Precise Synthesis and Thermoresponsive Property of Poly(ethyl glycidyl ether) and Its Block and Statistic Copolymers with Poly(glycidol)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PBnGE-stat-PEGE
2.3. Synthesis of PG-stat-PEGE
2.4. Synthesis of PBnGE-b-PEGE
2.5. Synthesis of PG-b-PEGE
2.6. Characterization
3. Results and Discussion
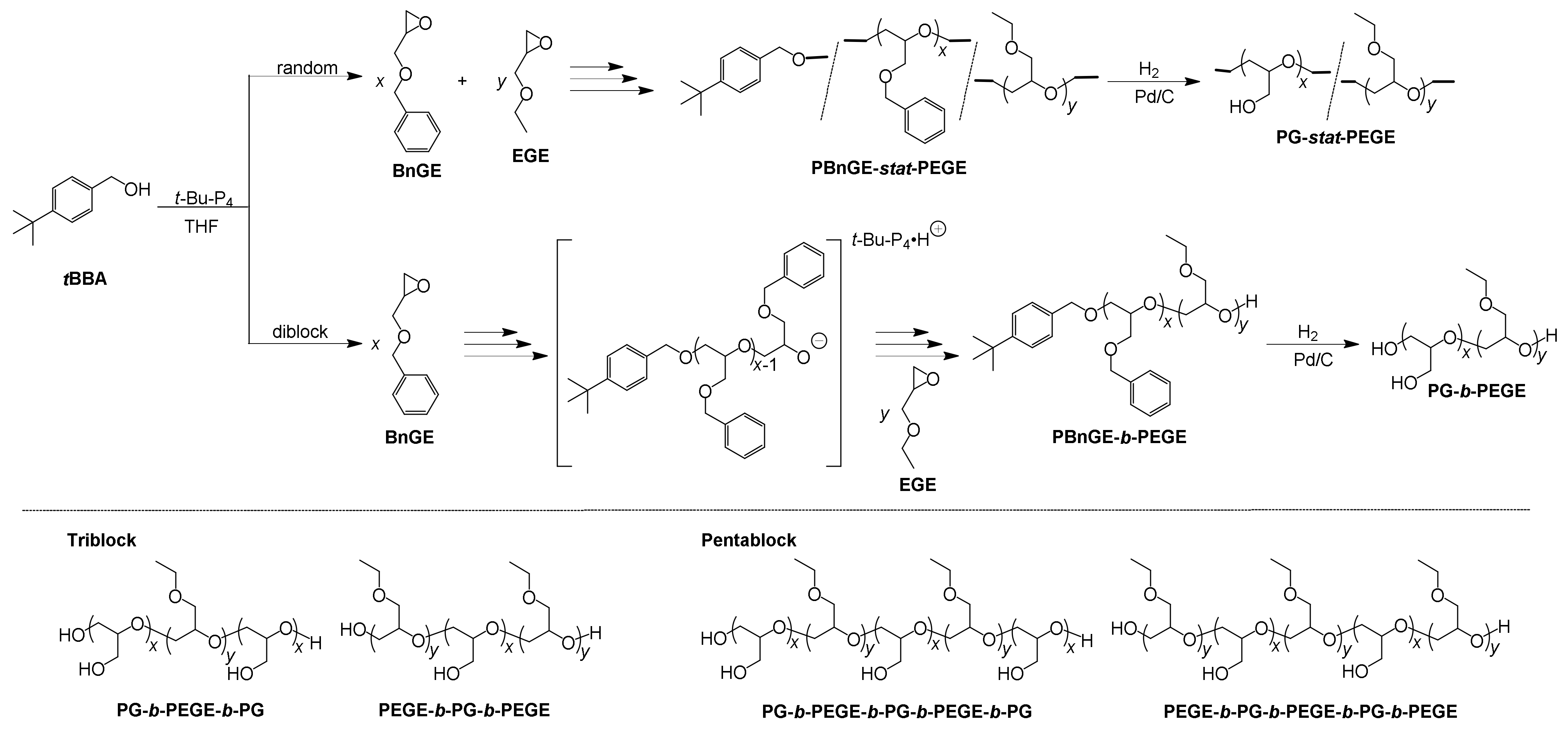
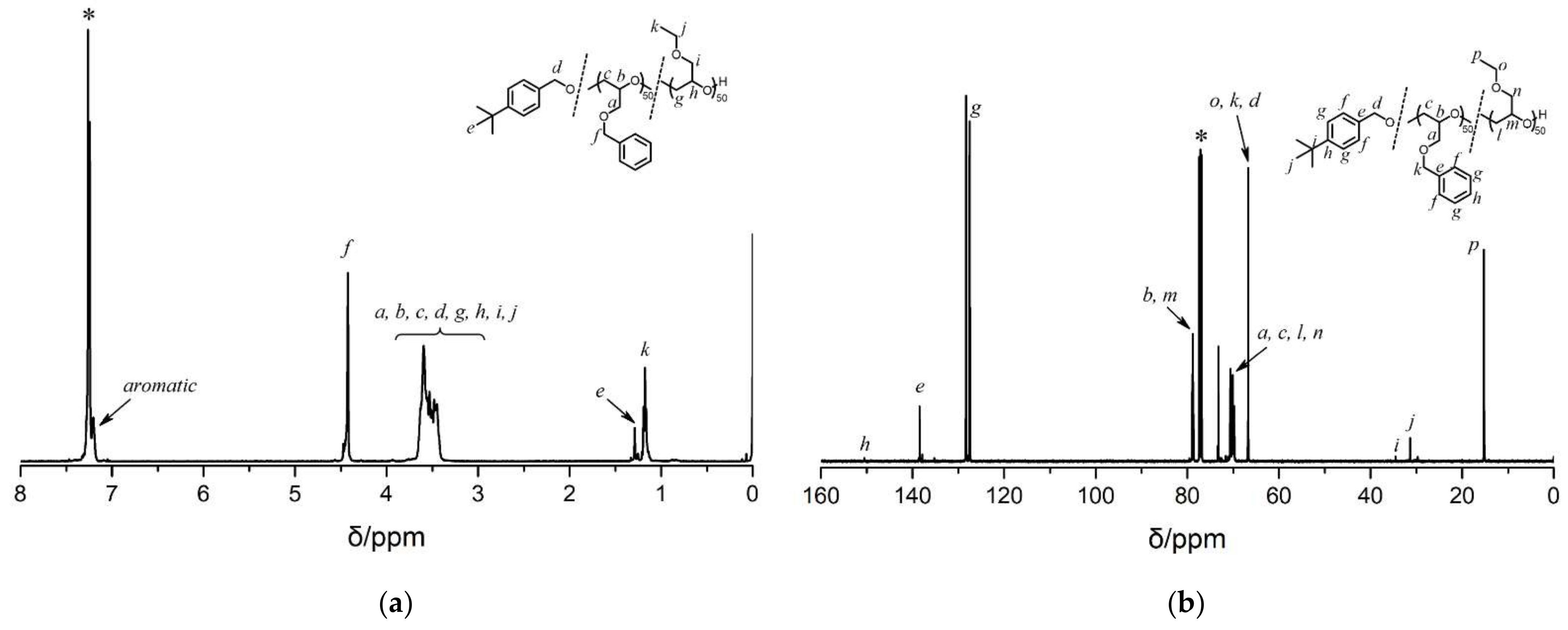
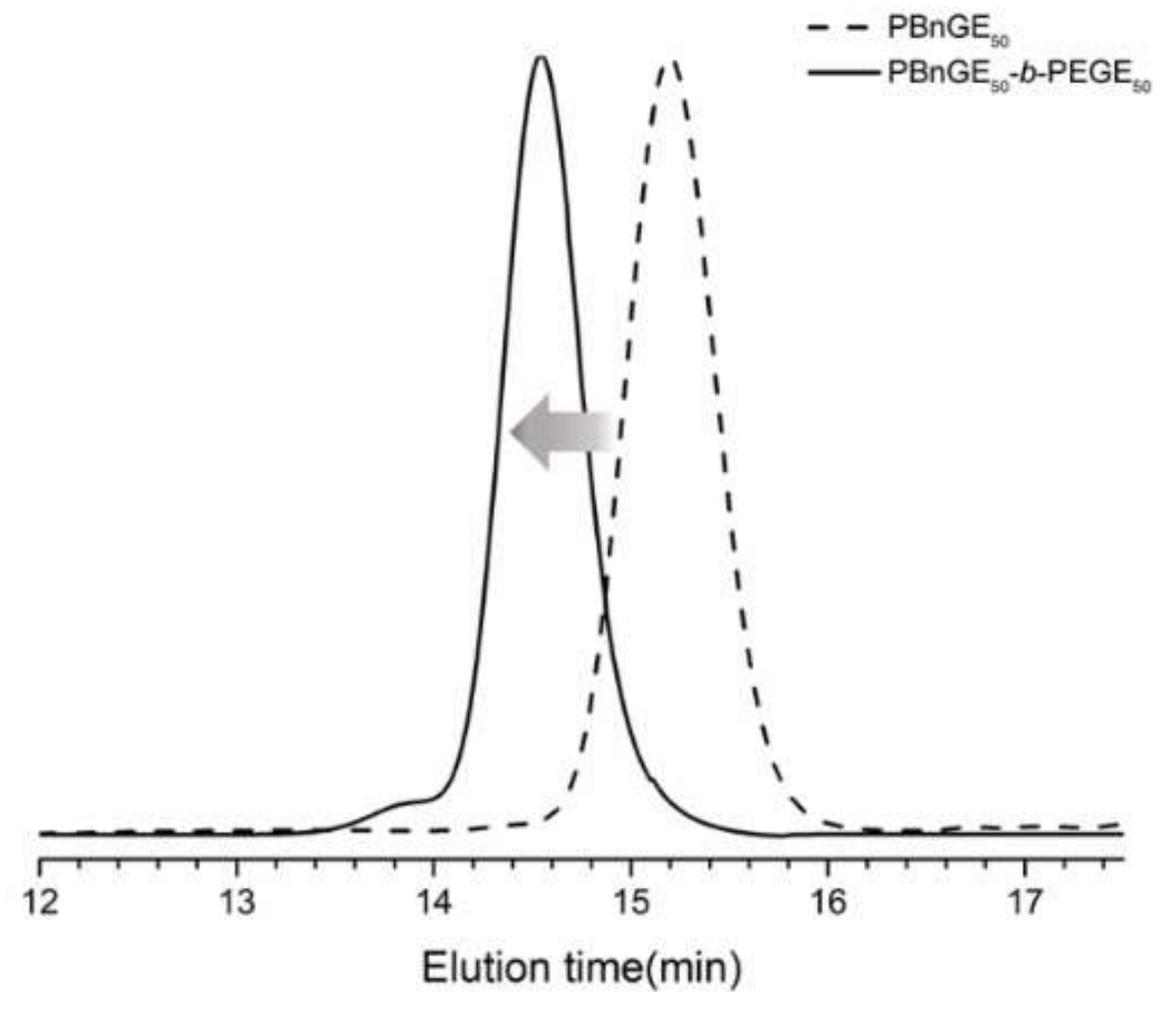
3.1. Synthesis of Statistic Copolymers and Block Copolymers
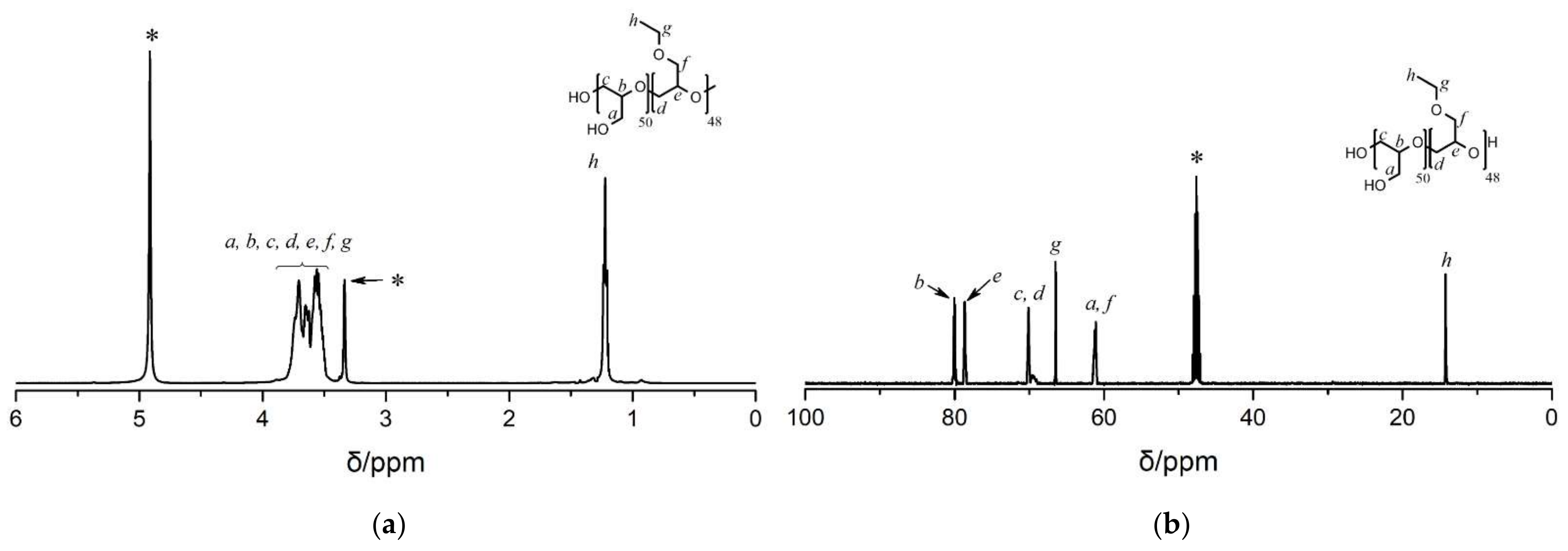
3.2. Synthesis and Characterizations of PG-stat-PEGE and PG-b-PEGE
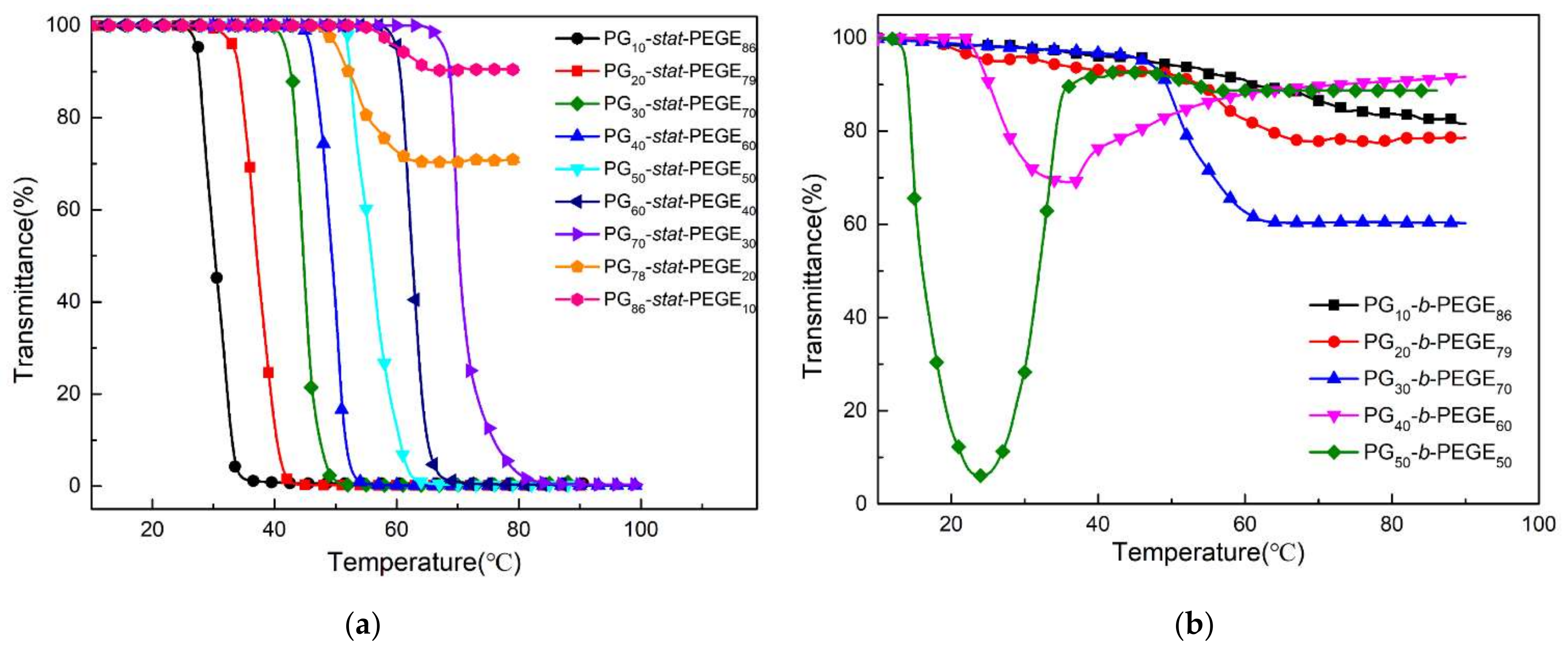
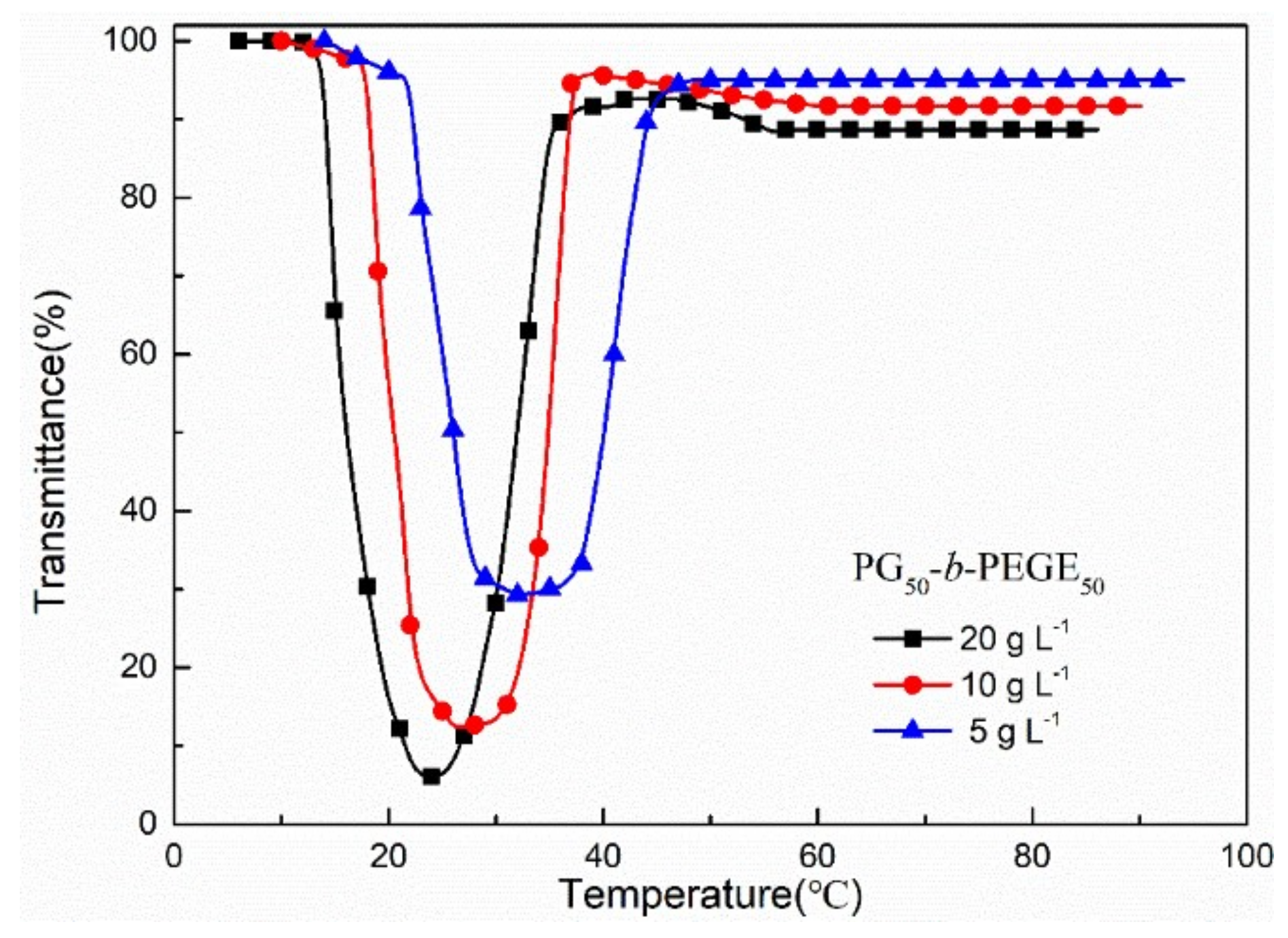
3.3. Thermoresponsive Properties of PGx-stat-PEGEy and PGx-b-PEGEy
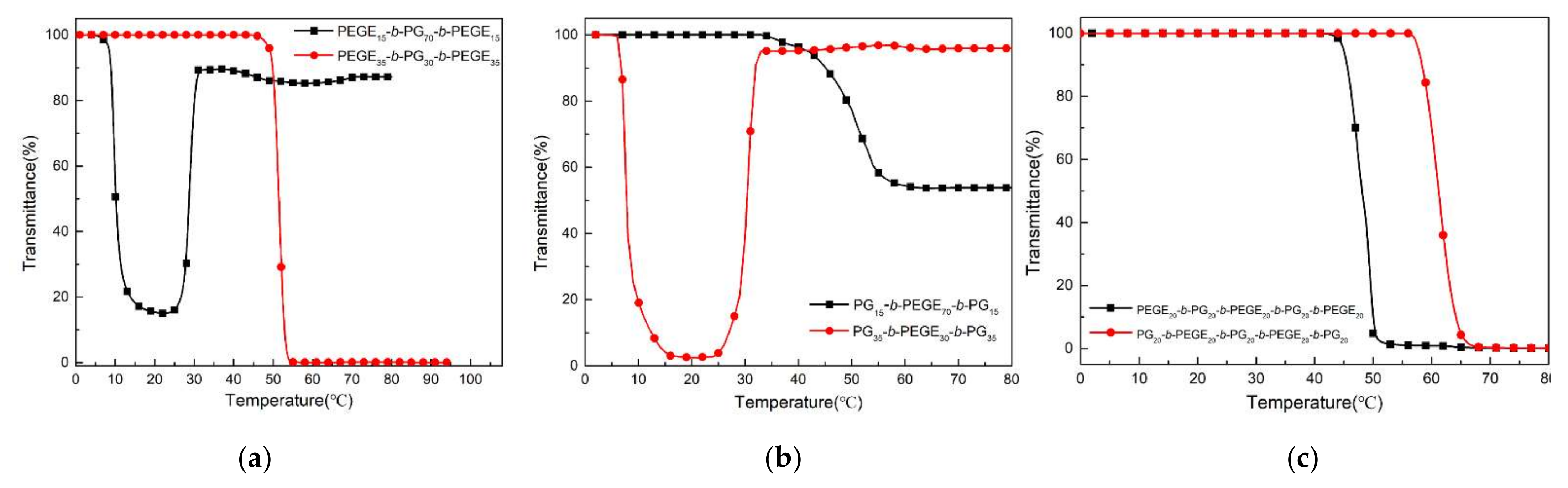
3.4. Thermoresponsive Properties of Multiblock Copolymer and Homopolymer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, R.; Tian, J.; Guan, Y.; Zhang, Y. Extraordinarily Large LCST Depression Converts Nonthermosensitive Polymer to Thermosensitive. Macromolecules 2019, 52, 365–375. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Hodorog, A.D.R.; Ibanescu, C.; Moleavin, L.; Hurduc, N. Thermo-responsiveness of polysiloxanes grafted with poly(dimethyl acrylamide) segments. Cen. Eur. J. Chem. 2012, 10, 1338–1348. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Liu, S. Responsive Polymers for Detection and Sensing Applications: Current Status and Future Developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.; McCormick, C.L. Stimuli-responsive amphiphilic (co)polymers via RAFT polymerization. Prog. Polym. Sci. 2010, 35, 45–91. [Google Scholar] [CrossRef]
