On the Applicability of Electrophoresis for Protein Quantification
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nice, E.C. The separation sciences, the front end to proteomics: An historical perspective. Biomed. Chromatogr. 2021, 35, e4995. [Google Scholar] [CrossRef]
- Agregan, R.; Echegaray, N.; Lopez-Pedrouso, M.; Kharabsheh, R.; Ruiz, D.J.F.; Lorenzo, J.M. Proteomic advances in milk and dairy products. Molecules 2021, 26, 3832. [Google Scholar] [CrossRef]
- Stadlmann, J.; Taubenschmid, J.; Wenzel, D.; Gattinger, A.; Dürnberger, G.; Dusberger, F.; Elling, U.; Mach, L.; Mechtler, K.; Penninger, M. Comparative glycoproteomics of stem cells identifies new players in ricin toxicity. Nature 2017, 549, 538–542. [Google Scholar] [CrossRef]
- Lopez-Pedrouso, M.; Lorenzo, J.M.; Gagaoua, M.; Ruiz, D.J.F. Current trends in proteomic advances for food allergen analysis. Biology 2020, 9, 247. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. A systematic evaluation of various methods for quantifying food protein hydrolysate peptides. Food Chem. 2019, 270, 25–31. [Google Scholar] [CrossRef]
- Gagnon, P.; Goricar, B.; Mencin, N.; Zvanut, T.; Peljhan, S.; Leskovec, M.; Strancar, A. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021, 13, 113. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.; Wang, B.; Wang, H.; Jin, S.; Yang, Z.; Chen, Y.; Quan, Y.; Xiang, X. A Sensitive HPLC-MS/MS method for the quantification of selegiline in Beagle dog plasma: Application to a pharmacokinetic study. Curr. Pharm. Anal. 2021, 17, 140–148. [Google Scholar] [CrossRef]
- Lagoutte-Renosi, J.; Royer, B.; Rabani, V.; Davani, S. Method for the determination of plasma ticagrelor and its active metabolite useful for research and clinical practice validation of an HPLC–MS/MS. Molecules 2021, 26, 278. [Google Scholar] [CrossRef]
- Faller, A.C.; Arunachalam, T.; Shanmughanandhan, D.; Kesanakurti, P.; Shehata, H.R.; Ragupathy, S.; Newmaster, S.G. Investigating appropriate molecular and chemical methods for ingredient identity testing of plant-based protein powder dietary supplements. Sci. Rep. 2019, 9, 12130. [Google Scholar] [CrossRef]
- De Silva Neto, G.F.; de Andrade Rodrigues, M.L.; Fonseca, A. A new quantitative gel electrophoresis method with image-based detection for the determination of food dyes and metallic ions. Talanta 2021, 221, 121602. [Google Scholar] [CrossRef]
- Peterson, G.L. Determination of total protein. Methods Enzymol. 1983, 91, 95–119. [Google Scholar]
- Serackis, A.; Matuzevicius, D.; Navakauskas, D.; Sabanovic, E.; Katkevicius, A.; Plonis, D. A robust identification of the protein standard bands in two-dimensional electrophoresis gel image. Electr. Control Commun. Eng. 2017, 13, 63–65. [Google Scholar] [CrossRef] [Green Version]
- Kurien, B.T.; Scofield, R.H. Electrophoretic Separation of Proteins: Methods and Protocols; Humana Press: New York, NY, USA, 2019; 523p. [Google Scholar]
- Tanford, C.; Reynolds, J.A. Characterization of membrane proteins in detergent solutions. Biochim. Biophys. Acta 1976, 457, 33–70. [Google Scholar] [CrossRef]
- Macheleidt, J.; Kniemeyer, O. Serological Proteome Analysis for the Characterization of Secreted Fungal Protein Antigens. In Host-Fungal Interactions. Methods in Molecular Biology; Bignell, E., Ed.; Humana: New York, NY, USA, 2021; Volume 2260, pp. 15–26. [Google Scholar]
- Dietrich, M.A.; Judycka, S.; Słowińska, M.; Kodzik, N.; Ciereszko, A. Short-term storage-induced changes in the proteome of carp (Cyprinus carpio L.) spermatozoa. Aquaculture 2021, 530, 735784. [Google Scholar] [CrossRef]
- Tans, R.; van Rijswijck, D.M.H.; Davidson, A.; Hannam, R.; Ricketts, B.; Tack, C.J.; Weccels, H.J.C.T.; Gloerich, J.; van Gool, A.J. Affimers as an alternative to antibodies for protein biomarker enrichment. Protein Expr. Purif. 2020, 174, 105677. [Google Scholar] [CrossRef]
