Forskolin-Loaded Halloysite Nanotubes as Osteoconductive Additive for the Biopolymer Tissue Engineering Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of MSCs
2.2. Differentiation of MSCs
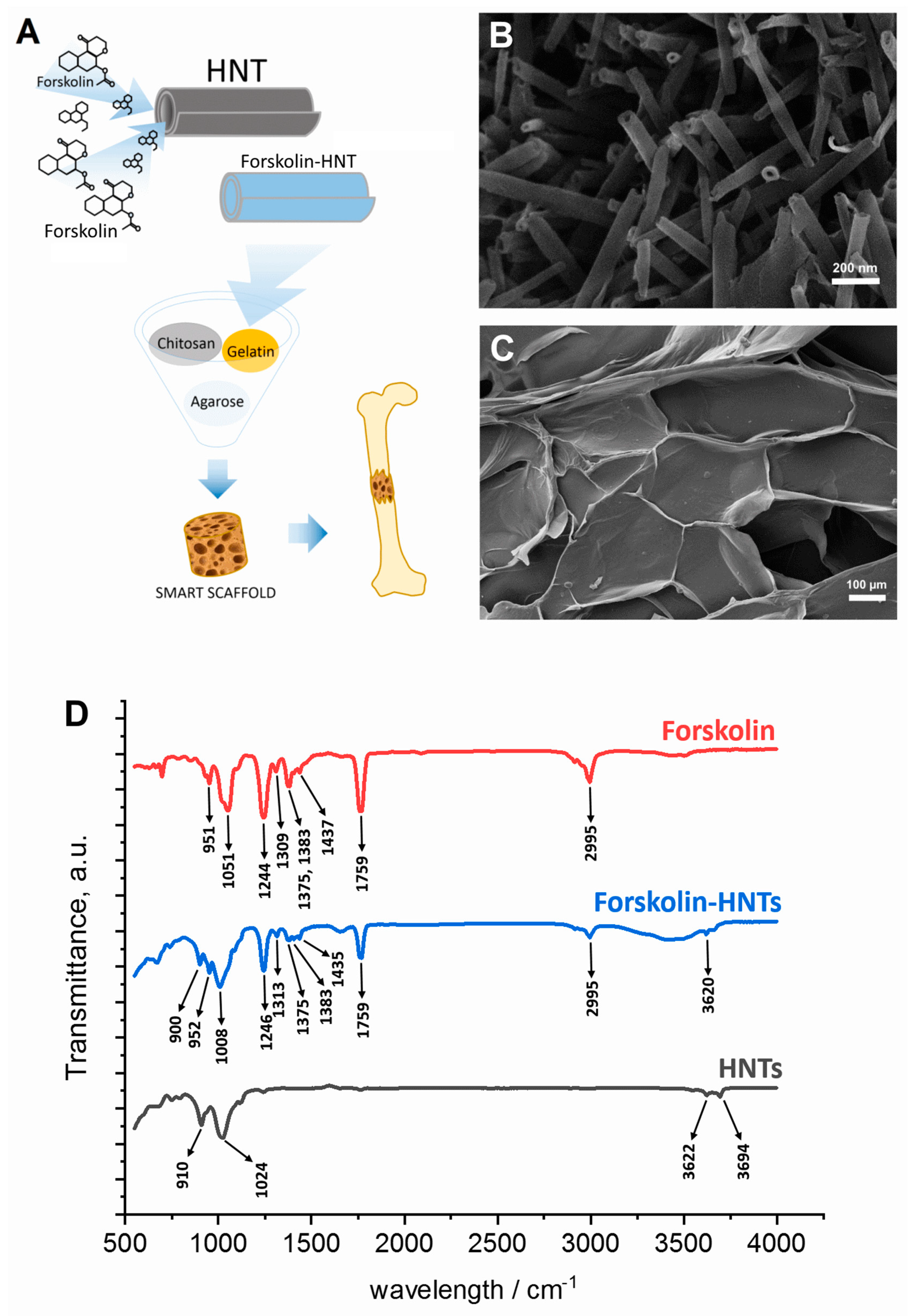
2.3. Forskolin-HNTs Fabrication
2.4. Fourier Transform Infrared Spectroscopy
2.5. Osteogenic Differentiation of MSCs on Polymeric Nanostructured Scaffolds
2.6. Dark-Field Microscopy
2.7. Atomic Force Microscopy (AFM)
2.8. 3D laser Scanning Confocal Microscopy
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moreira, A.; Kahlenberg, S.; Hornsby, P. Therapeutic Potential of Mesenchymal Stem Cells for Diabetes. J. Mol. Endocrinol. 2017, 59, R109–R120. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 982. [Google Scholar] [CrossRef]
- Lohan, P.; Treacy, O.; Griffin, M.D.; Ritter, T.; Ryan, A.E. Anti-Donor immune responses elicited by allogeneic mesenchymal stem cells and their extracellular vesicles: Are we still learning? Front. Immunol. 2017, 8, 1626. [Google Scholar] [CrossRef]
- Parekkadan, B.; Milwid, J.M. Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 2010, 12, 87–117. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. Npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Rakocevic, J.; Orlic, D.; Mitrovic-Ajtic, O.; Tomasevic, M.; Dobric, M.; Zlatic, N.; Milasinovic, D.; Stankovic, G.; Ostojić, M.; Labudovic-Borovic, M. Endothelial cell markers from clinician’s perspective. Exp. Mol. Pathol. 2017, 102, 303–313. [Google Scholar] [CrossRef]
- Nakano, A.; Harada, T.; Morikawa, S.; Kato, Y. Expression of leukocyte common antigen (CD45) on various human leukemia/lymphoma cell lines. Acta Pathol. Jpn. 1990, 40, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Szaraz, P.; Gratch, Y.S.; Iqbal, F.; Librach, C.L. In vitro differentiation of human mesenchymal stem cells into functional cardiomyocyte-like cells. J. Vis. Exp. 2017, 126, 55757. [Google Scholar] [CrossRef] [PubMed]
- Abd Allah, S.H.; Shalaby, S.M.; El-Shal, A.S.; El Nabtety, S.M.; Khamis, T.; Abd El Rhman, S.A.; Ghareb, M.A.; Kelani, H.M. Breast milk MSCs: An explanation of tissue growth and maturation of offspring. IUBMB Life 2016, 68, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Prockop, D.J. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair—Current views. Stem Cells 2007, 25, 2896–2902. [Google Scholar] [CrossRef]
- Alonso-Goulart, V.; Ferreira, L.; Duarte, C.A.; Lima, I.; Ferreira, E.R.; Oliveira, B.C.; Vargas, L.N.; Moraes, D.D.; Silva, I.; Faria, R.D.; et al. Mesenchymal stem cells from human adipose tissue and bone repair: A literature review. Biotechnol. Res. Innov. 2017, 2, 74–80. [Google Scholar] [CrossRef]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, E.S.; Barrett, J.G. The potential of mesenchymal stem cells to treat systemic inflammation in horses. Front. Vet. Sci. 2020, 6, 507. [Google Scholar] [CrossRef] [PubMed]