- Labbe, A.; Carlotti, S.; Deffieux, A.; Hirao, A. Controlled Polymerization of Glycidyl Methyl Ether Initiated by Onium Salt/Triisobutylaluminum and Investigation of the Polymer LCST. In Macromolecular Symposia; Wiley-VCH: Weinheim, Germany, 2007; Volume 249, pp. 392–397. [Google Scholar]
- Lee, S.; Lee, J.-S.; Lee, C.H.; Jung, Y.-S.; Kim, J.-M. Nonpolymeric Thermosensitive Benzenetricarboxamides. Langmuir 2011, 27, 1560–1564. [Google Scholar] [CrossRef]
- Ifuku, S.; Kadla, J.F. Preparation of a Thermosensitive Highly Regioselective Cellulose/N-Isopropylacrylamide Copolymer through Atom Transfer Radical Polymerization. Biomacromolecules 2008, 9, 3308–3313. [Google Scholar] [CrossRef]
- Danko, M.; Kronekova, Z.; Mrlik, M.; Osicka, J.; Yousaf, A.B.; Durackova, A.; Tkac, J.; Kasak, P. Sulfobetaines Meet Carboxybetaines: Modulation of Thermo- and Ion-Responsivity, Water Structure, Mechanical Properties, and Cell Adhesion. Langmuir 2019, 35, 1391–1430. [Google Scholar] [CrossRef]
- Woodfield, P.A.; Zhu, Y.; Pei, Y.; Roth, P.J. Hydrophobically Modified Sulfobetaine Copolymers with Tunable Aqueous UCST through Postpolymerization Modification of Poly(pentafluorophenyl acrylate). Macromolecules 2014, 47, 750–762. [Google Scholar] [CrossRef] [Green Version]
- Lewoczko, E.M.; Wang, N.; Lundberg, C.E.; Kelly, M.T.; Kent, E.W.; Wu, T.; Chen, M.-L.; Wang, J.-H.; Zhao, B. Effects of N-Substituents on the Solution Behavior of Poly(sulfobetaine methacrylate)s in Water: Upper and Lower Critical Solution Temperature Transitions. ACS Appl. Polym. Mater. 2021, 3, 867–878. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, N.; Fukuoka, M.; Yoshida, K.; Duan, Q.; Satoh, T.; Kakuchi, T. Synthesis and thermoresponsive properties of four-arm star-shaped poly(N-isopropylacrylamide)s bearing covalent and non-covalent cores. Polym. Chem. 2015, 6, 3608–3616. [Google Scholar] [CrossRef] [Green Version]