- Lu, Y.; Fu, T. Performance of commercial colorimetric assays for quantitation of total soluble protein in thermally treated milk samples. Food Anal. Methods 2020, 13, 1337–1345. [Google Scholar] [CrossRef]
- Laemmly, U.K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Hong, T.; Zhu, G. Proximity ligation assay: An ultrasensitive method for protein quantification and its applications in pathogen detection. Appl. Microbiol. Biotechnol. 2021, 105, 923–935. [Google Scholar] [CrossRef]
- Posch, A.; Kollmann, F.; Berkelman, T.; Dreskin, E. Sample preparation of secreted mammalian host cell proteins and their characterization by two-dimensional electrophoresis and western blotting. In Proteomic Profiling. Methods in Molecular Biology; Posch, A., Ed.; Humana: New York, NY, USA, 2021; Volume 2261, pp. 507–524. [Google Scholar]
- Gavrilova, K.V.; Bychkov, A.L.; Bychkova, E.S.; Akimenko, Z.A.; Chernonosov, A.A.; Kalambet, J.A.; Lomovskii, O.I. Mechanically activated hydrolysis of protein plant materials for the production of food components. Food Raw Mater. 2019, 7, 255–263. [Google Scholar] [CrossRef]
- Pogue, G.P.; Vojdani, F.; Palmer, K.E.; Whate, E.; Haydon, H.; Bratcher, B. Production of pharmaceutical grade recombinant native aprotinin and non-oxidized aprotinin variants under greenhouse and field conditions. In Commercial Plant-Produced Recombinant Protein Products; Howard, J., Hood, E., Eds.; Springer: Berlin, Germany, 2014; Volume 68, pp. 65–80. [Google Scholar]
- Sandberg, A.-S. Developing functional ingredients: A case study of pea protein. Funct. Foods 2011, 3, 358–382. [Google Scholar]
- Liu, S.; Li, Z.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Recent advances on protein separation and purification methods. Adv. Colloid Interface Sci. 2020, 284, 102254. [Google Scholar] [CrossRef]
- Shkuratova, N.A.; Neprienko, E.N.; Sharova, O.V.; Makarova, E.A.; Doroshenko, A.S. Comparative assessment of aprotinin quantification using different methods. Sib. J. Clin. Exp. Med. 2009, 2, 25–29. [Google Scholar]
- Boursier, B.; Delebarre, M.; Lis, J.; Marquilly, P.H. Textured Pea Proteins. RF Patent No. 2008108500/13, 10 September 2009. [Google Scholar]
- Kala, R.; Samkova, E.; Hanus, O.; Pecova, L.; Sekmokas, K.; Riaukiene, D. Milk protein analysis: An overview of the methods—Development and application. Acta Univ. Agric. Silvic. Mendel. Brun. 2019, 67, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Zemskova, L.A.; Sheveleva, I.V.; Sukhoverov, S.V.; Voit, F.V.; Sergienko, V.I.; Avramenko, V.A. The Method for Isolation and Purification of Bovine Serum Albumin. RF Patent No. 2005106613/13, 20 August 2006. [Google Scholar]
- Hickman, R.K.; Huang, Q.; Weed, C.L.; Ennis, S.T.; Perilli-Palmer, B.; Wan, M. Isolation and Purification of Antibodies Using Protein a Affinity Chromatography. RF Patent No. 2011120191/10, 27 November 2012. [Google Scholar]
- Aitekenov, S.; Gaipov, A.; Bukasov, R. Review: Detection and quantification of proteins in human urine. Talanta 2021, 223, 121718. [Google Scholar] [CrossRef] [PubMed]
- Londero, J.E.L.; Schavinski, C.R.; da Silva, F.D.; Piccoli, B.C.; Schuch, A.P. Development of a rapid electrophoretic assay for genomic DNA damage quantification. Ecotoxicol. Environ. Saf. 2021, 210, 111859. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, K.; Krasilov, V.; Malahova, I.; Kozmin, Y.; Kalambet, Y. Data processing in planar chromatography and gel electrophoresis using “Chrom & Spec” software. Analitika 2014, 19, 56–61. [Google Scholar]
- Ampersand International, Inc. Chrom & Spec Developer. Available online: http://www.chromandspec.com (accessed on 8 November 2021).
