textiles. Bioresour. Technol. 2009, 100, 1007–1010. [Google Scholar] [CrossRef]
- Sanchis-Sebastiá, M.; Novy, V.; Stigsson, L.; Galbe, M.; Wallberg, O. Towards circular fashion–transforming pulp mills into hubs for textile recycling. RSC Advances 2021, 11, 12321–12329. [Google Scholar] [CrossRef]
- Sanchis-Sebastiá, M.; Ruuth, E.; Stigsson, L.; Galbe, M.; Wallberg, O. Novel sustainable alternatives for the fashion industry: A method of chemically recycling waste textiles via acid hydrolysis. J. Waste Manag. 2021, 121, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Jeihanipour, A.; Karimi, K.; Niklasson, C.; Taherzadeh, M.J. A novel process for ethanol or biogas production from cellulose in blended-fibers waste textiles. J. Waste Manag. 2010, 30, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Lin, P.-J.; Wu, Y.-Q.; Ye, L.-Y.; Yang, D.-J.; Shieh, C.-J.; Lee, C.-K. Simultaneous saccharification and fermentation of waste textiles for ethanol production. BioResources 2014, 9, 2866–2875. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, S.; Vancov, T.; Palmer, J.; Morris, S. Ethanol production from cotton gin trash using optimised dilute acid pretreatment and whole slurry fermentation processes. Bioresour. Technol. 2014, 173, 42–51. [Google Scholar] [CrossRef]
- Yildirir, E. Chemical Recycling of Waste Plastics via Hydrothermal Processing. Ph.D. Thesis, University of Leeds, Leeds, UK, 2015. [Google Scholar]
- Wikberg, H.; Grönberg, V.; Jermakka, J.; Kemppainen, K.; Kleen, M.; Laine, C.; Paasikallio, V.; Oasmaa, A. Hydrothermal refining of biomass: An overview and future perspectives. Tappi J. 2015, 14, 195–207. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Lin, D.; Han, Y.; Chen, C.-T.A.; Wang, D.; Lin, Y.-S.; Sun, J.; Zheng, Q.; Jiao, N. Cultivation-independent and cultivation-dependent analysis of microbes in the shallow-sea hydrothermal system off Kueishantao Island, Taiwan: Unmasking heterotrophic bacterial diversity and functional capacity. Front. Microbiol. 2018, 9, 279. [Google Scholar] [CrossRef]
- Hongthong, S.; Leese, H.S.; Allen, M.J.; Chuck, C.J. Assessing the Conversion of Various Nylon Polymers in the Hydrothermal Liquefaction of Macroalgae. Environments 2021, 8, 34. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, X.; Yao, Z.; Chen, X. The properties and combustion behaviors of hydrochars derived from co-hydrothermal carbonization of sewage sludge and food waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Ling, C.; Shi, S.; Yan, Z.; Zhang, M.; Zhang, B.; Dai, J. Separation and characterization of waste cotton/polyester blend fabric with hydrothermal method. Fibers Polym. 2018, 19, 742–750. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, M.; Ling, C.; Hou, W.; Yan, Z. Extraction and characterization of microcrystalline cellulose from waste cotton fabrics via hydrothermal method. J. Waste Manag. 2018, 82, 139–146. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z. A mechanism study on hydrothermal carbonization of waste textile. Energy Fuels 2016, 30, 7746–7754. [Google Scholar] [CrossRef]