- Kolouchova, K.; Lobaz, V.; Benes, H.; Rosa, V.R.D.L.; Babuka, D.; Svec, P.; Cernoch, P.; Hruby, M.; Hoogenboom, R.; Stepanek, P.; et al. Thermoresponsive properties of polyacrylamides in physiological solutions. Polym. Chem. 2021, 12, 5077–5084. [Google Scholar] [CrossRef]
- Suzuki, T.; Kato, T.; Shinozaki, H. Photo-reversible Pb2+complexation of thermosensitive poly(N-isopropyl acrylamide-co-spiropyran acrylate) in water. Chem. Commun. 2004, 18, 2036–2037. [Google Scholar] [CrossRef] [PubMed]
- Beattie, D.A.; Mensah, J.A.; Beaussart, A.; Franks, G.V.; Yeap, K.-Y. In situ particle film ATR FTIR spectroscopy of poly(N-isopropyl acrylamide) (PNIPAM) adsorption onto talc. Phys. Chem. Chem. Phys. 2014, 16, 25143–25151. [Google Scholar] [CrossRef]
- Futscher, M.H.; Philipp, M.; Buschbaum, P.M.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 17012–17022. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.-W.; Chu, W.-C.; Chen, J.-K.; Kuo, S.-W. Complementary multiple hydrogen bonds stabilize thermo-sensitive supramolecular structures prepared from poly(N-isopropyl acrylamide) and adenine-functionalized poly(ethylene oxide). Eur. Polym. J. 2014, 50, 168–176. [Google Scholar] [CrossRef]
- Penas, A.G.; Wang, Y.; Bonilla, A.M.; García, M.F.; Stadler, F.J. Lower Critical Solution Temperature Sensitivity to Structural Changes in Poly(N-Isopropyl Acrylamide) Homopolymers. J. Polym. Sci. B Polym. Phys. 2019, 57, 1386–1393. [Google Scholar] [CrossRef]
- Swanson, J.P.; Cruz, M.A.; Monteleone, L.R.; Martinez, M.R.; Costanzo, P.J.; Joy, A. The Effect of Pendant Group Structure on the Thermoresponsive Properties of N-Substituted Polyesters. Polym. Chem. 2017, 8, 7195–7206. [Google Scholar] [CrossRef]