- Chrom & Spec. Chromatography Control Center. Manual. 2019. Available online: http://www.chromandspec.com/documentation/chromspec-documentation/ (accessed on 8 November 2021).
- Moldoveanu, S.C.; David, V. Selection of the HPLC Method in Chemical Analysis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; 598p. [Google Scholar]
- Bateman, A. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar]
- Bairoch, A.; Apweiler, R.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. The Universal Protein Resource (UniProt). Nucleic Acids Res. 2005, 33, D154–D159. [Google Scholar] [CrossRef] [Green Version]
- Novex Tris-Glycine Gels. TermoFisher Scientific. Available online: https://www.thermofisher.com/ru/ru/home/life-science/protein-biology/protein-gel-electrophoresis/protein-gels/novex-tris-glycine-gels.html (accessed on 8 November 2021).
- Gan, S.K.E. GelApp: Mobile gel electrophoresis analyser. Nat. Methods Appl. Notes 2015, 1–2, an9643. [Google Scholar]


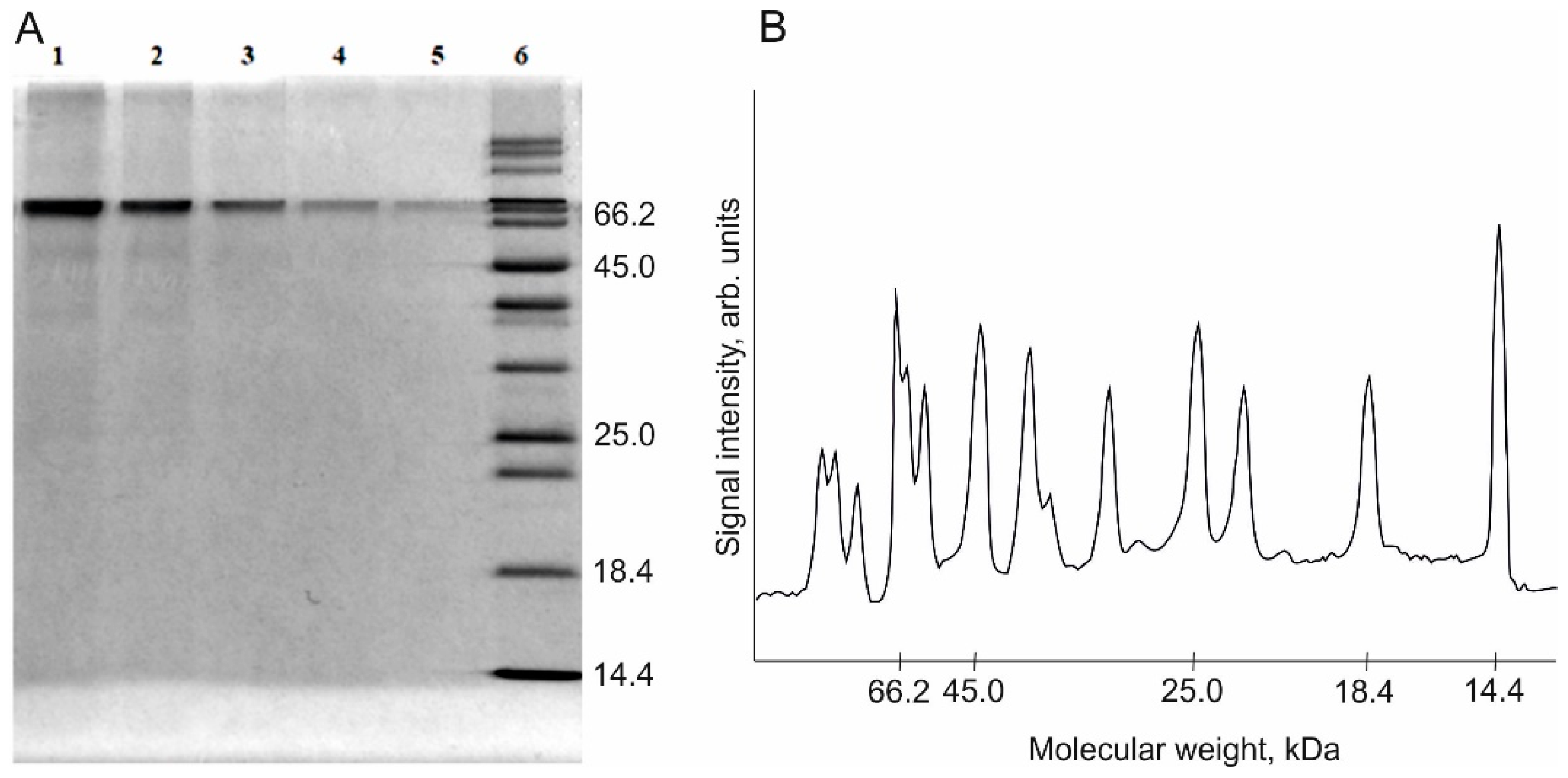