- Cassano, J.M.; Schnabel, L.V.; Goodale, M.B.; Fortier, L.A. Inflammatory licensed equine MSCs are chondroprotective and exhibit enhanced immunomodulation in an inflammatory environment. Stem Cell Res. Ther. 2018, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Sachs, P.C.; Mollica, P.A.; Bruno, R.D. Tissue specific microenvironments: A key tool for tissue engineering and regenerative medicine. J. Biol. Eng. 2017, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Bulstrode, N.W.; Whitaker, I.S.; Eastwood, D.M.; Dunaway, D.; Ferretti, P. Nanotechnology for stimulating osteoprogenitor differentiation. Open Orthop. J. 2016, 10, 849–861. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.; Gao, Y.; Cui, W.; Li, Y.; Xiao, D.; Zhou, R. Nanomaterials-based cell osteogenic differentiation and bone regeneration. Curr. Stem Cell Res. Ther. 2021, 16, 36–47. [Google Scholar] [CrossRef]
- Shuai, C.; Zan, J.; Deng, F.; Yang, Y.; Peng, S.; Zhao, Z. Core–shell-structured ZIF-8@ PDA-HA with controllable Zinc ion release and superior bioactivity for improving a poly-l-lactic acid scaffold. ACS Sustain. Chem. Eng. 2021, 9, 1814–1825. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng, Y.; Deng, F.; Shen, L.; Zhao, Z.; Peng, S.; Shuai, S.C. A bifunctional bone scaffold combines osteogenesis and antibacterial activity via in situ grown hydroxyapatite and silver nanoparticles. Bio-Des. Manuf. 2021, 3, 1–17. [Google Scholar]
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.; Joo, S.W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/β-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383–4392. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Y.; Yin, H.Y.; Guo, Z.Y.; Xu, F.; Xiao, B.; Jiang, W.L.; Guo, W.M.; Meng, H.Y.; Lu, S.-B.; et al. Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: Potential advantage of cellular transient memory function. Stem Cell Res. Ther. 2018, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Rooney, G.E.; Howard, L.; O’Brien, T.; Windebank, A.J.; Barry, F.P. Elevation of cAMP in mesenchymal stem cells transiently upregulates neural markers rather than inducing neural differentiation. Stem Cells Dev. 2009, 18, 387–398. [Google Scholar] [CrossRef]
- Insel, P.A.; Ostrom, R.S. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell. Mol. Neurobiol. 2003, 23, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.; Lu, W.; Louie, A.; Nissenson, R. Cyclic AMP signaling in bone marrow stromal cells has reciprocal effects on the ability of mesenchymal stem cells to differentiate into mature osteoblasts versus mature adipocytes. Endocrine 2012, 42, 622–636. [Google Scholar] [CrossRef]
- Doorn, J.; Siddappa, R.; van Blitterswijk, C.A.; de Boer, J. Forskolin enhances in vivo bone formation by human mesenchymal stromal cells. Tissue Eng. Part A 2012, 18, 558–567. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Price, R.R. Halloysite nanotubules, a novel substrate for the controlled delivery of bioactive molecules. In Bio-Inorganic Hybrid Nanomaterials; Ruiz-Hitzky, E., Ariga, K., Lvov, Y.M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 419–441. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Arul, M.R.; Rudraiah, S.; Kalajzic, I.; Kumbar, S.G. Aligned microchannel polymer-nanotube composites for peripheral nerve regeneration: Small molecule drug delivery. J. Control. Release 2019, 296, 54–67. [Google Scholar] [CrossRef]