- Li, Y.; Shao, M.; Huang, M.; Sang, W.; Zheng, S.; Jiang, N.; Gao, Y. Enhanced remediation of heavy metals contaminated soils with EK-PRB using β-CD/hydrothermal biochar by waste cotton as reactive barrier. Chemosphere 2022, 286, 131470. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Yuan, Z.; Zhang, D.; Sun, Z.; Huang, Y.; Chen, W.; Tian, D.; Deng, H.; Zhou, Y. Fabrication of cotton textile waste-based magnetic activated carbon using FeCl 3 activation by the Box–Behnken design: Optimization and characteristics. RSC Adv. 2018, 8, 38081–38090. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhou, Y.; Sun, Z.; Zhang, D.; Huang, Y.; Gu, S.; Chen, W. Understanding reactions and pore-forming mechanisms between waste cotton woven and FeCl3 during the synthesis of magnetic activated carbon. Chemosphere 2020, 241, 125120. [Google Scholar] [CrossRef]
- Kawamura, K.; Sako, K.; Ogata, T.; Tanabe, K. Environmentally friendly, hydrothermal treatment of mixed fabric wastes containing polyester, cotton, and wool fibers: Application for HMF production. Bioresour. Technol. Rep. 2020, 11, 100478. [Google Scholar] [CrossRef]
- Asaoka, Y.; Funazukuri, T. Hydrothermal saccharification of cotton cellulose in dilute aqueous formic acid solution. Res. Chem. Intermed. 2011, 37, 233–242. [Google Scholar] [CrossRef]
- Keh, E.Y.M.; Yao, L.; Liao, X.; Liu, Y.; Cheuk, K.; Chan, A. Method for Separating and Recycling a Waste Polyester-Cotton Textile by Means of a Hydrothermal Reaction Catalyzed by an Organic Acid. U.S. Patent 2020/0262108 A1, 20 August 2020. [Google Scholar]
- McKinney, R. Ammonolysis of Nylon. US 5302756A, 12 April 1994. [Google Scholar]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef] [Green Version]
- Labaki, M.; Jeguirim, M. Thermochemical conversion of waste tyres—A review. Environ. Sci. Pollut. Res. 2017, 24, 9962–9992. [Google Scholar] [CrossRef]
- Samolada, M.; Zabaniotou, A. Potential application of pyrolysis for the effective valorisation of the end of life tires in Greece. Environ. Dev. 2012, 4, 73–87. [Google Scholar] [CrossRef]
- Portofino, S.; Donatelli, A.; Iovane, P.; Innella, C.; Civita, R.; Martino, M.; Matera, D.A.; Russo, A.; Cornacchia, G.; Galvagno, S. Steam gasification of waste tyre: Influence of process temperature on yield and product composition. J. Waste Manag. 2013, 33, 672–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasin, S. Eco-Design for End-of-Life Phase of Flame Retardant Textiles. Ph.D. Thesis, Université Lille 1-Sciences et Technologies, Lille, France, 2017. [Google Scholar]
- Cañete Vela, I.; Maric, J.; Seemann, M. Valorisation of textile waste via steam gasification in a fluidized bed reactor. In Proceedings of the 7th International Conference on Sustainable Solid Waste Management, Crete Island, Greece, 26–29 June 2019. [Google Scholar]
- Wen, C.; Wu, Y.; Chen, X.; Jiang, G.; Liu, D. The pyrolysis and gasification performances of waste textile under carbon dioxide atmosphere. J. Therm. Anal. Calorim. 2017, 128, 581–591. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, C.; Chen, X.; Jiang, G.; Liu, G.; Liu, D. Catalytic pyrolysis and gasification of waste textile under carbon dioxide atmosphere with composite Zn-Fe catalyst. Fuel Process. Technol. 2017, 166, 115–123. [Google Scholar] [CrossRef]
