Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Methods
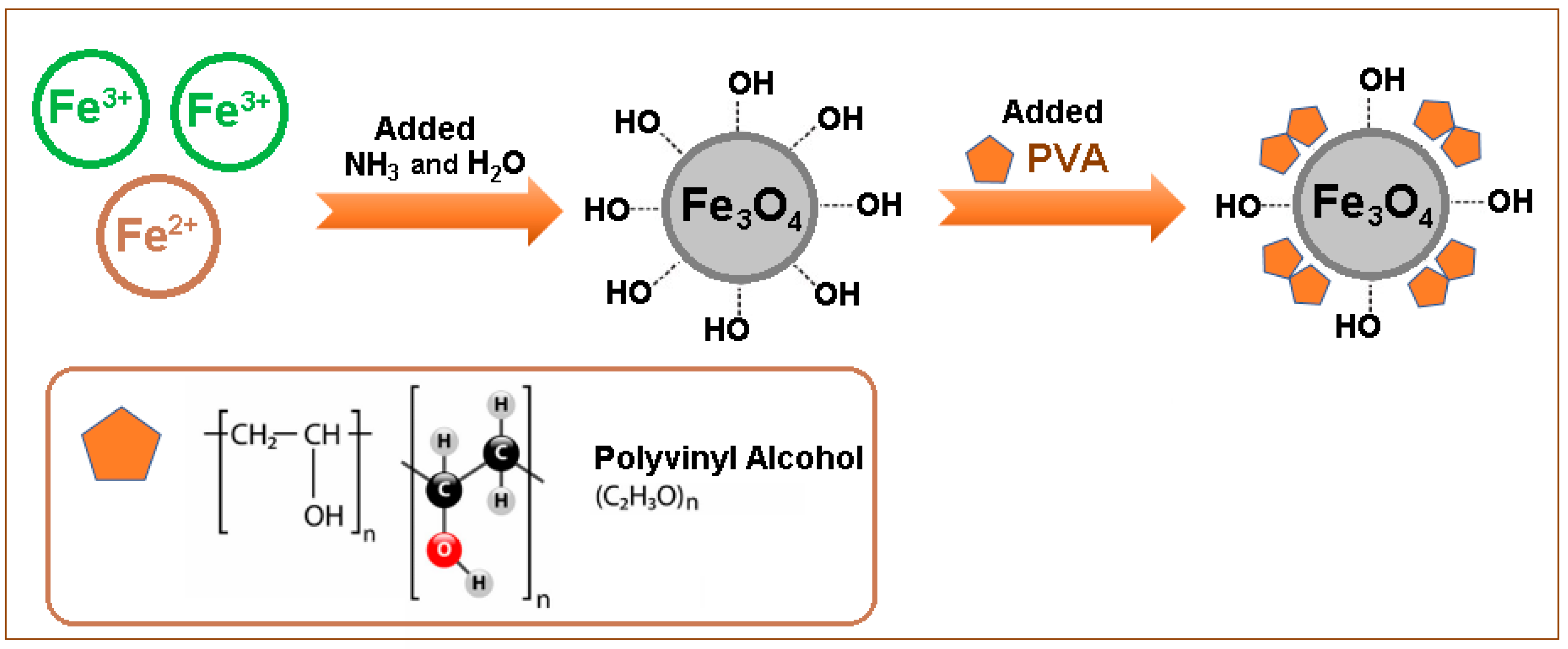
2.2. Synthesis of the Composite
2.3. Photocatalytic Experimental Procedure
2.4. Characterization of the Composites
3. Results and Discussion
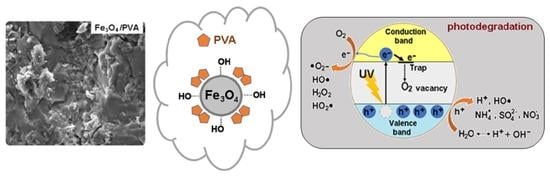
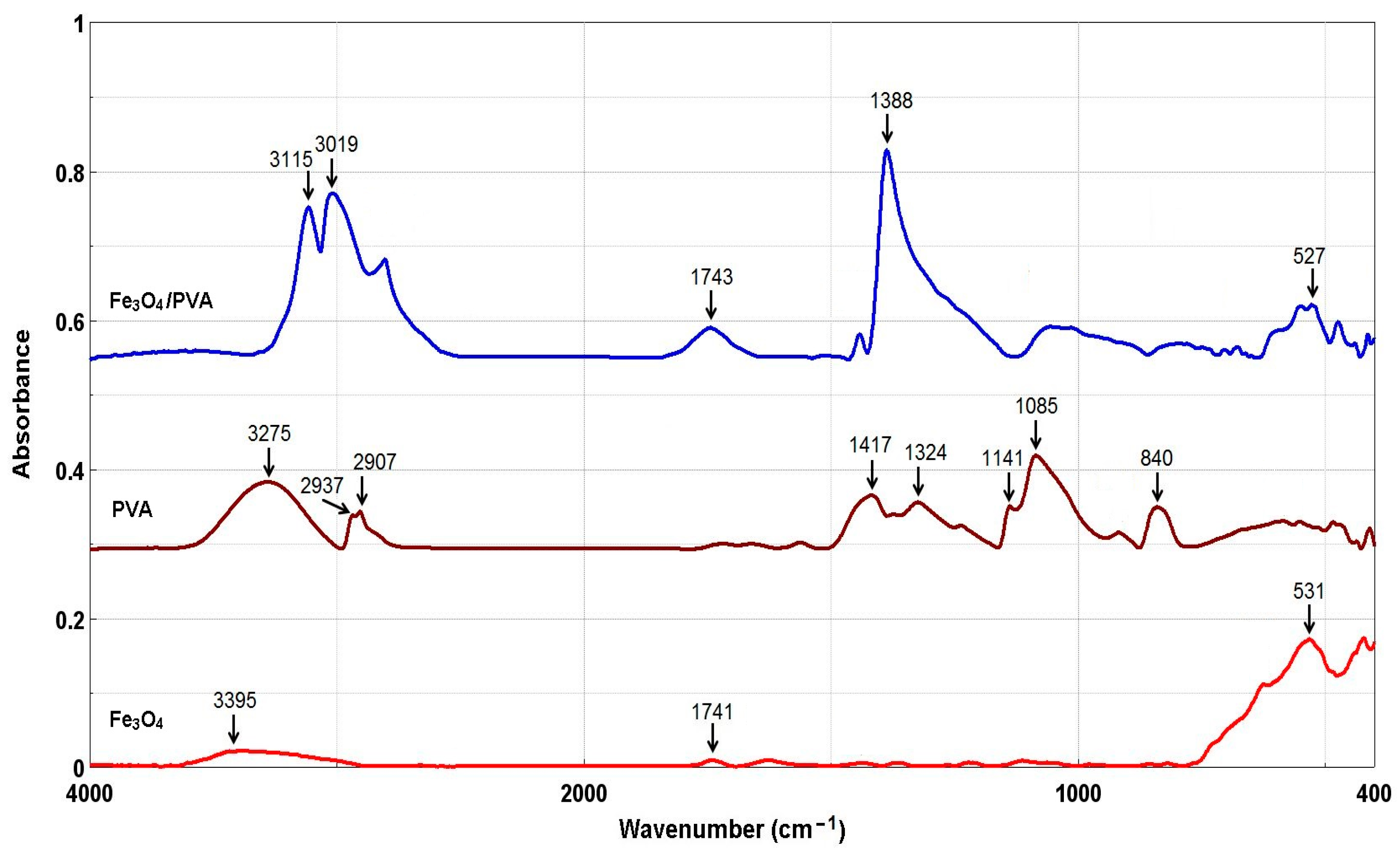
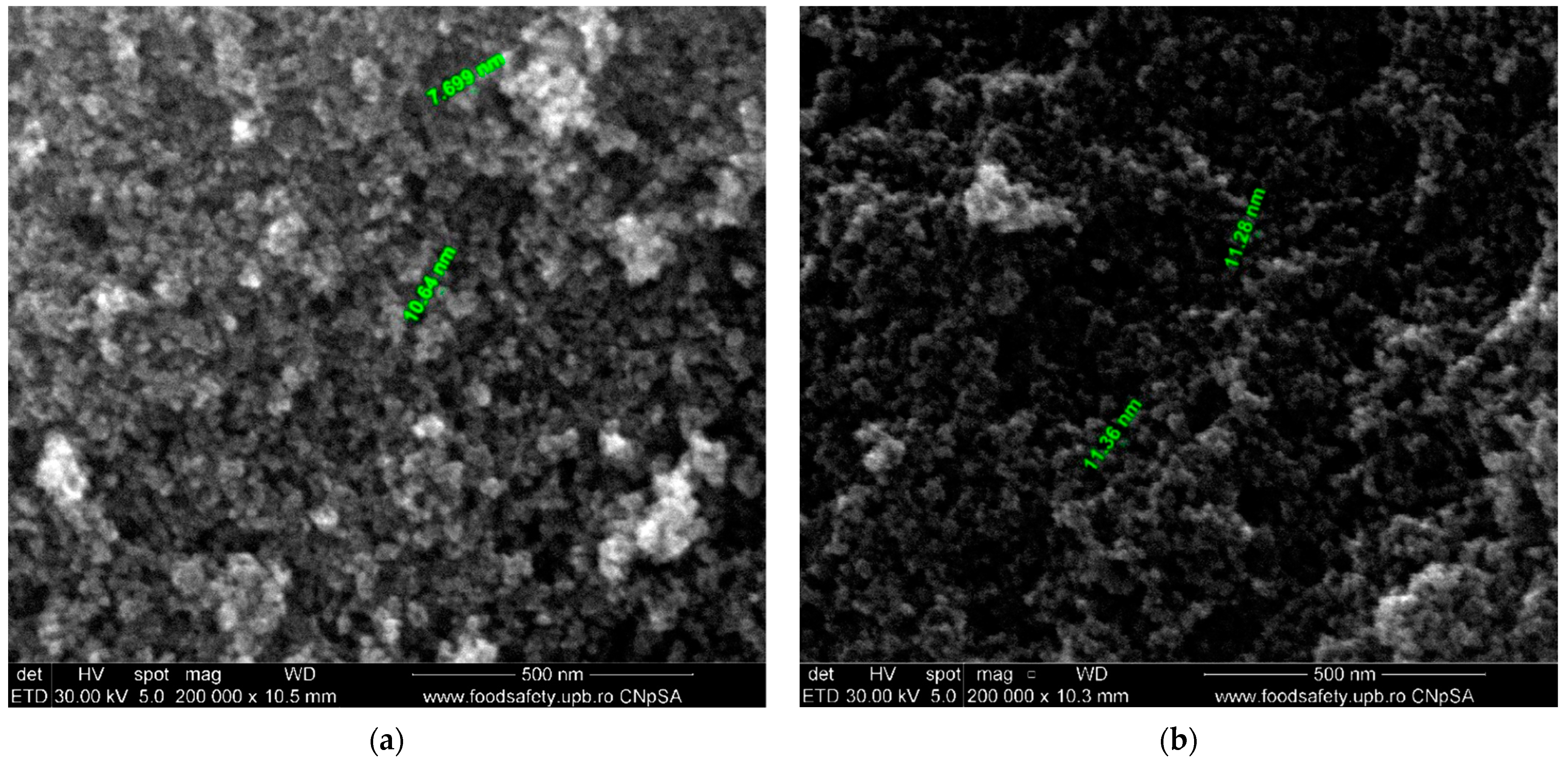
3.1. Characterization of Composites
3.2. Photocatalytic Degradation
3.2.1. Effect of Composite Dosage on Dye Removal
3.2.2. Effect of the Amount of H2O2
3.2.3. Effect of pH
3.2.4. Effect of the Fe3O4/PVA Composite under UV Irradiation
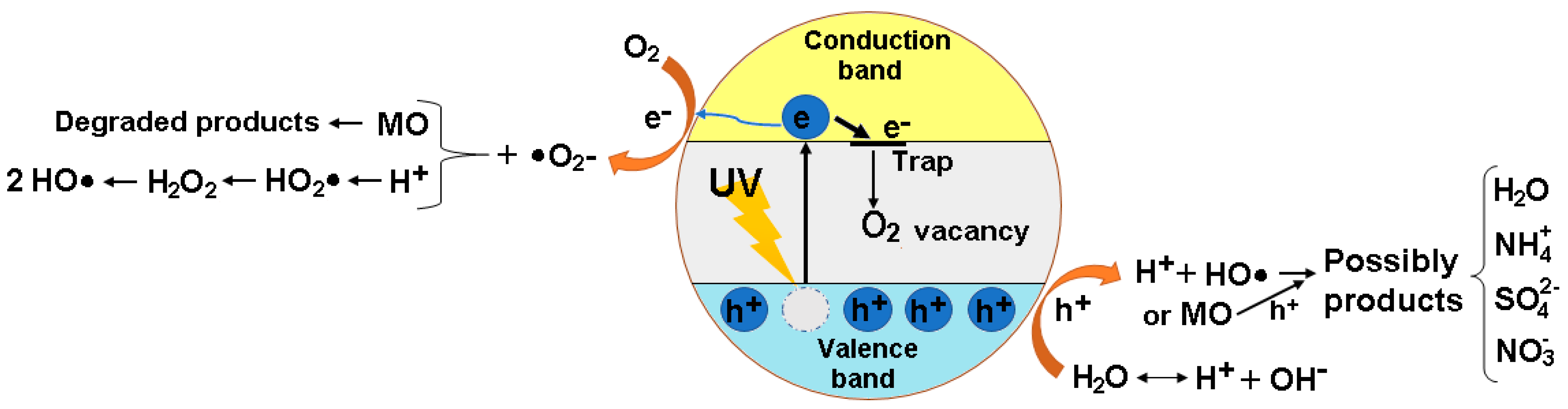
3.2.5. Photodegradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, X.; Hana, J.; Gong, H.; Meng, L.; Mei, X.; Sun, Y.; Qi, L.; Gan, L. Degradation of emerging contaminants by sono-Fenton process with in situ generated H2O2 and the improvement by P25-mediated visible light irradiation. J. Hazard. Mater. 2020, 391, 122229. [Google Scholar] [CrossRef]
- Golshan, M.; Zare, M.; Goudarzi, G.; Abtahi, M.; Babaei, A.A. Fe3O4@HAP-enhanced photocatalytic degradation of Acid Red73 in aqueous suspension: Optimization, kinetic, and mechanism studies. Mater. Res. Bull. 2017, 91, 59–67. [Google Scholar] [CrossRef]
- Salim, S.; Hadibarata, T.; Elwina, E.; Dewi, R.; Alaraidh, I.A.; Al-Ghamdi, A.A.; Alsahli, A.A. Development of activated carbon from Eichhornia Crassipes via chemical activation and its application to remove a synthetic dye. Biointerface Res. Appl. Chem. 2019, 9, 4394–4400. [Google Scholar]
- Ali, S.F.A.; Gad, E.S. Investigation of an adsorbent based on novel starch/chitosan nanocomposite in extraction of indigo carmine dye from aqueous solutions. Biointerface Res. Appl. Chem. 2020, 10, 5556–5563. [Google Scholar]
