Obtaining Active Polylactide (PLA) and Polyhydroxybutyrate (PHB) Blends Based Bionanocomposites Modified with Graphene Oxide and Supercritical Carbon Dioxide (scCO2)-Assisted Cinnamaldehyde: Effect on Thermal-Mechanical, Disintegration and Mass Transport Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Strains
2.2. Microorganisms, Culture Medium, and Obtained PHB
2.3. Preparation of Blends and Bionanocomposites
2.3.1. PLA/GO Masterbatch
2.3.2. Extrusion of Blends and Bionanocomposites
2.4. Supercritical Impregnation of Cinnamaldehyde (Ci) in Blends and Bionanocomposites
2.5. Characterization of Graphene Oxide (GO), Blends and Bionanocomposites
2.5.1. Quantification of Cinnamaldehyde in Blends and Bionanocomposites
2.5.2. Attenuated Total Reflectance Fourier Transforms Infrared (ATR-FTIR) Spectroscopy
2.5.3. Thermal Properties
2.5.4. Mechanical Properties
2.6. Disintegration under Composting Conditions
2.7. Kinetic Release of Active Compound from PLA/PHB Blends and Bionanocomposites
2.7.1. Mathematical Modeling of Active Release Kinetic from Impregnated Blends and Bionanocomposites
- (1)
- The initial Ci concentration in the different materials is known and is homogeneously distributed in the polymer.
- (2)
- The ethanolic solutions are initially Ci free.
- (3)
- There is no chemical interaction between the simulant solutions and polymer (not swelling).
- (4)
- No mass transfer limitation in the release medium, so the Ci is homogeneously distributed in the bulk of them.
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Active Impregnated Blends and Bionanocomposites
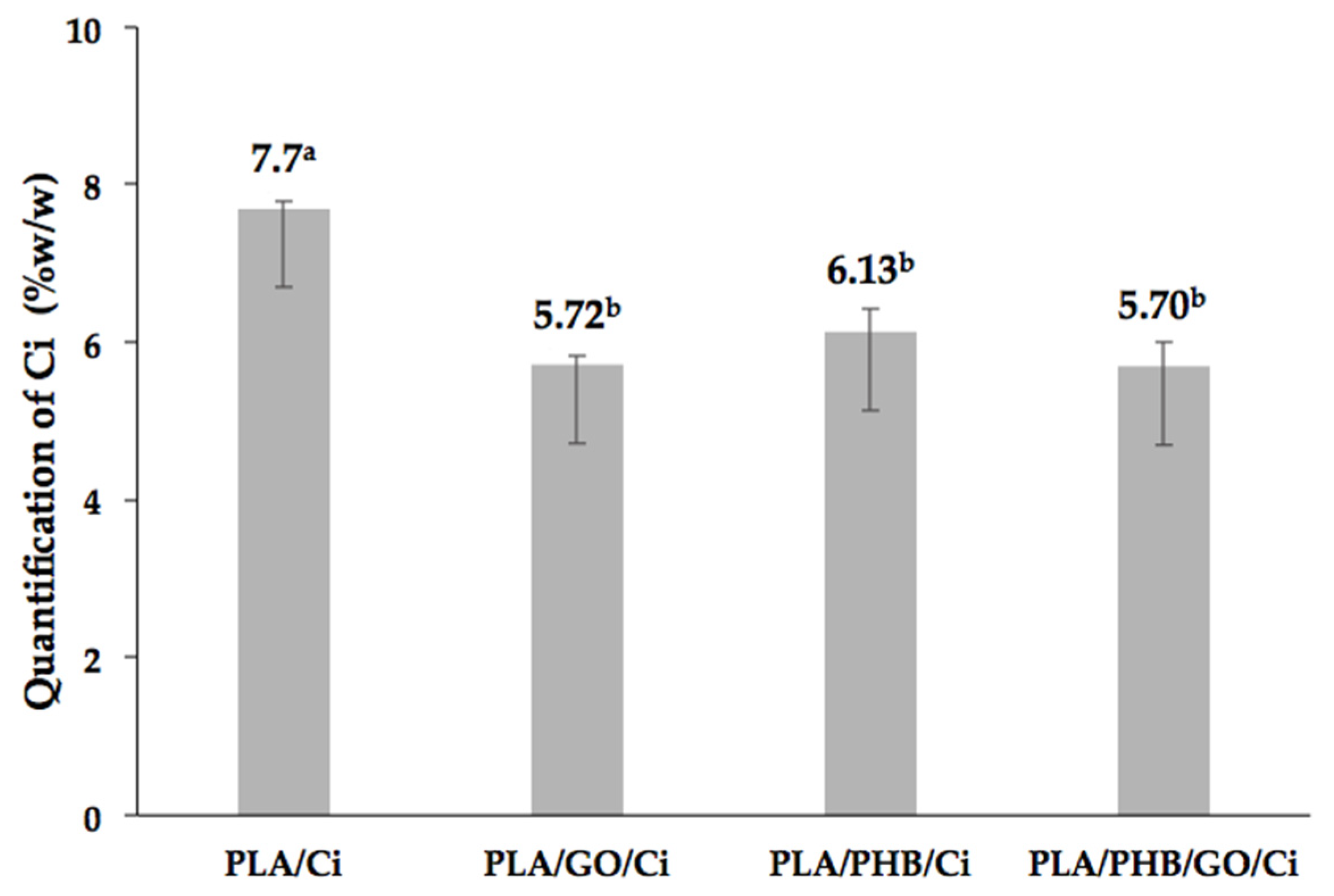
3.1.1. Supercritical Impregnation of Cinnamaldehyde in Blends and Bionanocomposites
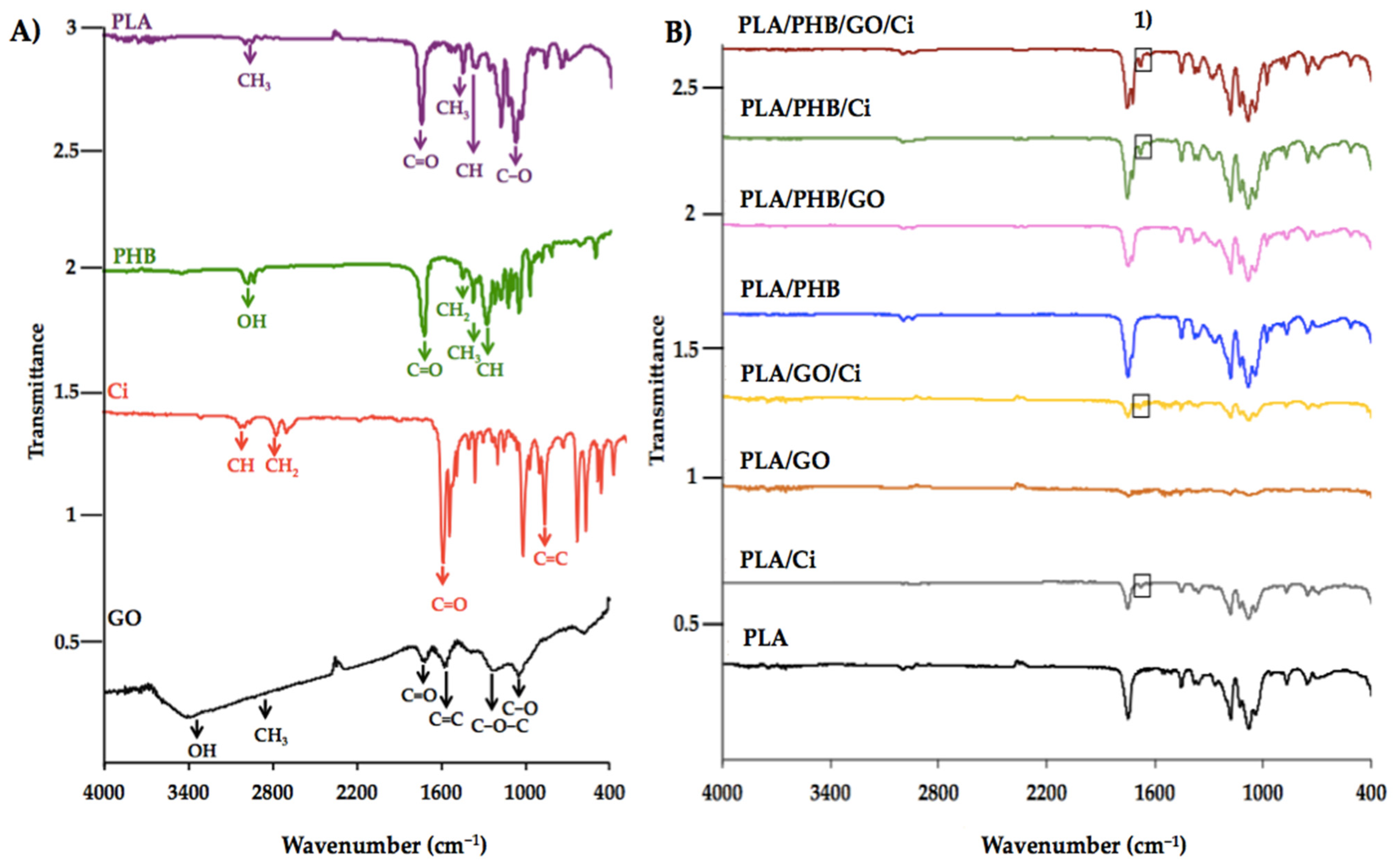
3.1.2. Attenuated Total Reflectance Fourier Transforms Infrared (ATR-FTIR) Spectroscopy
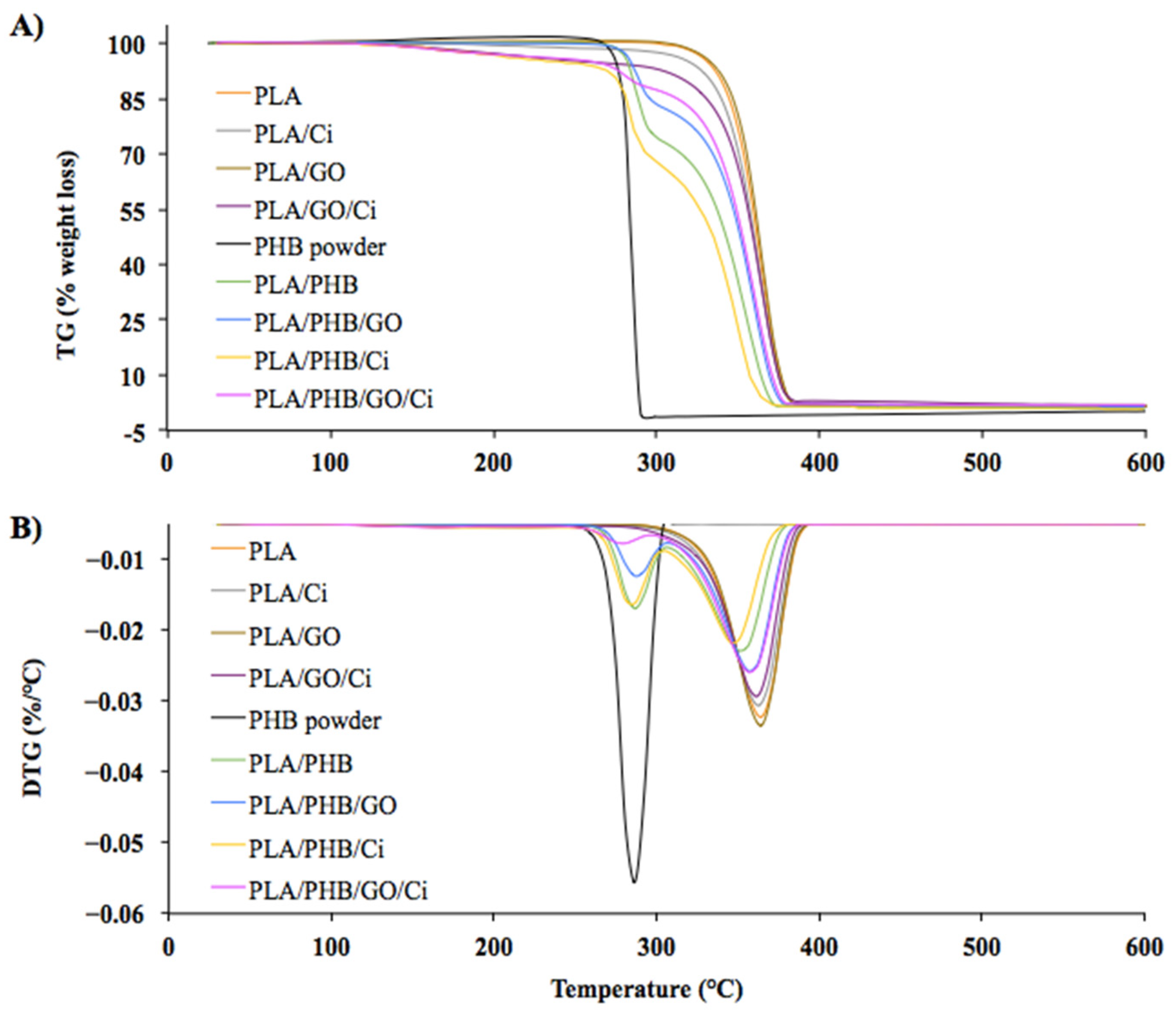
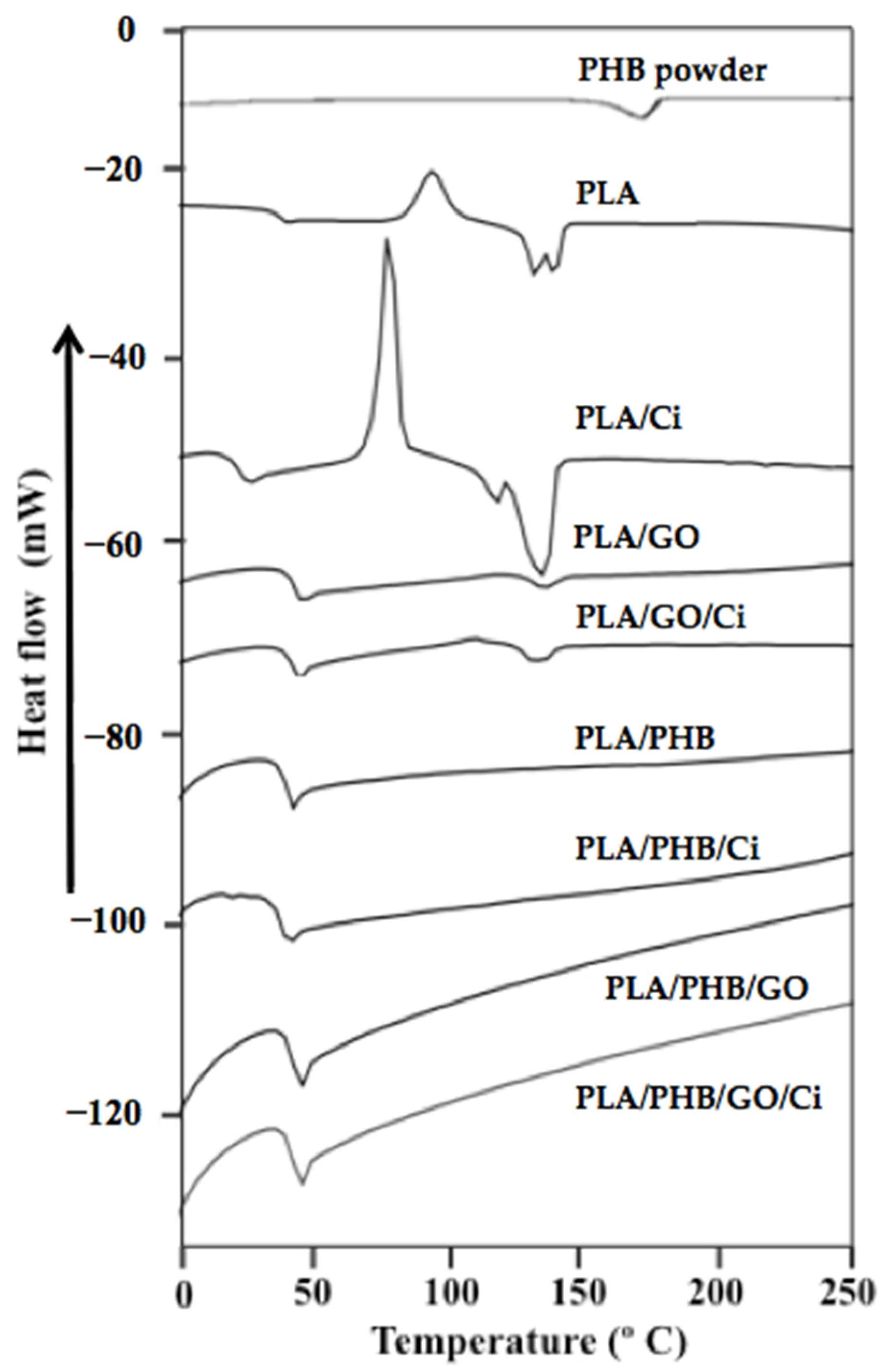
3.1.3. Thermal Properties
3.1.4. Mechanical Properties
