Nanocomposites of Chitosan/Graphene Oxide/Titanium Dioxide Nanoparticles/Blackberry Waste Extract as Potential Bone Substitutes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Chitosan Beads
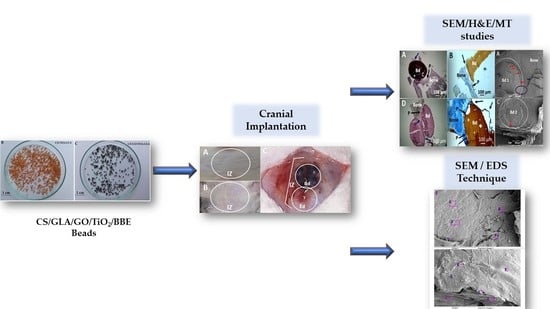
2.3. Critical Bone Defect Studies
3. Results
3.1. Characterization of the Synthesized Chitosan Beads
3.2. Critical Bone Defect Studies
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Florencio-Silva, R.; da Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonzo, M.; Alvarez Primo, F.; Anil Kumar, S.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone tissue engineering techniques, advances, and scaffolds for treatment of bone defects. Curr. Opin. Biomed. Eng. 2021, 17, 100248. [Google Scholar] [CrossRef] [PubMed]
- Dinçel, Y.M. Bone Graft Types. In Bone Grafting-Recent Advances with Special References to Cranio-Maxillofacial Surgery; IntechOpen: London, UK, 2018; ISBN 1789848830. [Google Scholar]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnol. 2020, 18, 23. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Erol-Taygun, M.; Unalan, I.; Idris, M.I.B.; Mano, J.F.; Boccaccini, A.R. Bioactıve Glass-Polymer Nanocomposites for Bone Tıssue Regeneration Applicatıons: A Revıew. Adv. Eng. Mater. 2019, 21, 1900287. [Google Scholar] [CrossRef]
- Idumah, C.I. Progress in polymer nanocomposites for bone regeneration and engineering. Polym. Polym. Compos. 2021, 29, 509–527. [Google Scholar]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2020, 41, 397–402. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, A.A.; Ahmad, A.; Ibrahim, M.N.M.; Rashid, M. Chitosan-based nanocomposites for gene delivery: Application and future perspectives. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 245–262. [Google Scholar]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.; Valencia, C.; Zuluaga, F.; Valencia, M.; Mina, J.; Grande-Tovar, C. Synthesis and Application of Scaffolds of Chitosan-Graphene Oxide by the Freeze-Drying Method for Tissue Regeneration. Molecules 2018, 23, 2651. [Google Scholar] [CrossRef] [Green Version]
- Grande Tovar, D.C.; Castro, I.J.; Valencia, H.C.; Zapata, A.P.; Solano, A.M.; Florez López, E.; Chaur, N.M.; Valencia Zapata, E.M.; Mina Hernandez, H.J. Synthesis of Chitosan Beads Incorporating Graphene Oxide/Titanium Dioxide Nanoparticles for In Vivo Studies. Molecules 2020, 25, 2308. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 183–191. [Google Scholar] [CrossRef]
- Mahshid, S.; Askari, M.; Ghamsari, M.S. Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. J. Mater. Process. Technol. 2007, 189, 296–300. [Google Scholar] [CrossRef]
- Grande-Tovar, C.; Araujo-Pabón, L.; Flórez-López, E.; Aranaga-Arias, C. Determinación de la actividad antioxidante y antimicrobiana de residuos de mora (Rubus glaucus Benth). Inf. Técnico 2021, 85, 64–82. [Google Scholar] [CrossRef]
- McGrath, J.C.; Drummond, G.B.; McLachlan, E.M.; Kilkenny, C.; Wainwright, C.L. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [Green Version]
- Deev, R.V.; Drobyshev, A.Y.; Bozo, I.Y.; Isaev, A.A. Ordinary and activated bone grafts: Applied classification and the main features. Biomed Res. Int. 2015, 2015, 365050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucchi, C.; Del Fabbro, M.; Arias, A.; Fuentes, R.; Mendes, J.M.; Ordonneau, M.; Orti, V.; Manzanares-Céspedes, M.-C. Multicenter study of patients’ preferences and concerns regarding the origin of bone grafts utilized in dentistry. Patient Prefer. Adherence 2019, 13, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanchetta, P.; Lagarde, N.; Uguen, A.; Marcorelles, P. Mixture of hyaluronic acid, chondroitin 6 sulphate and dermatan sulphate used to completely regenerate bone in rat critical size defect model. J. Cranio-Maxillofac. Surg. 2012, 40, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Bouaicha, S.; von Rechenberg, B.; Osterhoff, G.; Wanner, G.A.; Simmen, H.-P.; Werner, C.M.L. Histological remodelling of demineralised bone matrix allograft in posterolateral fusion of the spine—An ex vivo study. BMC Surg. 2013, 13, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Dupoirieux, L.; Pourquier, D.; Picot, M.C.; Neves, M. Comparative study of three different membranes for guided bone regeneration of rat cranial defects. Int. J. Oral Maxillofac. Surg. 2001, 30, 58–62. [Google Scholar] [CrossRef]
- Senos, R.; Hankenson, K.D. Calvaria critical-size defects in rats using piezoelectric equipment: A comparison with the classic trephine. Injury 2020, 51, 1509–1514. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.-L.; Lai, Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Vajgel, A.; Mardas, N.; Farias, B.C.; Petrie, A.; Cimões, R.; Donos, N. A systematic review on the critical size defect model. Clin. Oral Implants Res. 2014, 25, 879–893. [Google Scholar] [CrossRef]