- Gangarajula, Y.; Kedharnath, R.; Gopal, B. Investigation of photocatalytic activity of pure strontium hydroxyapatite and its Ti-substituted and TiO2 loaded forms. Appl. Catal. A Gen. 2015, 506, 100–108. [Google Scholar] [CrossRef]
- Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.; Luan, J.; Tang, Y.; Fan, H.; Yuan, Z.; Chen, J. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 5749–5760. [Google Scholar] [CrossRef]
- Chen, H.; Chen, N.; Gao, Y.; Feng, C. Photocatalytic degradation of methylene blue by magnetically recoverable Fe3O4/Ag6Si2O7 under simulated visible light. Powder Technol. 2018, 326, 247–254. [Google Scholar] [CrossRef]
- Xu, L.; Meng, L.; Zhang, X.; Mei, X.; Guo, X.; Li, W.; Wang, P.; Gan, L. Promoting Fe3+/Fe2+ cycling under visible light by synergistic interactions between P25 and small amount of Fenton reagents. J. Hazard. Mater. 2019, 379, 120795. [Google Scholar] [CrossRef]
- De Oliveira Guidolin, T.; Possolli, N.M.; Polla, M.B.; Wermuth, T.B.; Franco de Oliveira, T.; Eller, S.; Montedo, O.R.K.; Arcaro, S.; Cechinel, M.A.P. Photocatalytic pathway on the degradation of methylene blue from aqueous solutions using magnetite nanoparticles. J. Clean. Prod. 2021, 318, 128556. [Google Scholar] [CrossRef]
- Rivera, F.L.; Recio, F.J.; Palomares, F.J.; Sánchez-Marcos, J.; Menéndez, N.; Mazarío, E.; Herrasti, P. 2020. Fenton-like degradation enhancement of methylene blue dye with magnetic heating induction. J. Electroanal. Chem. 2020, 879, 114773. [Google Scholar] [CrossRef]
- Arifin, S.A.; Jalaludin, S.; Saleh, R. Photocatalytic Decolorization of Malachite Green in the Presence of Fe3O4/TiO2/CuO Nanocomposites. Mater. Sci. Forum 2015, 1123, 264–269. [Google Scholar]