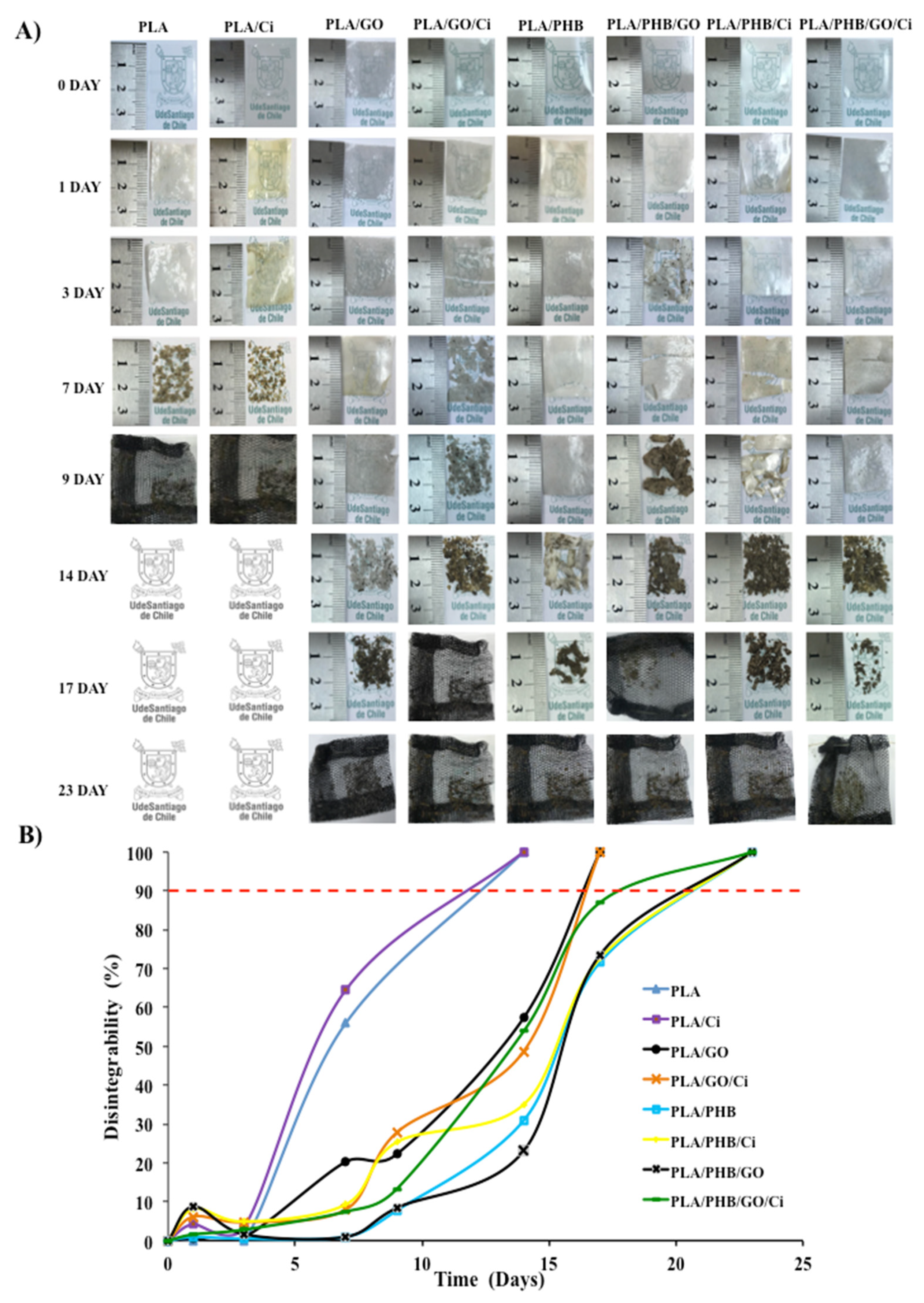
3.2. Disintegration under Composting Conditions
3.3. Release Kinetics of the Active Compound from Materials with and without Nano-Reinforcement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Ausejo, J.; Sanchez-Safont, E.; Lagaron, J.M.; Olsson, R.T.; Gamez-Perez, J.; Cabedo, L. Assessing the thermoformability of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(acid lactic) blends compatibilized with diisocyanates. Polym. Test 2017, 62, 235–245. [Google Scholar] [CrossRef]
- Plavec, R.; Hlaváčiková, S.; Omaníková, L.; Feranc, J.; Vanovčanová, Z.; Tomanová, K.; Bočkaj, J.; Kruželák, J.; Medlenová, E.; Gálisová, I.; et al. Recycling possibilities of bioplastics based on PLA/PHB blends. Polym. Test 2020, 92, 106880. [Google Scholar] [CrossRef]
- Olejnik, O.; Masek, A.; Zawadziłło, J. Processability and Mechanical Properties of Thermoplastic Polylactide/Polyhydroxybutyrate (PLA/PHB) Bioblends. Mater 2021, 14, 898. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Y.; Chen, G.Q. Medical application of microbial biopolyesters polyhydroxyalkanoates. Artif. Cell. Blood. Sub 2009, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.R.; Patel, R.P. Role of biodegradable polymers in drug delivery. Int. J. Curr. Pharm. Res. 2012, 4, 74–81. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Akoulina, E.A.; Chesnokova, D.V.; Wenhao, Y.; Makhina, T.K.; Demyanova, I.V.; Zhuikova, Y.V.; Voinova, V.V.; Belishev, N.V.; Surmenev, R.A.; et al. The Growth of 3T3 Fibroblasts on PHB, PLA and PHB/PLA Blend Films at Different Stages of Their Biodegradation In Vitro. Polymers 2021, 13, 108. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.R. Poly (lactic acid) blends in biomedical applications. Adv. Drug Delivery Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Ploypetchara, N.; Suppakul, P.; Atong, D.; Pechyen, C. Blend of polypropylene/poly (lactic acid) for medical packaging application: Physicochemical, thermal, mechanical, and barrier properties. Energy Procedia 2014, 56, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Tănase, E.E.; Popa, M.E.; Râpă, M.; Popa, O. Preparation and characterization of biopolymer blends based on polyvinyl alcohol and starch. Rom. Biotechnol. Lett. 2015, 20, 10306–10315. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and characterization of plasticized PLA/PHB blends for biodegradable multiphase systems. eXPRESS Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Tech. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Gonzalez Ausejo, J.; Rydz, J.; Musioł, M.; Sikorska, W.; Sobota, M.; Włodarczyk, J.; Adamus, G.; Janeczek, H.; Kwiecień, I.; Hercog, A.; et al. A comparative study of three-dimensional printing directions: The degradation and toxicological profile of a PLA/PHA blend. Polym. Degrad. Stab 2018, 152, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Butt, F.I.; Muhammad, N.; Hamid, A.; Moniruzzaman, M.; Sharif, F. Recent progress in the utilization of biosynthesized polyhydroxyalkanoates for biomedical applications—Review. Int. J. Biol. Macromol. Part A 2018, 120, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.P.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. Electric Phenomenon: A Disregarded Tool in Tissue Engineering and Regenerative Medicine. Trends Biotechnol. 2020, 38, 24–49. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Basu, A.; Nazarkovsky, M.; Ghadi, R.; Khan, W.; Domb, A.J. Poly(lactic acid)-based nanocomposites. Polym. Adv. Technol. 2017, 28, 919–930. [Google Scholar] [CrossRef]
- Luzi, F.; Dominici, F.; Armentano, I.; Fortunati, E.; Burgos, N.; Fiori, S.; Torre, L. Combined effect of cellulose nanocrystals, carvacrol and oligomeric lactic acid in PLA_PHB polymeric films. Carbohydr. Polym. 2019, 223, 115–131. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Daisy, E.A.C.; Rajendran, N.K.; Houreld, N.N.; Marraiki, N.; Elgorban, A.M.; Rajan, M. Curcumin and Gymnema sylvestre extract loaded graphene oxide-polyhydroxybutyrate-sodium alginate composite for diabetic wound regeneration. React. Funct. Polym. 2020, 154, 104671. [Google Scholar] [CrossRef]