- Naumenko, E.A.; Fakhrullin, R.F. Toxicological evaluation of clay nanomaterials and polymer-clay nanocomposites. In Functional Polymer Composites with Nanoclays; Lvov, Y.M., Guo, B., Fakhrullin, R.F., Eds.; Royal Society of Chemistry: London, UK, 2017; pp. 399–419. [Google Scholar]
- Zakirova, E.Y.; Aimaletdinov, A.M.; Tambovsky, M.A.; Rizvanov, A.A. Comparative characteristics of mesenchymal stem cell lines from different animal species. Tsitologiya 2021, 63, 139–146. [Google Scholar]
- Cho, J.S.; Park, J.H.; Kang, J.H.; Kim, S.E.; Park, I.H.; Lee, H.M. Isolation and characterization of multipotent mesenchymal stem cells in nasal polyps. Exp. Biol. Med. 2015, 240, 185–193. [Google Scholar] [CrossRef]
- Meloan, S.N.; Puchtler, H. Chemical mechanisms of staining methods: Von Kossa’s technique: What von Kossa really wrote and a modified reaction for selective demonstration of inorganic phosphates. J. Histotechnol. 1985, 8, 11–13. [Google Scholar] [CrossRef]
- Guryanov, I.; Naumenko, E.; Akhatova, F.; Lazzara, G.; Cavallaro, G.; Nigamatzyanova, L.; Fakhrullin, R. Selective cytotoxic activity of Prodigiosin@halloysite nanoformulation. Front. Bioeng. Biotechnol. 2020, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, E.A.; Guryanov, I.D.; Yendluri, R.; Lvov, Y.M.; Fakhrullin, R.F. Clay nanotube-biopolymer composite scaffolds for tissue engineering. Nanoscale 2016, 8, 7257–7271. [Google Scholar] [CrossRef]
- Akhatova, F.; Danilushkina, A.; Kuku, G.; Saricam, M.; Culha, M.; Fakhrullin, R. Simultaneous intracellular detection of plasmonic and non-plasmonic nanoparticles using dark-field hyperspectral microscopy. Bull. Chem. Soc. Jpn. 2018, 91, 1640–1645. [Google Scholar] [CrossRef]
- Akhatova, F.; Fakhrullina, G.; Khakimova, E.; Fakhrullin, R. Atomic force microscopy for imaging and nanomechanical characterisation of live nematode epicuticle: A comparative Caenorhabditis elegans and Turbatrix aceti study. Ultramicroscopy 2018, 194, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.A.; Robinson, S.O.; Lopez, M.J. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet. Surg. 2008, 37, 713–724. [Google Scholar] [CrossRef]
- Naumenko, E.; Fakhrullin, R. Halloysite Nanoclay/Biopolymers composite materials in tissue engineering. Biotechnol. J. 2019, 14, 1900055. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J.; Little, D.G.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur. Cells Mater. 2013, 26, 120–132. [Google Scholar] [CrossRef]
- Fakhrullina, G.I.; Akhatova, F.S.; Lvov, Y.M.; Fakhrullin, R.F. Toxicity of halloysite clay nanotubes in vivo: A Caenorhabditis elegans study. Environ. Sci. Nano 2015, 2, 54–59. [Google Scholar] [CrossRef]
- Rozhina, E.; Panchal, A.; Akhatova, F.; Lvov, Y.; Fakhrullin, R. Cytocompatibility and cellular uptake of alkylsilane-modified hydrophobic halloysite nanotubes. Appl. Clay Sci. 2020, 185, 105371. [Google Scholar] [CrossRef]