- Liu, X.; Zhang, T.; Xu, D.; Zhang, L. Microwave-Assisted Catalytic Degradation of Crystal Violet with Barium Ferrite Nanomaterial. Ind. Eng. Chem. Res. 2016, 55, 11869–11877. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Stroeve, P.; Sohrabi, A. Templated growth of superparamagnetic iron oxide nanoparticles by temperature programming in the presence of poly (vinyl alcohol). Thin Solid Films 2010, 518, 4281–4289. [Google Scholar] [CrossRef]
- Seo, K.; Sinha, K.; Novitskaya, E.; Graeve, O.A. Polyvinylpyrrolidone (PVP) effects on iron oxide nanoparticle formation. Mater. Lett. 2018, 215, 203–206. [Google Scholar] [CrossRef]
- Usawattanakul, N.; Torgbo, S.; Sukyai, P.; Khantayanuwong, S.; Puangsin, B.; Srichola, P. Development of Nanocomposite Film Comprising of Polyvinyl Alcohol (PVA) Incorporated with Bacterial Cellulose Nanocrystals and Magnetite Nanoparticles. Polymers 2021, 13, 1778. [Google Scholar] [CrossRef]
- Gulgun, M.A.; Nguyen, M.H.; Kriven, W.M. Polymerized organic-inorganic synthesis of mixed oxides. J. Am. Ceram. Soc. 1999, 82, 556. [Google Scholar] [CrossRef]
- Stoia, M.; Istratie, R.; Păcurariu, C. Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J. Therm. Anal. Calorim. 2016, 125, 1185–1198. [Google Scholar] [CrossRef]
- Orbulet, O.D.; Borda, C.; Garleanu, D.; Garleanu, G.; Stancu, A.; Modrogan, C. Fe3O4 particles functionalized with EDTA and PVA—Preparation, characterization and their use in removal of manganese ions from synthetic aqueous solutions. UPB Sci. Bull. Ser. B 2021, 83, 101–116. [Google Scholar]
- Adak, A.K.; Pathak, A.; Pramanik, P. Polyvinyl alcohol evaporation method for preparation of submicron chromite powders. Br. Ceram. Trans. 1999, 98, 200. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539. [Google Scholar] [CrossRef]