- Ziyaei, M.D.; Barikani, M.; Honarkar, H. Recycling of Polyethylene Terephthalate (PET) via Glycolysis Method for Synthesis Waterborne Polyurethane. In Proceedings of the International Seminar on Polymer Science and Technology, Tehran, Iran, 10–22 November 2018; pp. 520–523. [Google Scholar]
- Guo, Z.; Adolfsson, E.; Tam, P.L. Nanostructured micro particles as a low-cost and sustainable catalyst in the recycling of PET fiber waste by the glycolysis method. J. Waste Manag. 2021, 126, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabagh, A.; Yehia, F.; Eshaq, G.; Rabie, A.; ElMetwally, A. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Heiran, R.; Ghaderian, A.; Reghunadhan, A.; Sedaghati, F.; Thomas, S.; hossein Haghighi, A. Glycolysis: An efficient route for recycling of end of life polyurethane foams. J. Polym. Res. 2021, 28, 1–19. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eissa, A.-M.M.; Moustafa, M.E.; Eshaq, G.; Rabie, A.-R.M.; ElMetwally, A.E. Glycolysis of poly (ethylene terephthalate) catalyzed by the Lewis base ionic liquid [Bmim][OAc]. Ind. Eng. Chem. Res. 2014, 53, 18443–18451. [Google Scholar] [CrossRef]
- Guo, Z.; Eriksson, M.; de la Motte, H.; Adolfsson, E. Circular recycling of polyester textile waste using a sustainable catalyst. J. Clean. Prod. 2021, 283, 124579. [Google Scholar] [CrossRef]
- Hommez, B.; Goethals, E.J. Degradation of nylon-6 by glycolysis. Part 1: Identification of degradation products. J. Macromol. Sci.—Pure Appl. Chem. 1998, 35, 1489–1505. [Google Scholar] [CrossRef]
- Huczkowski, P.; Kapko, J.; Olesiak, R. Degradation of nylon-6 in ethylene glycol. Polymer 1978, 19, 77–80. [Google Scholar] [CrossRef]
- Huczkowski, P.; Kapko, J. Degradation of nylon-6 in ethylene glycol: 2. Mathematical illustration of degradation. Polymer 1980, 21, 86–88. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. Thermal degradation of nylon polymers. Polym. Int. 2000, 49, 943–948. [Google Scholar] [CrossRef]
- Kim, K.J.; Dhevi, D.M.; Lee, J.S.; Dal Cho, Y.; Choe, E.K. Mechanism of glycolysis of nylon 6, 6 and its model compound by ethylene glycol. Polym. Degrad. Stab. 2006, 91, 1545–1555. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Zhang, X.; Qian, J.; Xing, X.; Wang, X. Synthesis of poly (ethylene terephthalate) based on glycolysis of waste PET fiber. J. Macromol. Sci. A 2020, 57, 430–438. [Google Scholar] [CrossRef]
- Shukla, S.; Harad, A.M. Glycolysis of polyethylene terephthalate waste fibers. J. Appl. Polym. Sci. 2005, 97, 513–517. [Google Scholar] [CrossRef]
- Maryan, A.S.; Montazer, M.; Damerchely, R. Discoloration of denim garment with color free effluent using montmorillonite based nano clay and enzymes: Nano bio-treatment on denim garment. J. Clean. Prod. 2015, 91, 208–215. [Google Scholar] [CrossRef]
- Groves, E.; Palenik, C.S.; Palenik, S. A survey of extraction solvents in the forensic analysis of textile dyes. Forensic Sci. Int. 2016, 268, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Yang, Y. Complete separation of colorants from polymeric materials for cost-effective recycling of waste textiles. Chem. Eng. Sci. 2022, 427, 131570. [Google Scholar] [CrossRef]