- Chen, S.; Wang, K.; Zhang, W. A new thermoresponsive polymer of poly(N-acryloylsarcosine methyl ester) with tunable LCST. Polym. Chem. 2017, 8, 3090–3101. [Google Scholar] [CrossRef]
- Tong, J.-G.; Wei, Z.-Y.; Yang, H.-L.; Yang, Z.-Y.; Chen, Y. Study on the phase transition behaviors of thermoresponsive hyperbranched polyampholytes in water. Polymer 2016, 84, 107–116. [Google Scholar] [CrossRef]
- Dong, Q.; Luo, C.; Li, N.; Chi, J.; Zhang, Q. Temperature and Recognition Dual Responsive Poly(N-Isopropylacrylamide) and Poly(N, N-Dimethylacrylamide) with Adamantyl Side Group. Materials 2018, 11, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Chao, H.; Li, G.; Tang, R.; Liu, Z.; Liu, Z.; Jiang, J. Backbone-Based LCST-Type Hyperbranched Poly(oligo(ethylene glycol)) with CO2-Reversible Iminoboronate Linkers. Macromol. Chem. Phys. 2018, 219, 1800346. [Google Scholar] [CrossRef]
- Topp, M.D.C.; Dijkstra, P.J.; Talsma, H.; Feijen, J. Thermosensitive Micelle-Forming Block Copolymers of Poly(ethylene glycol) and Poly(N-isopropylacrylamide). Macromolecules 1997, 30, 8518–8520. [Google Scholar] [CrossRef]
- Pelletier, M.; Babin, J.; Tremblay, L.; Zhao, Y. Investigation of a New Thermosensitive Block Copolymer Micelle: Hydrolysis, Disruption, and Release. Langmuir 2008, 24, 12664–12670. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, J.; Jiang, X.; Zhu, Z.; Rao, J.; Yin, J.; Wu, T.; Liu, H.; Liu, S. Thermosensitive Unimolecular Micelles Surface-Decorated with Gold Nanoparticles of Tunable Spatial Distribution. Chem. Mater. 2007, 19, 2489–2494. [Google Scholar] [CrossRef]
- Huynh, V.; Ifraimov, N.; Wylie, R.G. Modulating the Thermoresponse of Polymer-Protein Conjugates with Hydrogels for Controlled Release. Polymers 2021, 13, 2772. [Google Scholar] [CrossRef] [PubMed]