- Galinato, M.G.I.; Whaley, C.M.; Lehnert, N. Vibrational Analysis of the Model Complex (μ-edt) [Fe (CO)3]2 and Comparison to Iron-only Hydrogenase: The Activation Scale of Hydrogenase Model Systems. Inorg. Chem. 2010, 49, 3201. [Google Scholar] [CrossRef] [Green Version]
- Stoia, M.; Pacurariu, C.; Istratie, R.; Niznansky, D. Solvothermal synthesis of magnetic FexOy/C nanocomposites used as adsorbents for the removal of methylene blue from wastewater. J. Therm. Anal. Calorim. 2015, 121, 989. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Lungulescu, E.M.; Nicula, N.; Somoghi, R.; Ditu, L.M.; Ungureanu, C.; Sutan, A.N.; Draghiceanu, O.A.; Paunescu, A.; et al. Phytosynthesis and radiation-assisted methods for obtaining metal nanoparticles. J. Mater. Sci. 2020, 55, 1915–1932. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Jiao, C.; Zhao, Y.; Zhang, J.; Wang, H. Self-Assembled Polyvinyl Alcohol–Tannic Acid Hydrogels with Diverse Microstructures and Good Mechanical Properties. ACS Omega 2018, 3, 11788–11795. [Google Scholar] [CrossRef] [Green Version]
- Aziz, W.N.A.; Bumajdad, A.; Sagheer, F.A.; Madkour, M. Selective synthesis and characterization of iron oxide nanoparticles via PVA/PVP polymer blend as structure-directing agent. Mater. Chem. Phys. 2020, 249, 122927. [Google Scholar] [CrossRef]
- Nigam, B.; Mittal, S.; Prakash, A.; Satsangi, S.; Mahto, P.K.; Swain, B.P. Synthesis and characterization of Fe3O4 nanoparticles for nanofluid applications-A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 012187. [Google Scholar] [CrossRef]
- de Alves, F.H.O.; Araújo, O.A.; de Oliveira, A.C.; Garg, V.K. Preparation and characterization of PAni(CA)/Magnetic iron oxide hybrids and evaluation in adsorption/photodegradation of blue methylene dye. Surf. Interfaces 2021, 23, 100954. [Google Scholar] [CrossRef]