- Haslinger, S.; Wang, Y.; Rissanen, M.; Lossa, M.B.; Tanttu, M.; Ilen, E.; Määttänen, M.; Harlin, A.; Hummel, M.; Sixta, H. Recycling of vat and reactive dyed textile waste to new colored man-made cellulose fibers. Green Chem. 2019, 21, 5598–5610. [Google Scholar] [CrossRef] [Green Version]
- Padmanaban, A.; Murugadoss, G.; Venkatesh, N.; Hazra, S.; Rajesh Kumar, M.; Tamilselvi, R.; Sakthivel, P. Electrochemical determination of harmful catechol and rapid decolorization of textile dyes using ceria and tin doped ZnO nanoparticles. J. Environ. Chem. Eng. 2021, 9, 105976. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Lu, J.; Li, X.; Ge, M. Decoloration of waste PET alcoholysis liquid by an electrochemical method. Water Sci. Technol. 2018, 77, 2463–2473. [Google Scholar] [CrossRef]
- Huang, J.; Yan, D.; Dong, H.; Li, F.; Lu, X.; Xin, J. Removal of trace amount impurities in glycolytic monomer of polyethylene terephthalate by recrystallization. J. Environ. Chem. Eng. 2021, 106277. [Google Scholar] [CrossRef]


| Textile | Analyzer | Mathematic Algorithms | Recognition Rate, % | Ref. |
|---|---|---|---|---|
| Pure (polyester slash; wool; cotton; polyester normal; nylon). Mix (polyester/nylon; polyester/wool; polyester/cotton slash; polyester/cotton). | NIR | SVM, MLP + CNN | 92–98 | [74] |
| Cotton, polyester, polyamide, acrylic, silk, wool. | NIR | SIMCA | 93–97 | [79] |
| Polypropylene, polyethylene terephthalate, polylactic acid, cashmere, Tencel, cotton, wool | NIR | PCA + SIMCA | 100 | [80] |
| Wool | NIR | PLS, ECR | [81] | |
| Satin, twill, plain | ResNet-50 | CNN | 99.3 | [82] |
| Satin, twill, plain | Epson Scan V330 | TILT, HOG, FCM clustering, histogram equalization data | 94.5 | [83] |
| Non-woven fabric, plain weave, twill weave, double jersey, satin weave | Canon scanner 9950F, Self-organizing map (SOM) network | CIE + Co-occurrence matrix | 92.6 | [84] |
| Cotton, polyester, viscose | NIR | NA | 76–100 | [85] |
| Company | Material | Method | Country | Ref. |
|---|---|---|---|---|
| Ambercycle | Textile | Enzymatic treatment | Los Angeles | [110] |
| BlockTexx | Cotton and polyester (PET) | Fiber separation technology | Australia | [111] |
| FENC | PET and textile | Hydrolysis | Taiwan | [112] |
| Infinited Fiber | Cellulose and polyester (PET) | Alkaline extraction | Finland | [113,114] |
| Ioncell | Cotton and polyester (PET) | Ionic liquid solvent polymer dissolution | Finland | [115] |
| Lenzing | Pre-consumer cotton and post-consumer garments | Closed loops and chemical recovery | Austria | [116] |
| Tyton BioSciences | Cotton pulp, polyester (PET), poly-cotton blends | Subcritical water treatment | Danville | [117] |
| Worn Again Technologies | Cotton and polyester (PET) | Dissolution polymer Solvent separation | UK | [118] |
| Type of Technology | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Pyrolysis |
|
| [132,133] |
| Enzymatic Hydrolysis |
|
| [120] |
| Hydrothermal |
|
| [134,135,136] |
| Gasification |
|
| [137] |
| Glycolysis |
|
| [138] |
| Ammonolysis |
|
| [139] |