- Karesoja, M.; Karjalainen, E.; Hietala, H.; Tenhu, H. Phase Separation of Aqueous Poly(2-dimethylaminoethyl methacrylate-block-N-vinylcaprolactams). J. Phys. Chem. B 2014, 118, 10776–10784. [Google Scholar] [CrossRef]
- Marsili, L.; Bo, M.D.; Eisele, G.; Donati, I.; Berti, F.; Toffoli, G. Characterization of Thermoresponsive Poly-N-Vinylcaprolactam Polymers for Biological Applications. Polymers 2021, 13, 2639. [Google Scholar] [CrossRef]
- Kehrle, J.; Hçhlein, I.M.D.; Yang, Z.; Jochem, A.-R.; Helbich, T.; Kraus, T.; Veinot, J.G.C.; Rieger, B. Thermoresponsive and Photoluminescent Hybrid Silicon Nanoparticles by Surface-Initiated Group Transfer Polymerization of Diethyl Vinylphosphonate. Angew. Chem. Int. Ed. 2014, 53, 12494–12497. [Google Scholar]
- Grishkewich, N.; Akhlaghi, S.P.; Yao, Z.; Berry, R.; Tam, K.C. Cellulose nanocrystal-poly(oligo(ethylene glycol) methacrylate) brushes with tunable LCSTs. Carbohydr. Polym. 2016, 144, 215–222. [Google Scholar] [CrossRef]
- Moers, C.; Wrazidlo, R.; Natalello, A.; Netz, I.; Mondeshki, M.; Frey, H. (1-Adamantyl) methyl Glycidyl Ether: A Versatile Building Block for Living Polymerization. Macromol. Rapid Commun. 2014, 35, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.S.; Moers, C.; Frey, H. A Challenging Comonomer Pair: Copolymerization of Ethylene Oxide and Glycidyl Methyl Ether to Thermoresponsive Polyethers. Macromolecules 2017, 47, 5490–5500. [Google Scholar] [CrossRef]
- Isono, T.; Miyachi, K.; Satoh, Y.; Sato, S.; Kakuchia, T.; Satoh, T. Design and synthesis of thermoresponsive aliphatic polyethers with a tunable phase transition temperature. Polym. Chem. 2017, 8, 5698–5707. [Google Scholar] [CrossRef]