- Kumar, A.; Zo, S.M.; Kim, J.H.; Kim, S.C.; Han, S.S. Enhanced physical, mechanical, and cytocompatibility behavior of polyelectrolyte complex hydrogels by reinforcing halloysite nanotubes and graphene oxide. Compos. Sci. Technol. 2019, 175, 35–45. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Teng, L.; Wang, L.; Zhu, G.; Liu, S.; Luo, Z.; Shi, X.; Wang, Y.; Ren, L. The antibacterial applications of graphene and its derivatives. Small 2016, 12, 4165–4184. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, A.H.; Mazinani, S.; Sharif, F.; Ranaei-Siadat, S.O. GO nanosheets localization by morphological study on PLA-GO electrospun nanocomposite nanofibers. J. Polym. Res. 2018, 25, 1–11. [Google Scholar] [CrossRef]
- Pinto, A.M.; Cabral, J.; Tanaka, D.A.P.; Mendes, A.M.; Magalhães, F.D. Effect of incorporation of graphene oxide and graphene nanoplatelets on mechanical and gas permeability properties of poly (lactic acid) films. Polym. Int. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Torres, A.; Peñaloza, A.; Sepulveda, H.; Galotto, M.J.; Guarda, A.; Bruna, J. Development of an antimicrobial material based on a nanocomposite cellulose acetate film for active food packaging. Food Addit. Contam. A 2014, 31, 342–353. [Google Scholar] [CrossRef]
- Hauser, C.; Wunderlich, J. Antimicrobial packaging films with a sorbic acid based coating. Procedia Food Sci. 2011, 1, 197–202. [Google Scholar] [CrossRef]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun nanofibres containing antimicrobial plant extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Romero, J.; Macan, A.; Guarda, A.; Galotto, M.J. Near critical and supercritical impregnation and kinetic release of thymol in LLDPE films used for food packaging. J. Supercrit. Fluids 2014, 85, 41–48. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Martínez, F.; Salazar, L.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Assessment of kinetic release of thymol from LDPE nanocomposites obtained by supercritical impregnation: Effect of depressurization rate and nanoclay content. Eur. Polym. J. 2017, 93, 294–306. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Villegas, C.; Garrido, L.; Roa, K.; Torres, A.; Galotto, M.; Rojas, A.; Romero, J. Modifying an Active Compound’s Release Kinetic Using a Supercritical Impregnation Process to Incorporate an Active Agent into PLA Electrospun Mats. Polymers 2018, 10, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, A.; Torres, A.; Añazco, A.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Effect of pressure and time on scCO2-assisted incorporation of thymol into LDPE-based nanocomposites for active food packaging. J. CO2 Util. 2018, 26, 434–444. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. Part B Eng. 2019, 176, 107366. [Google Scholar] [CrossRef]
- Sepulveda, J.; Villegas, C.; Torres, A.; Vargas, E.; Rodriguez, F.; Baltazar, S.; Prada, A.; Rojas, A.; Romero, J.; Faba, S.; et al. Effect of functionalized silica nanoparticles on the mass transfer process in active PLA nanocomposite films obtained by supercritical impregnation for sustainable food packaging. J. Supercrit. Fluids 2020, 161, 104844. [Google Scholar] [CrossRef]
- Cerro, D.; Bustos, G.; Villegas, C.; Buendia, N.; Truffa, G.; Godoy, M.P.; Rodríguez, F.; Rojas, A.; Galotto, M.J.; Constandil, L.; et al. Effect of supercritical incorporation of cinnamaldehyde on physical-chemical properties, disintegration and toxicity studies of PLA/lignin nanocomposites. Int. J. Biol. Macromol. 2021, 167, 255–266. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Galotto, M.J.; Guarda, A.; Romero, J. Supercritical impregnation for food applications: A review of the effect of the operational variables on the active compound loading. Crit Rev. Food Sci. Nutr. 2020, 60, 1290–1301. [Google Scholar] [CrossRef]