| Raw Materials | Reactors | Catalyst | Reaction Time, Min | Temperature, °C | Yield (%) | Major Products | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Solid | Liquid | Gas | |||||||
| Egyptian banknote ELCBs Cotton, 100% | Batch | NA | 30 | 700 | 15.65 | 29.28 | 55.07 | 2-Propanone, toluene, 3-furaldehyde, 2-furalmethanol, benzaldehyde, phenol, acetophenone | [147] |
| Hemp, 100% | Batch | NA | 120 | 750 | 24 | 48.5 | 27.50 | Activated carbon | [155] |
| Flax, 100% | Fixed bed | ZnCl2 | 220 | 450 | 44.80 | NA | NA | Activated carbon | [156] |
| Egyptian banknote ELCBs Cotton, 100% | Batch | NA | 30 | 600 | 17.26 | 30.28 | 52.46 | 1,3-Dioxolane-2-propanal, 2-methyl, furfural, 2-furanmethanol, 4,4-ethylenedioxy-1-pentylamine | [147] |
| Flax, 100% | Fixed bed | ZnCl2 | 220 | 450 | 44.80 | NA | NA | Activated carbon | [156] |
| Cotton, 70% Polyester, 30% | Batch | Dye-Originating Heavy Metals | 35 | 500–700 | 17.79 | 37.59 | 44.62 | Activated carbon | [147] |
| Egyptian banknote ELCBs Cotton, 100% | Batch | NA | 30 | 500 | 20.74 | 39.75 | 39.51 | Toluene, furfural, 2-furanmethanol, 2-furancarboxaldehyde, 5-methyl | [147] |
| Hemp, 100% | Fixed bed | ZnCl2 | 220 | 450 | 41.60 | NA | NA | Activated carbon | [156] |
| Cotton, 100% | Fixed bed | Na2CO3 | 120 | 600 | 16.25 | 29.49 | 54.26 | Furans, ketones | [130] |
| Coir, 100% | Fixed bed | NA | 240 | 800 | 37.40 | 47.40 | 18.20 | Activated carbon | [157] |
| Abaca, 100% | Fixed bed | NA | 240 | 800 | 28.60 | 48.10 | 23.60 | Activated carbon | [157] |
| Cotton, 100% | Batch | NA | 70 | 700 | 12.50 | 74.00 | 13.50 | Double bond carboxyl and carbonyl liquid | [143] |
| Low Grade biomass fiber (Flax, 100%) | Batch | NA | 120 | 900 | 20 | 55 | 25 | Activated carbon | [155] |
| Flax, 100% | Fixed bed | H3PO4 | 220 | 450 | 39.20 | NA | NA | Activated carbon | [156] |
| Biomass fiber waste Jute, 100% | Fixed bed | NA | 240 | 800 | 24.60 | 59.60 | 15.90 | Activated carbon | [157] |
| Textile Materials | Type of Pretreatment | Condition of Pretreatment | Enzyme | Condition of Hydrolysis | Glucose Yield, % | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| T (°C) | T (h) | T (°C) | T (h) | |||||
| Cotton, 90% Wool, 5% Polyester, 5% | Protease (Enzymatic) | 50 | 48 | Cellic CTec3® and Savinase 12T® | 50 | 70 | 95.0 | [175] |
| Used Jeans | Phosphoric acid (Acid) | 50 | 7 | Cellulase Trichoderma reesei and Aspergillus niger | 50 | 96 | 79.2 | [176] |
| Cotton red T-shirt, 100% cotton black T-shirt, 100% | N-methyl-morpholine-N-oxide& Phosphoric Acid (Acid) | 50 | 1 | Cellulase AP3, Aspergillus niger | 50 | 72 | 87.0–95.0 | [177] |
| Cotton red T-shirt, 100% cotton black T-shirt, 100% | NaOH/Urea (Base) | 50 | 1 | Cellulase AP3, Aspergillus niger | 50 | 72 | 48.0–55.0 | [177] |
| Cotton T-shirts, 100% | 1-allyl-3-methylimidazolium chloride (Ionic liquid) | 110 | 1.5 | G. xylinus (Acetobacter aceti subsp. xylinus or A. xylinus) | 50 | 24 | 81.6 | [168] |
| Towels, cellulose content 87.8% | Untreated Pretreatment | NA | NA | Cellulase | 200 | 0.03 | 74.2 | [169] |
| Waste blue jeans (polyester/cotton), 40%/60% | Sodium carbonate (Base) | 150 | 2 | Celluclast 1.5 L and β-glucosidase | 45 | 72 | 81.71 | [170] |