- Aoki, S.; Koide, A.; Imabayashi, S.; Watanabe, M. Novel Thermosensitive Polyethers Prepared by Anionic Ring-Opening Polymerization of Glycidyl Ether Derivatives. Chem. Lett. 2002, 32, 1128–1129. [Google Scholar] [CrossRef]
- Heinen, S.; Rackow, S.; Schafer, A.; Weinhart, M. A Perfect Match: Fast and Truly Random Copolymerization of Glycidyl Ether Monomers to Thermoresponsive Copolymers. Macromolecules 2016, 50, 44–53. [Google Scholar] [CrossRef]
- Ogura, M.; Tokuda, H.; Imabayashi, S.; Watanabe, M. Preparation and Solution Behavior of a Thermoresponsive Diblock Copolymer of Poly(ethyl glycidyl ether) and Poly(ethylene oxide). Langmuir 2007, 23, 9429–9434. [Google Scholar] [CrossRef]
- Reinicke, S.; Schmelz, J.; Lapp, A.; Karg, M.; Hellweg, T.; Schmalz, H. Smart hydrogels based on double responsive triblock terpolymers. Soft Matter. 2009, 5, 2648–2657. [Google Scholar] [CrossRef]
- Weinhart, M.; Becherer, T.; Haag, R. Switchable, biocompatible surfaces based on glycerol copolymers. Chem. Commun. 2011, 47, 1553–1555. [Google Scholar] [CrossRef]
- Becherer, T.; Heinen, S.; Wei, Q.; Haag, R.; Weinhart, M. In-Depth Analysis of Switchable Glycerol Based Polymeric Coatings for Cell Sheet Engineering. Acta Biomater. 2015, 25, 43–55. [Google Scholar] [CrossRef]
- Cao, X.; Chen, Y.; Chai, W.; Zhang, W.; Wang, Y.; Fu, P.-F. Thermoresponsive Self-Assembled Nanovesicles Based on Amphiphilic Triblock Copolymers and Their Potential Applications as Smart Drug Release Carriers. J. Appl. Polym. Sci. 2015, 11, 41361. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Xu, L.; Fu, X.; Narumi, A.; Sato, S.; Shen, X.; Kakuchi, T. Poly[glycidyl oligo(oxyethylene)carbamate]s (PGn-EOmR’ and RPGn-EOmR’): Controlled Synthesis and Effects of Molecular Parameters (n and m), Side Group (R’), and End-Group (R) on Thermoresponsive Property. Polym. Chem. 2021, 12, 2580–2591. [Google Scholar] [CrossRef]