- Díaz-Barrera, A.; Andler, R.; Martínez, I.; Peña, C. Poly-3-hydroxybutyrate production by Azotobacter vinelandii strains in batch cultures at different oxygen transfer rates. J. Chem. Technol. Biotechnol. 2016, 91, 1063–1071. [Google Scholar] [CrossRef]
- García, A.; Pérez, D.; Castro, M.; Urtuvia, V.; Castillo, T.; Díaz-Barrera, A.; Peña, C. Production and recovery of poly-3-hydroxybutyrate [P(3HB)] of ultra-high molecular weight using fed-batch cultures of Azotobacter vinelandii OPNA strain. J. Chem. Technol. Biotechnol. 2019, 94, 1853–1860. [Google Scholar] [CrossRef]
- Stoffers, N.H.; Brandsch, R.; Bradley, E.L.; Cooper, I.; Dekker, M.; Störmer, A.; Franz, R. Feasibility study for the development of certified reference materials for specific migration testing. Part 2: Estimation of diffusion parameters and comparison of experimental and predicted data. Food Addit. Contam. 2005, 22, 173–184. [Google Scholar] [CrossRef]
- STM D882-12, Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2012. [CrossRef]
- UNE-EN ISO-20200. Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Effect of chitosan and catechin addition on the structural, thermal, mechanical and disintegration properties of plasticized electrospun PLA-PHB biocomposites. Polym. Degrad. Stab. 2016, 132, 145–156. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Tensile properties, barrier properties, and biodegradation in soil of compression-molded gelatin-dialdehyde starch films. J. Appl. Polym. Sci. 2009, 112, 2166–2178. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- C.E. 13130-1. Materials and Articles in Contact with Foodstuffs-Plastic Substances Subject to Limitation. Part 1: Guide to Test Methods for the Specific Migration of Substances from Plastics to Foods and Food Simulants and the Determination of substances in Plastics and the Selection of Exposure to Food Simulants; CEN: Brussels, Belgium, 2004.