- Sanad, M.M.S.; Farahat, M.M.; El-Hout, S.I.; El-Sheikh, S.M. Preparation and characterization of magnetic photocatalyst from the banded iron formation for effective photodegradation of methylene blue under UV and visible illumination. J. Environ. Chem. Eng. 2021, 9, 105127. [Google Scholar] [CrossRef]
- Oliveira, P.N.; Bini, R.D.; Dias, G.S.; Alcouffe, P.; Santos, I.A.; David, L.; Cótica, L.F. Magnetite nanoparticles with controlled sizes via thermal degradation of optimized PVA/Fe(III) complexes. J. Magn. Magn. Mater. 2018, 460, 381–390. [Google Scholar] [CrossRef]
- Jia, H.; Huang, F.; Gao, Z.; Zhong, C.; Zhou, H.; Jiang, M.; Wei, P. Immobilization of v-transaminase by magnetic PVA-Fe3O4 nanoparticles. Biotechnol. Rep. 2016, 10, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmorsi, Y.M.; Riyad, Z.H.; Mohamed, H.M.; Abid, H.; Bary, E. Decolorization of mordant red 73 azo dye in water using H2O2/UV photo Fenton treatment. J. Hazard. Mater. 2010, 174, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qian, G.M.; Zhang, B.; Liu, L.L.; Liu, H. Facile synthesis of spherical nanohydroxyapatite and its application in photocatalytic degradation of methyl orange dye under UV irradiation. Mater. Lett. 2016, 178, 15–17. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A critical review of eggshell waste: An effective source of hydroxyapatite as photocatalyst. J. Met. Mater. Miner. 2018, 28, 124–135. [Google Scholar]