- Thomas, A.; Müller, S.S.; Frey, H. Beyond Poly(ethylene glycol): Linear Polyglycerol as a Multifunctional Polyether for Biomedical and Pharmaceutical Applications. Boomacromolecules 2014, 15, 1935–1954. [Google Scholar] [CrossRef] [PubMed]
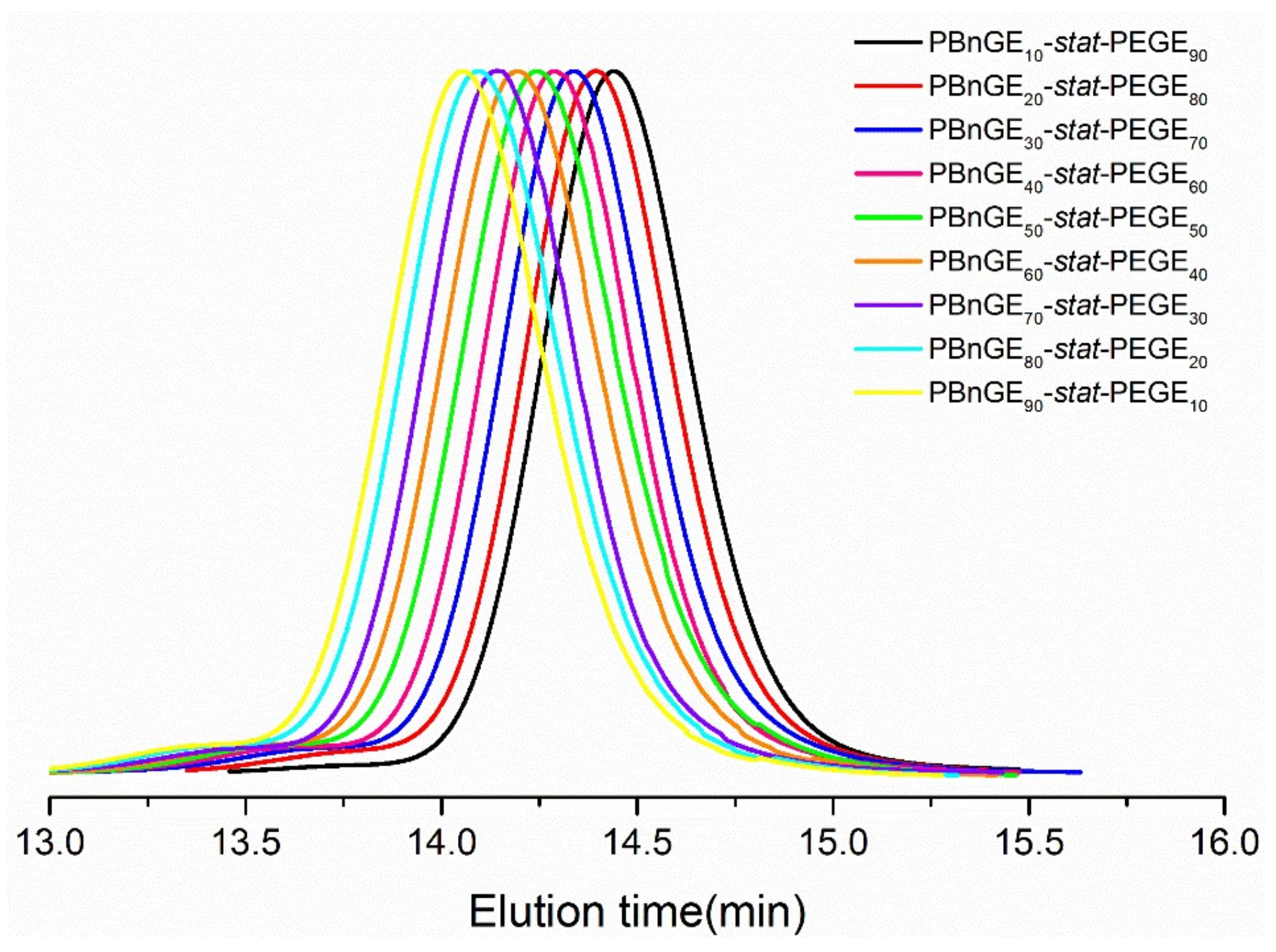


| Entry b | Code | [BnGE]0/[EGE]0 | Mn,calcd (kg mol−1) c | Mn,NMR (kg mol−1) d | Mw/Mn e | x, y f |
|---|---|---|---|---|---|---|
| 1 | PBnGEx-stat-PEGEy | 10/90 | 11.0 | 10.8 | 1.10 | 10, 88 |
| 2 | 20/80 | 11.6 | 11.5 | 1.09 | 20, 79 | |
| 3 | 30/70 | 12.2 | 12.2 | 1.09 | 30, 70 | |
| 4 | 40/60 | 12.9 | 12.9 | 1.08 | 40, 60 | |
| 5 | 50/50 | 13.5 | 13.5 | 1.07 | 50, 50 | |
| 6 | 60/40 | 14.1 | 14.1 | 1.08 | 60, 40 | |
| 7 | 70/30 | 14.7 | 14.7 | 1.08 | 70, 30 | |
| 8 | 80/20 | 15.3 | 15.0 | 1.10 | 78, 20 | |
| 9 | 90/10 | 16.0 | 15.6 | 1.09 | 88, 10 | |
| 10 | PBnGEx-b-PEGEy | 10/90 | 11.0 | 10.8 | 1.08 | 10, 88 |
| 11 | 20/80 | 11.6 | 11.5 | 1.05 | 20, 79 | |
| 12 | 30/70 | 12.2 | 12.2 | 1.04 | 30, 70 | |
| 13 | 40/60 | 12.9 | 12.9 | 1.07 | 40, 60 | |
| 14 | 50/50 | 13.5 | 13.5 | 1.05 | 50, 50 |
| Entry | Starting Material | Product | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | Mw,MALSb (kg mol−1) | Mw/Mnc | Yield (%) | Tcpd (°C) | Dh (nm) e | ||||
| 5 °C | 30 °C | 90 °C | |||||||
| 15 | PBnGEx-stat-PEGEy | PG10-stat-PEGE86 | 9.5 | 1.12 | 93.5 | 30.5 | 10.5 | - | 690 |
| 16 | PG20-stat-PEGE79 | 9.5 | 1.11 | 91.4 | 37.0 | 8.9 | - | 704 | |
| 17 | PG30-stat-PEGE70 | 9.4 | 1.13 | 90.1 | 45.4 | 9.6 | - | 725 | |
| 18 | PG40-stat-PEGE60 | 9.1 | 1.11 | 91.9 | 50.5 | 9.9 | - | 719 | |
| 19 | PG50-stat-PEGE50 | 8.8 | 1.10 | 93.4 | 56.1 | 10.4 | - | 722 | |
| 20 | PG60-stat-PEGE40 | 8.5 | 1.13 | 92.6 | 62.2 | 10.1 | - | 731 | |