- Nagata, M.J.H.; Santinoni, C.S.; Pola, N.M.; De Campos, N.; Messora, M.R.; Bomfim, S.R.M.; Ervolino, E.; Fucini, S.E.; Faleiros, P.L.; Garcia, V.G. Bone marrow aspirate combined with low-level laser therapy: A new therapeutic approach to enhance bone healing. J. Photochem. Photobiol. B Biol. 2013, 121, 6–14. [Google Scholar] [CrossRef]
- Farnezi Bassi, A.P.; Bizelli, V.F.; de Mendes Brasil, L.F.; Pereira, J.C.; Al-Sharani, H.M.; Momesso, G.A.C.; Faverani, L.P.; de Almeida Lucas, F. Is the Bacterial Cellulose Membrane Feasible for Osteopromotive Property? Membranes 2020, 10, 230. [Google Scholar] [CrossRef]
- Ghiacci, G.; Graiani, G.; Ravanetti, F.; Lumetti, S.; Manfredi, E.; Galli, C.; Cacchioli, A.; Macaluso, G.M.; Sala, R. “Over-inlay” block graft and differential morphometry: A novel block graft model to study bone regeneration and host-to-graft interfaces in rats. J. Periodontal Implant Sci. 2016, 46, 220–233. [Google Scholar] [CrossRef] [Green Version]
- Luvizuto, E.R.; de Oliveira, J.C.S.; Gomes-Ferreira, P.H.S.; Pereira, C.C.S.; Faverani, L.P.; Antoniali, C.; Okamoto, R. Immunohistochemical response in rats of beta-tricalcium phosphate (TCP) with or without BMP-2 in the production of collagen matrix critical defects. Acta Histochem. 2017, 119, 302–308. [Google Scholar] [CrossRef] [Green Version]
- López Tenorio, D.; Valencia, H.C.; Valencia, C.; Zuluaga, F.; Valencia, E.M.; Mina, H.J.; Grande Tovar, D.C. Evaluation of the Biocompatibility of CS-Graphene Oxide Compounds In Vivo. Int. J. Mol. Sci. 2019, 20, 1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, R.; She, Z.; Wang, M.; Yu, X.; Jin, H.; Feng, Q. Repair of rat calvarial bone defects by controlled release of rhBMP-2 from an injectable bone regeneration composite. J. Tissue Eng. Regen. Med. 2012, 6, 614–621. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Q.; Xu, C.-B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int. J. Clin. Exp. Med. 2017, 10, 14904–14910. [Google Scholar]
- Sroga, G.E.; Vashishth, D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr. Osteoporos. Rep. 2012, 10, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuong, J.; Hellmich, C. Bone fibrillogenesis and mineralization: Quantitative analysis and implications for tissue elasticity. J. Theor. Biol. 2011, 287, 115–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezirganlı, Ş.; Kazancıoğlu, H.O.; Mihmanlı, A.; Aydın, M.Ş.; Sharifov, R.; Alkan, A. The effect of local simvastatin application on critical size defects in the diabetic rats. Clin. Oral Implants Res. 2014, 25, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, J.; Tuo, Z.; Ren, G. Repair of long bone defects of large size using a tissue-engineered periosteum in a rabbit model. J. Mater. Sci. Mater. Med. 2021, 32, 105. [Google Scholar] [CrossRef]
- Torquato, L.C.; Suárez, E.A.C.; Bernardo, D.V.; Pinto, I.L.R.; Mantovani, L.O.; Silva, T.I.L.; Jardini, M.A.N.; Santamaria, M.P.; De Marco, A.C. Bone repair assessment of critical size defects in rats treated with mineralized bovine bone (Bio-Oss®) and photobiomodulation therapy: A histomorphometric and immunohistochemical study. Lasers Med. Sci. 2021, 36, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Kim, J.; Lim, J.; Lee, E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology 2010, 74, 777–785. [Google Scholar] [CrossRef] [PubMed]







| Name | System | Formulation |
|---|---|---|
| F1 | CS/GLA | Chitosan, glutaraldehyde |
| F2 | CS/GLA/GO | Chitosan, glutaraldehyde, graphene oxide |
| F3 | CS/GLA/TiO2 | Chitosan, glutaraldehyde, titanium dioxide nanoparticles |
| F4 | CS/GLA/GO/TiO2 | Chitosan, glutaraldehyde, graphene oxide, titanium dioxide nanoparticles |
| F5 | CS/GLA/GO/TiO2/BBE | Chitosan, glutaraldehyde, graphene oxide, titanium dioxide nanoparticles, blackberry waste extract |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Llano, C.H.; Solano, M.A.; Grande-Tovar, C.D. Nanocomposites of Chitosan/Graphene Oxide/Titanium Dioxide Nanoparticles/Blackberry Waste Extract as Potential Bone Substitutes. Polymers 2021, 13, 3877. https://doi.org/10.3390/polym13223877
Valencia-Llano CH, Solano MA, Grande-Tovar CD. Nanocomposites of Chitosan/Graphene Oxide/Titanium Dioxide Nanoparticles/Blackberry Waste Extract as Potential Bone Substitutes. Polymers. 2021; 13(22):3877. https://doi.org/10.3390/polym13223877
Chicago/Turabian StyleValencia-Llano, Carlos Humberto, Moisés A. Solano, and Carlos David Grande-Tovar. 2021. "Nanocomposites of Chitosan/Graphene Oxide/Titanium Dioxide Nanoparticles/Blackberry Waste Extract as Potential Bone Substitutes" Polymers 13, no. 22: 3877. https://doi.org/10.3390/polym13223877