- Galotto, M.J.; Torres, A.; Guarda, A.; Moraga, N.; Romero, J. Experimental and theoretical study of LDPE versus different concentrations of Irganox 1076 and different thickness. Food Res. Int. 2011, 44, 566–574. [Google Scholar] [CrossRef]
- Halász, K.; Csóka, L. Plasticized biodegradable poly (lactic acid) based composites containing cellulose in micro-and nanosize. J. Eng. 2013, 2013, 329379. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Liu, L.; Zhang, J. Manipulating dispersion and distribution of graphene in PLA through novel interface engineering for improved conductive properties. ACS Appl. Mater. Interfaces 2014, 6, 14069–14075. [Google Scholar] [CrossRef]
- Maurer, F.H.; Arza, C.R. Dispersion and interaction of graphene oxide in amorphous and semi-crystalline nano-composites: A PALS study. J. Phys. Conf. Ser. 2015, 618, 012020. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Nie, R.; Chen, P.; Hou, Z. Selective hydrogenation of cinnamaldehyde over reduced graphene oxide supported Pt catalyst. Catal. Commun. 2013, 41, 101–105. [Google Scholar] [CrossRef]
- Akgün, M.; Başaran, İ.; Suner, S.C.; Oral, A. Geraniol and cinnamaldehyde as natural antibacterial additives for poly (lactic acid) and their plasticizing effects. J. Polym. Eng. 2020, 40, 38–48. [Google Scholar] [CrossRef]
- Gong, M.; Zhao, Q.; Dai, L.; Li, Y.; Jiang, T. Fabrication of polylactic acid/hydroxyapatite/graphene oxide composite and their thermal stability, hydrophobic and mechanical properties. J. Asian Ceram. Soc. 2017, 5, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and characterization of graphene oxide (GO) and reduced graphene oxide (rGO) for gas sensing application. Macromol. Symp. 2017, 376, 1700006. [Google Scholar] [CrossRef]
- Riba, J.R.; Cantero, R.; García-Masabet, V.; Cailloux, J.; Canals, T.; Maspoch, M.L. Multivariate identification of extruded PLA samples from the infrared spectrum. J. Mater. Sci. 2020, 55, 1269–1279. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly(lactic acid)-modified films for food packaging application: Physical, mechanical, and barrier behavior. J. Appl. Polym. Sci. 2012, 125, E390–E401. [Google Scholar] [CrossRef]
- Ramezani, M.; Amoozegar, M.A.; Ventosa, A. Screening and comparative assay of poly-hydroxyalkanoates produced by bacteria isolated from the Gavkhooni Wetland in Iran and evaluation of poly-β-hydroxybutyrate production by halotolerant bacterium Oceanimonas sp. GK1. Ann. Microbiol. 2015, 65, 517–526. [Google Scholar] [CrossRef]
- Lai, S.M.; Liu, Y.H.; Huang, C.T.; Don, T.M. Miscibility and toughness improvement of poly(lactic acid)/poly (3-Hydroxybutyrate) blends using a melt-induced degradation approach. J. Polym. Res. 2017, 24, 1–12. [Google Scholar] [CrossRef]
- Strong, P.J.; Laycock, B.; Mahamud, S.N.S.; Jensen, P.D.; Lant, P.A.; Tyson, G.; Pratt, S. The opportunity for high-performance biomaterials from methane. Microorganisms 2016, 4, 11. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA–PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Zou, H.; Yi, C.; Wang, L.; Liu, H.; Xu, W. Thermal degradation of poly (lactic acid) measured by thermogravimetry coupled to Fourier transform infrared spectroscopy. J. Therm. Anal. Calorim. 2009, 97, 929–935. [Google Scholar] [CrossRef]
- Pachekoski, W.M.; Dalmolin, C.; Agnelli, J.A.M. The influence of the industrial processing on the degradation of poly (hydroxybutyrate)—PHB. Mater. Res. 2013, 16, 237–332. [Google Scholar] [CrossRef] [Green Version]
- Reis, K.C.; Pereira, L.; Melo, I.C.N.A.; Marconcini, J.M.; Trugilho, P.F.; Tonoli, G.H.D. Particles of coffee wastes as reinforcement in polyhydroxybutyrate (PHB) based composites. Mater. Res. 2015, 18, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Bian, J.; Lin, H.L.; Wang, G.; Zhou, Q.; Wang, Z.J.; Zhou, X.; Lu, Y.; Zhao, X.W. Morphological, Mechanical and Thermal Properties of Chemically Bonded Graphene Oxide Nanocomposites with Biodegradable Poly(3-hydroxybutyrate) by Solution Intercalation. Polym. Polym. Compos. 2016, 24, 133–141. [Google Scholar] [CrossRef]
- Huang, H.D.; Ren, P.G.; Xu, J.Z.; Xu, L.; Zhong, G.J.; Hsiao, B.S.; Li, Z.M. Improved barrier properties of poly(lactic acid) with randomly dispersed graphene oxide nanosheets. J. Memb. Sci. 2014, 464, 110–118. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Wang, Y. Development of PLA-PHB-based biodegradable active packaging and its application to salmon. Packag. Technol. Sci. 2018, 31, 739–746. [Google Scholar] [CrossRef]
- Sridhar, V.; Lee, I.; Chun, H.H.; Park, H. Graphene reinforced biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) nano-composites. eXPRESS Polym. Lett. 2013, 7, 320–328. [Google Scholar] [CrossRef]