| 21 | PG70-stat-PEGE30 | 8.2 | 1.11 | 89.3 | 70.4 | 10.8 | - | 754 | |
| 22 | PG78-stat-PEGE20 | 7.8 | 1.13 | 90.7 | - | 12.3 | - | 92 | |
| 23 | PG86-stat-PEGE10 | 7.4 | 1.12 | 89.0 | - | 15.5 | - | 87 | |
| 24 | PBnGEx-b-PEGEy | PG10-b-PEGE88 | 9.7 | 1.14 | 89.3 | - | 19.3 | - | 54 |
| 25 | PG20-b-PEGE79 | 9.5 | 1.13 | 92.4 | - | 26.8 | - | 76 | |
| 26 | PG30-b-PEGE70 | 9.4 | 1.13 | 93.1 | - | 24.5 | - | 58 | |
| 27 | PG40-b-PEGE60 | 9.1 | 1.12 | 91.3 | - | 32.4 | 178 | 42 | |
| 28 | PG50-b-PEGE50 | 8.8 | 1.13 | 90.5 | - | 38.2 | 268 | 40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Wang, Y.; Narumi, A.; Xu, L.; Sato, S.-i.; Shen, X.; Kakuchi, T. Precise Synthesis and Thermoresponsive Property of Poly(ethyl glycidyl ether) and Its Block and Statistic Copolymers with Poly(glycidol). Polymers 2021, 13, 3873. https://doi.org/10.3390/polym13223873
He T, Wang Y, Narumi A, Xu L, Sato S-i, Shen X, Kakuchi T. Precise Synthesis and Thermoresponsive Property of Poly(ethyl glycidyl ether) and Its Block and Statistic Copolymers with Poly(glycidol). Polymers. 2021; 13(22):3873. https://doi.org/10.3390/polym13223873
Chicago/Turabian StyleHe, Tingyu, Yanqiu Wang, Atsushi Narumi, Liang Xu, Shin-ichiro Sato, Xiande Shen, and Toyoji Kakuchi. 2021. "Precise Synthesis and Thermoresponsive Property of Poly(ethyl glycidyl ether) and Its Block and Statistic Copolymers with Poly(glycidol)" Polymers 13, no. 22: 3873. https://doi.org/10.3390/polym13223873
APA StyleHe, T., Wang, Y., Narumi, A., Xu, L., Sato, S.-i., Shen, X., & Kakuchi, T. (2021). Precise Synthesis and Thermoresponsive Property of Poly(ethyl glycidyl ether) and Its Block and Statistic Copolymers with Poly(glycidol). Polymers, 13(22), 3873. https://doi.org/10.3390/polym13223873