| Cotton, 100% | Sodium carbonate (Base) | 150 | 2 | Celluclast 1.5 L and β-glucosidase | 45 | 72 | 88.0 | [170] |
| Textile from End-of-life euro banknotes | NaOH/Urea (Base) | −20 | 6 | Cellulase | 50 | 382 | 96.0 | [178] |
| Textile l | Type of Pretreatment | Hydrolysis | Enzyme for Hydrolysis | Fermentation Process | Microorganism | Type of Fermentation | EthanolYield, % | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | T (h) | T (°C) | T (h) | |||||||
| Polyester/cotton (50%/50%) | N-methyl morpholine-N-oxide (Cellulose solvent) | NA | 48 | Cellulase and β-glucosidase | 30 | 24 | Saccharomyces cerevisiae | SHF | 91.91 | [197] |
| Polyester/cotton (40%/60%) | NaOH/Urea (Alkali) | 45 | 72 | 30 FPU C ellulase and 60 IU β-glucosidase | 36 | 72 | Saccharomyces cerevisiae | SSF | 70.00 | [163] |
| Cotton, 100% | Na2S2O4 and Na2CO3 (Alkali) | 50 | 48 | Cellulase AP3 | 37 | 48 | Zymomonas mobilis | SSF | 90.00 | [198] |
| Viscose/polyester (60%/40%) | N-methylmorpholine-N-oxide (Cellulose solvent) | NA | 48 | Cellulase and β-glucosidase | 30 | 24 | Saccharomyces cerevisiae | SHF | 94.99 | [197] |
| Cotton, 100% | Na2CO3 (Alkali) | 45 | 72 | Cellulase and β-glucosidase | 32 | 24 | Saccharomyces cerevisiae CCUG 53310 | NA | 69.40 | [170] |
| Cotton ginning trash, 100% | Sulfuric acid (Acid) | 50 | 96 | Cellic CTec 2 cellulase | 30 | NA | Saccharomyces cerevisiae | SSF | 70.00 | [199] |
| Feedstock | Reactor | Catalyst | Time, h | Temperature, °C | Major Product | Yield, % | Ref. |
|---|---|---|---|---|---|---|---|
| A blue cotton/polyester, | Batch | Hydrochloric acid | 3 | 150 | Cellulose powder | 49.3 | [205] |
| Waste cotton fabrics | Batch | Hydrochloric acid | 1.6 | 150 | Microcrystalline cellulose | 85.5 | [206] |
| Cotton/synthetic fibers, | Autoclave | NA | 90 | 280 | Volatile compound (CH4, C2H4) | 98.0 | [207] |
| Waste cottons | Hydrothermal reactor | β-cyclodextrin | 3 | 250 | Activated carbon | NA | [208] |
| Cotton textile waste | Tubular | Ferric chloride | 1 | 700 | Nanopowder | 32.6 | [209] |
| Waste cotton woven | Tubular | Ferric chloride | 1.5 | 700 | Activated carbon | NA | [210] |
| Light blue and white uniform with polyester/cotton, | Hydrothermal reactor | Citric acid | 1 | 225 | 5′-hydroxymethylfurfural | 12.5 | [211] |
| Cotton | Semi-batch | Formic acid | 1 | 250 | Glucose | 83.8 | [212] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damayanti, D.; Wulandari, L.A.; Bagaskoro, A.; Rianjanu, A.; Wu, H.-S. Possibility Routes for Textile Recycling Technology. Polymers 2021, 13, 3834. https://doi.org/10.3390/polym13213834
Damayanti D, Wulandari LA, Bagaskoro A, Rianjanu A, Wu H-S. Possibility Routes for Textile Recycling Technology. Polymers. 2021; 13(21):3834. https://doi.org/10.3390/polym13213834
Chicago/Turabian StyleDamayanti, Damayanti, Latasya Adelia Wulandari, Adhanto Bagaskoro, Aditya Rianjanu, and Ho-Shing Wu. 2021. "Possibility Routes for Textile Recycling Technology" Polymers 13, no. 21: 3834. https://doi.org/10.3390/polym13213834
APA StyleDamayanti, D., Wulandari, L. A., Bagaskoro, A., Rianjanu, A., & Wu, H.-S. (2021). Possibility Routes for Textile Recycling Technology. Polymers, 13(21), 3834. https://doi.org/10.3390/polym13213834