- de Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of cinnamaldehyde into cassava starch biocomposite films using supercritical fluid technology for the development of food active packaging. Carbohydr. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez-Díaz, M.; Meneses-Acosta, A.; Romo-Uribe, A.; Peña, C.; Segura, D.; Espin, G. Thermo-mechanical properties, microstructure and biocompatibility in poly-β-hydroxybutyrates (PHB) produced by OP and OPN strains of Azotobacter vinelandii. Eur. Polym. J. 2015, 63, 101–112. [Google Scholar] [CrossRef]
- Berwig, K.H.; Baldasso, C.; Dettmer, A. Production and characterization of poly (3-hydroxybutyrate) generated by Alcaligenes latus using lactose and whey after acid protein precipitation process. Bioresour. Technol. 2016, 218, 31–37. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef]
- Zhang, X.; Geng, B.; Chen, H.; Chen, Y.; Wang, Y.; Zhang, L.; Liu, H.; Yang, H.; Chen, J. Extraordinary toughness enhancement of poly (lactic acid) by incorporating very low loadings of noncovalent functionalized graphene-oxide via masterbatch-based melt blending. Chem. Eng. J. 2018, 334, 2014–2020. [Google Scholar] [CrossRef]
- Fortunati, E.; Puglia, D.; Santulli, C.; Sarasini, F.; Kenny, J.M. Biodegradation of Phormium tenax/poly(lactic acid) composites. J. Appl. Polym. Sci. 2012, 125, E562–E572. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA–PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.T.; Choi, M.K.; Sim, Y.; Lim, J.T.; Kim, G.S.; Seong, M.J.; Hyung, J.H.; Kim, K.S.; Umar, A.; Lee, S.K. Effect of graphene oxide ratio on the cell adhesion and growth behavior on a graphene oxide-coated silicon substrate. Sci. Rep. 2016, 6, 33835. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Han, M.; Ryu, B.D.; Hyung, J.H.; Han, N.; Park, Y.J.; Ko, K.B.; Kang, K.K.; Cuong, T.V.; Hong, C.H. Enhanced thermal stability of reduced graphene oxide-Silicon Schottky heterojunction solar cells via nitrogen doping. Mater. Sci. Semicond. Process. 2017, 59, 45–49. [Google Scholar] [CrossRef]
- Rojas, A.; Velásquez, E.; Garrido, L.; Galotto, M.J.; López de Dicastillo, C. Design of active electrospun mats with single and core-shell structures to achieve different curcumin release kinetics. J. Food Eng. 2020, 273, 1288. [Google Scholar] [CrossRef]

| Materials | Tensile Strength (MPa) | Tensile Modulus (MPa) | Elongation of Break (%) |
|---|---|---|---|
| PLA | 2677 ± 190 a | 50 ± 4 a | 4.7 ± 1.2 a |
| PLA/Ci | 119 ± 50 c | 20 ± 4 b | 12.5 ± 5 b |
| PLA/GO | 532 ± 248 b | 40 ± 4 c | 4.4 ± 0.2 a |
| PLA/GO/Ci | 111 ± 68 c | 26 ± 4 b | 5.3 ± 0.6 a |
| PLA/PHB | 850 ± 413 d | 34 ± 12 c | 12 ± 5 b |
| PLA/PHB/Ci | 526 ± 264 b | 57 ± 4 d | 4.6 ± 0.5 a |
| PLA/PHB/GO | 951 ± 366 d | 38 ± 9 c | 8 ± 5 a |
| PLA/PHB/GO/Ci | 302 ± 163 c | 24 ± 12 b | 16 ± 8 b |
| Simulant | Polymeric System | KP/S | Deff (m2 s−1) | RMSE (%) |
|---|---|---|---|---|
| EtOH 10% | PLA/Ci | 950 ± 31 | 5.0 × 10−15 | 1.5 |
| PLA/GO/Ci | 450 ± 38 | 8.0 × 10−15 | 2.0 | |
| PLA/PHB/Ci | 460 ± 37 | 6.2 × 10−15 | 1.9 | |
| PLA/PHB/GO/Ci | 380 ± 36 | 6.0 × 10−15 | 2.4 | |
| EtOH 50% | PLA/Ci | 135 ± 20 | 4.7 × 10−14 | 1.1 |
| PLA/GO/Ci | 160 ± 19 | 9.9 × 10−14 | 0.8 | |
| PLA/PHB/Ci | 57 ± 11 | 8.7 × 10−14 | 0.9 | |
| PLA/PHB/GO/Ci | 24 ± 5 | 1.5 × 10−13 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas, C.; Torres, A.; Bruna, J.; Bustos, M.I.; Díaz-Barrera, A.; Romero, J.; Rojas, A.; Guarda, A. Obtaining Active Polylactide (PLA) and Polyhydroxybutyrate (PHB) Blends Based Bionanocomposites Modified with Graphene Oxide and Supercritical Carbon Dioxide (scCO2)-Assisted Cinnamaldehyde: Effect on Thermal-Mechanical, Disintegration and Mass Transport Properties. Polymers 2021, 13, 3968. https://doi.org/10.3390/polym13223968
Villegas C, Torres A, Bruna J, Bustos MI, Díaz-Barrera A, Romero J, Rojas A, Guarda A. Obtaining Active Polylactide (PLA) and Polyhydroxybutyrate (PHB) Blends Based Bionanocomposites Modified with Graphene Oxide and Supercritical Carbon Dioxide (scCO2)-Assisted Cinnamaldehyde: Effect on Thermal-Mechanical, Disintegration and Mass Transport Properties. Polymers. 2021; 13(22):3968. https://doi.org/10.3390/polym13223968
Chicago/Turabian StyleVillegas, Carolina, Alejandra Torres, Julio Bruna, María Ignacia Bustos, Alvaro Díaz-Barrera, Julio Romero, Adrián Rojas, and Abel Guarda. 2021. "Obtaining Active Polylactide (PLA) and Polyhydroxybutyrate (PHB) Blends Based Bionanocomposites Modified with Graphene Oxide and Supercritical Carbon Dioxide (scCO2)-Assisted Cinnamaldehyde: Effect on Thermal-Mechanical, Disintegration and Mass Transport Properties" Polymers 13, no. 22: 3968. https://doi.org/10.3390/polym13223968