- Tarasova, E.; Naumenko, E.; Rozhina, E.; Akhatova, F.; Fakhrullin, F. Cytocompatibility and uptake of polycations-modified halloysite clay nanotubes. Appl. Clay Sci. 2019, 169, 21–30. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Halloysite clay nanotubes as a ceramic “skeleton” for functional biopolymer composites with sustained drug release. J. Mater. Chem. B 2013, 1, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Yendluri, R.; Lvov, Y.; de Villiers, M.M.; Vinokurov, V.; Naumenko, E.; Tarasova, E.; Fakhrullin, R. Paclitaxel encapsulated in halloysite clay nanotubes for intestinal and intracellular delivery. J. Pharm. Sci. 2017, 106, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullina, G.; Khakimova, E.; Akhatova, F.; Lazzara, G.; Parisi, F.; Fakhrullin, R.F. Selective antimicrobial effects of curcumin@halloysite nanoformulation: A Caenorhabditis elegans study. ACS Appl. Mater. Interfaces 2019, 11, 23050–23064. [Google Scholar] [CrossRef] [PubMed]
- Dzamukova, M.; Naumenko, E.; Lvov, Y.; Guryanov, I.; Fakhrullin, R. Enzyme-activated intracellular drug delivery with tubule clay nanoformulation. Sci. Rep. 2015, 5, 10560. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Exploiting the colloidal stability and solubilization ability of clay nanotubes/ionic surfactant hybrid nanomaterials. J. Phys. Chem. C 2012, 116, 21932–21938. [Google Scholar] [CrossRef]
- Zhao, L.; Li, G.; Zhou, G.Q. SOX9 directly binds CREB as a novel synergism with the PKA pathway in BMP-2-induced osteochondrogenic differentiation. J. Bone Miner. Res. 2009, 24, 826–836. [Google Scholar] [CrossRef]
- Edlund, C.; Jackson, T.R.; Khalid, N.; Bevan, N.; Dale, T.; Dengel, A.; Ahmed, S.; Trygg, J.; Sjögren, R. LIVECell—A large-scale dataset for label-free live cell segmentation. Nat. Methods 2021, 18, 1038–1045. [Google Scholar] [CrossRef]




| Specimen | Sq/µm | Ssk | Sku | Svk/µm |
|---|---|---|---|---|
| HNTs-free scaffolds | 27.1 ± 9.1 | 0.35 ± 0.4 | 3.12 ± 0.5 | 19.9 ± 4.1 |
| HNTs-doped scaffolds | 13.4 ± 1.4 | −0.04 ± 0.1 | 3.23 ± 0.5 | 12.4 ± 0.9 |
| Control − HNT | Control + HNT | Osteo + HNT | Forskolin + HNT | ||||
|---|---|---|---|---|---|---|---|
| Adhesion nN | Modulus MPa | Adhesion nN | Modulus MPa | Adhesion nN | Modulus MPa | Adhesion nN | Modulus MPa |
| 3.2 ± 0.4 | 24.6 ± 3.2 | 3.9 ± 0.6 | 25.1 ± 5.1 | 5.7 ± 3.1 | 26.8 ± 3.6 | 4.7 ± 0.8 | 31.4 ± 3.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naumenko, E.; Guryanov, I.; Zakirova, E.; Fakhrullin, R. Forskolin-Loaded Halloysite Nanotubes as Osteoconductive Additive for the Biopolymer Tissue Engineering Scaffolds. Polymers 2021, 13, 3949. https://doi.org/10.3390/polym13223949
Naumenko E, Guryanov I, Zakirova E, Fakhrullin R. Forskolin-Loaded Halloysite Nanotubes as Osteoconductive Additive for the Biopolymer Tissue Engineering Scaffolds. Polymers. 2021; 13(22):3949. https://doi.org/10.3390/polym13223949
Chicago/Turabian StyleNaumenko, Ekaterina, Ivan Guryanov, Elena Zakirova, and Rawil Fakhrullin. 2021. "Forskolin-Loaded Halloysite Nanotubes as Osteoconductive Additive for the Biopolymer Tissue Engineering Scaffolds" Polymers 13, no. 22: 3949. https://doi.org/10.3390/polym13223949
APA StyleNaumenko, E., Guryanov, I., Zakirova, E., & Fakhrullin, R. (2021). Forskolin-Loaded Halloysite Nanotubes as Osteoconductive Additive for the Biopolymer Tissue Engineering Scaffolds. Polymers, 13(22), 3949. https://doi.org/10.3390/polym13223949