- Chai, Y.; Ding, J.; Wang, L.; Liu, Q.; Ren, J.; Dai, W.-L. Enormous enhancement in photocatalytic performance of Ag3PO4/HAp composite: A Z-scheme mechanism insight. Appl. Catal. B Environ. 2015, 179, 29–36. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Faria, J.L. Photochemical and photocatalytic degradation ofan azo dye in aqueous solution by UV irradiation. J. Photochem. Photobiol. A Chem. 2003, 155, 133–143. [Google Scholar] [CrossRef]




| Amount of Composite, g | Fe3O4 | Fe3O4/PVA |
|---|---|---|
| Dye Removal, % | ||
| 1.5 | 65.89 ± 0.05 | 78.32 ± 0.03 |
| 2 | 77.56 ± 0.07 | 89.25 ± 0.04 |
| 3 | 87.71 ± 0.04 | 92.54 ± 0.04 |
| 5 | 91.37 ± 0.04 | 98.65 ± 0.03 |
| Amount of H2O2, mL | Fe3O4 | Fe3O4/PVA |
|---|---|---|
| Dye Removal, % | ||
| 3.5 | 58.41 ± 0.06 | 60.62 ± 0.04 |
| 6.7 | 71.77 ± 0.03 | 80.78 ± 0.05 |
| 10 | 67.83 ± 0.04 | 75.14 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrogan, C.; Cǎprǎrescu, S.; Dǎncilǎ, A.M.; Orbuleț, O.D.; Grumezescu, A.M.; Purcar, V.; Radițoiu, V.; Fierascu, R.C. Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater. Polymers 2021, 13, 3911. https://doi.org/10.3390/polym13223911
Modrogan C, Cǎprǎrescu S, Dǎncilǎ AM, Orbuleț OD, Grumezescu AM, Purcar V, Radițoiu V, Fierascu RC. Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater. Polymers. 2021; 13(22):3911. https://doi.org/10.3390/polym13223911
Chicago/Turabian StyleModrogan, Cristina, Simona Cǎprǎrescu, Annette Madelene Dǎncilǎ, Oanamari Daniela Orbuleț, Alexandru Mihai Grumezescu, Violeta Purcar, Valentin Radițoiu, and Radu Claudiu Fierascu. 2021. "Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater" Polymers 13, no. 22: 3911. https://doi.org/10.3390/polym13223911
APA StyleModrogan, C., Cǎprǎrescu, S., Dǎncilǎ, A. M., Orbuleț, O. D., Grumezescu, A. M., Purcar, V., Radițoiu, V., & Fierascu, R. C. (2021). Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater. Polymers, 13(22), 3911. https://doi.org/10.3390/polym13223911