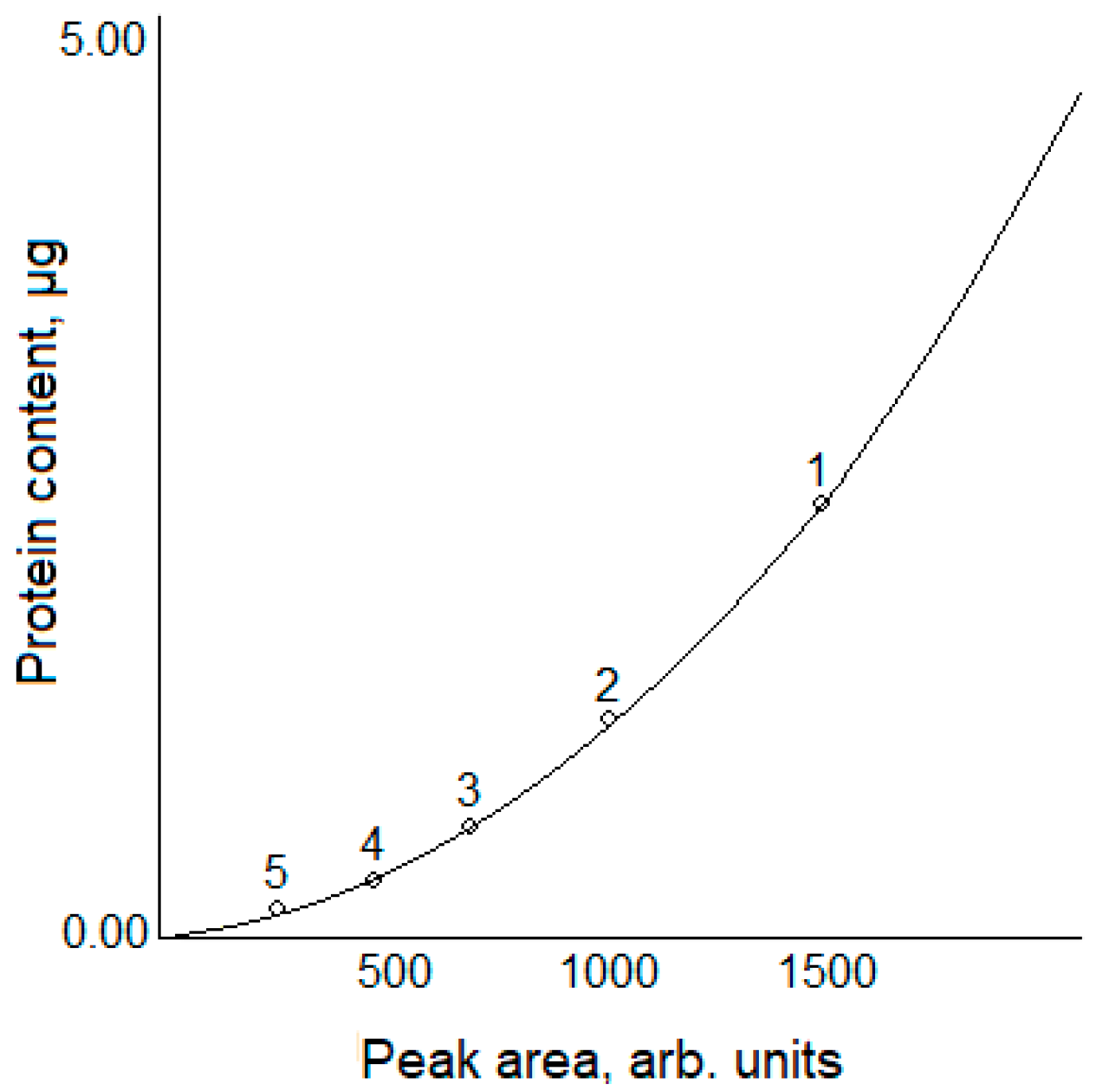
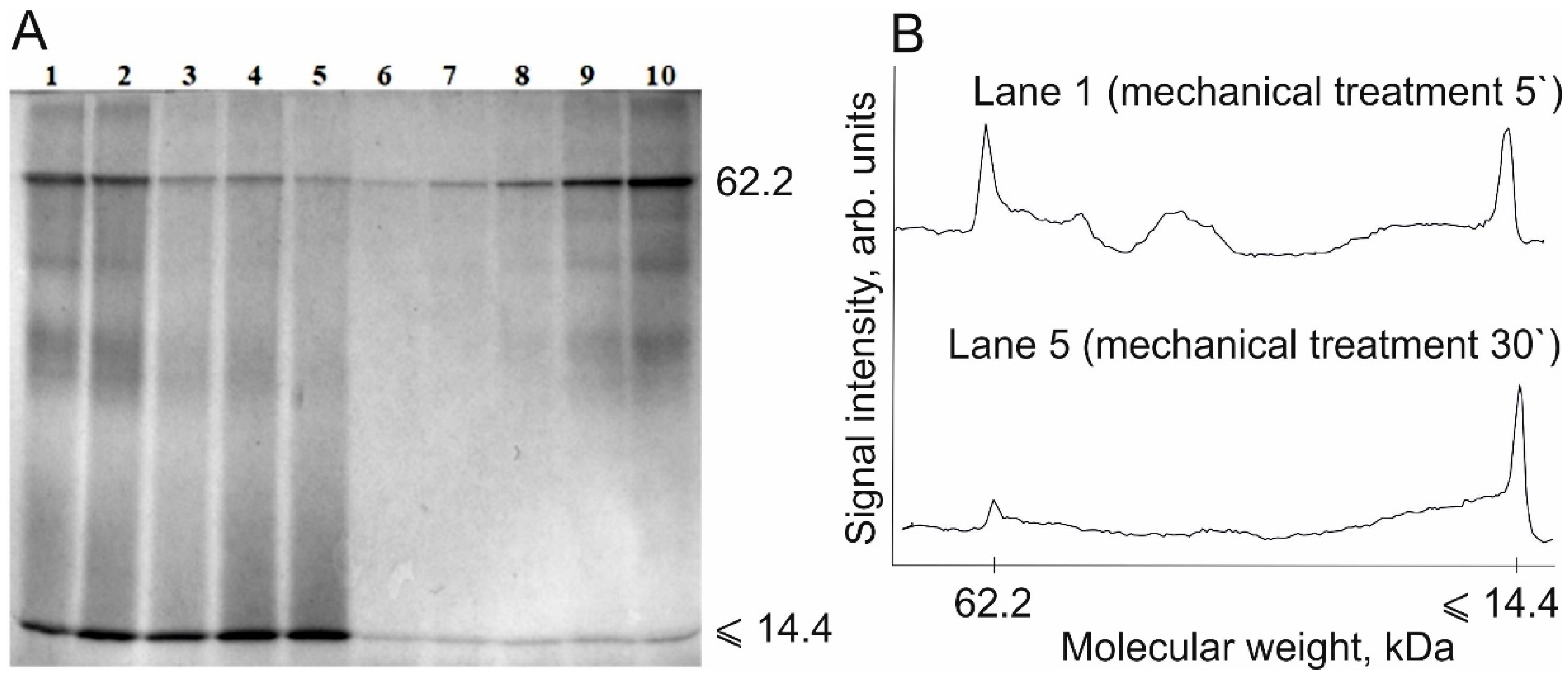
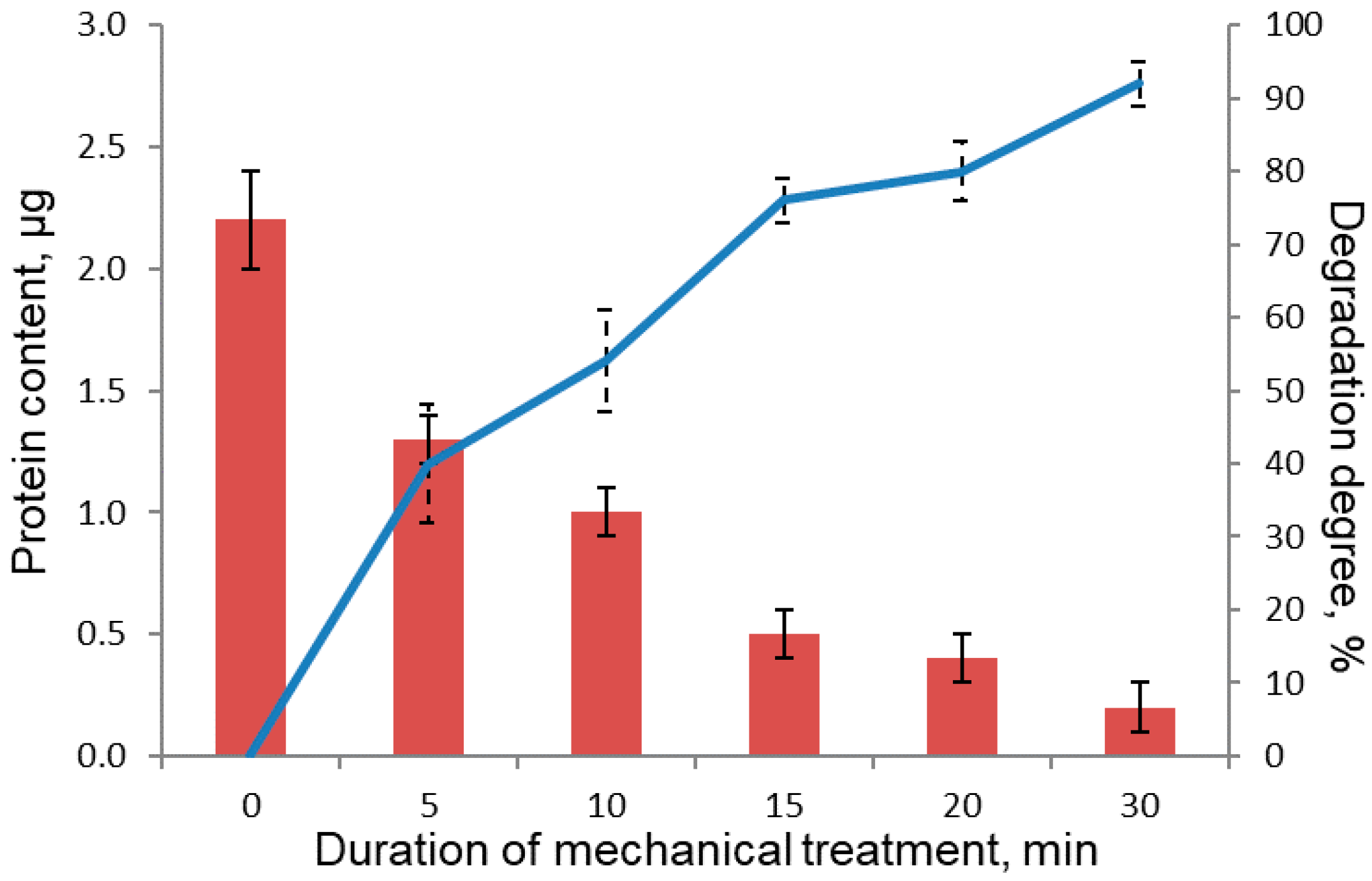


| Lane Number | Protein Content in the Sample, µg | Peak Area, Arb. Units |
|---|---|---|
| 1 | 2.0 | 1288 ± 49 |
| 2 | 1.0 | 934 ± 35 |
| 3 | 0.5 | 763 ± 29 |
| 4 | 0.25 | 525 ± 20 |
| 5 | 0.125 | 348 ± 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dome, K.; Akimenko, Z.; Bychkov, A.; Kalambet, Y.; Lomovsky, O. On the Applicability of Electrophoresis for Protein Quantification. Polymers 2021, 13, 3971. https://doi.org/10.3390/polym13223971
Dome K, Akimenko Z, Bychkov A, Kalambet Y, Lomovsky O. On the Applicability of Electrophoresis for Protein Quantification. Polymers. 2021; 13(22):3971. https://doi.org/10.3390/polym13223971
Chicago/Turabian StyleDome, Karina, Zoya Akimenko, Aleksey Bychkov, Yuri Kalambet, and Oleg Lomovsky. 2021. "On the Applicability of Electrophoresis for Protein Quantification" Polymers 13, no. 22: 3971. https://doi.org/10.3390/polym13223971






