Efficient Film Fabrication and Characterization of Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) (PEDOT:PSS)-Metalloporphine Nanocomposite and Its Application as Semiconductor Material
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
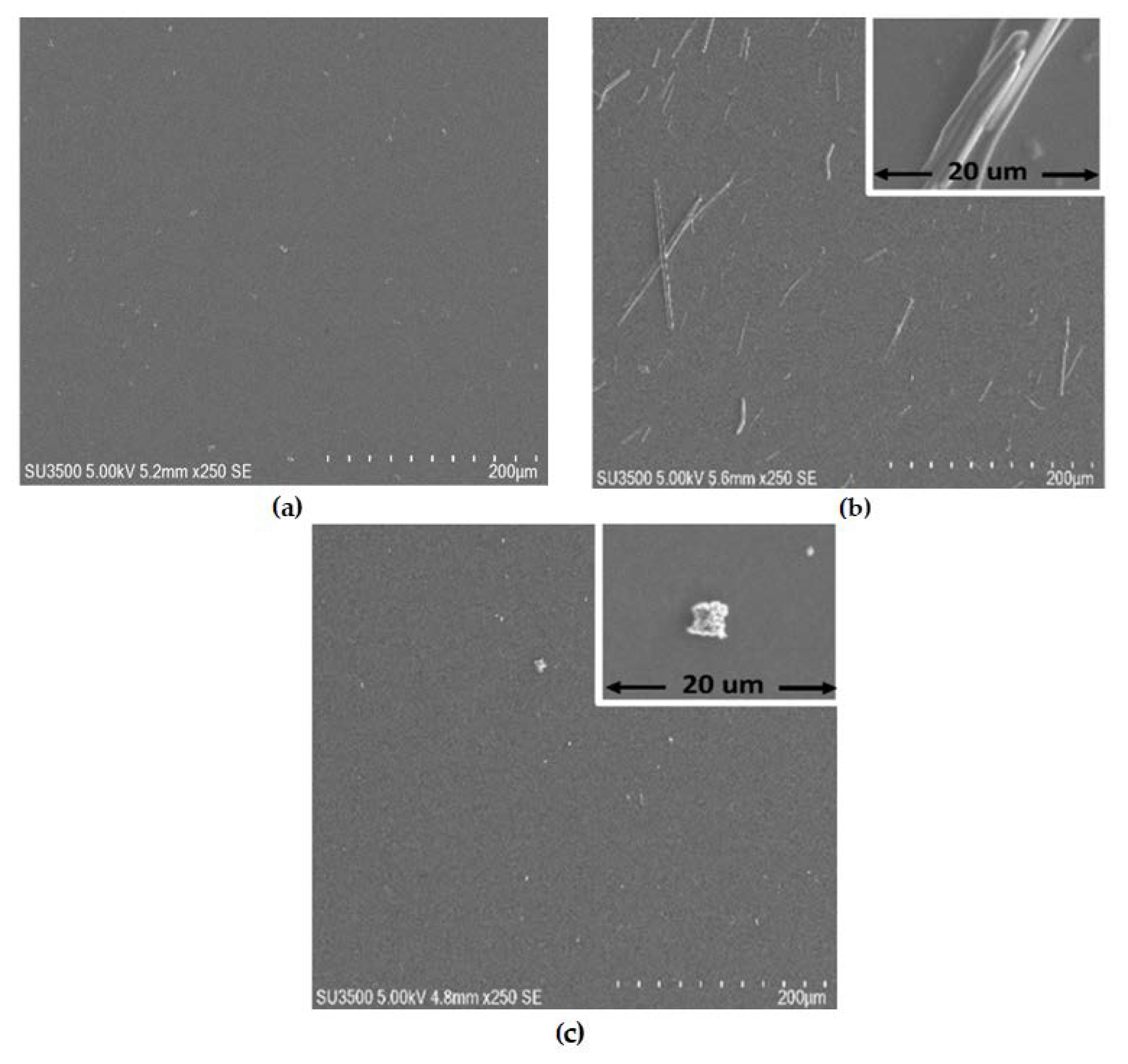
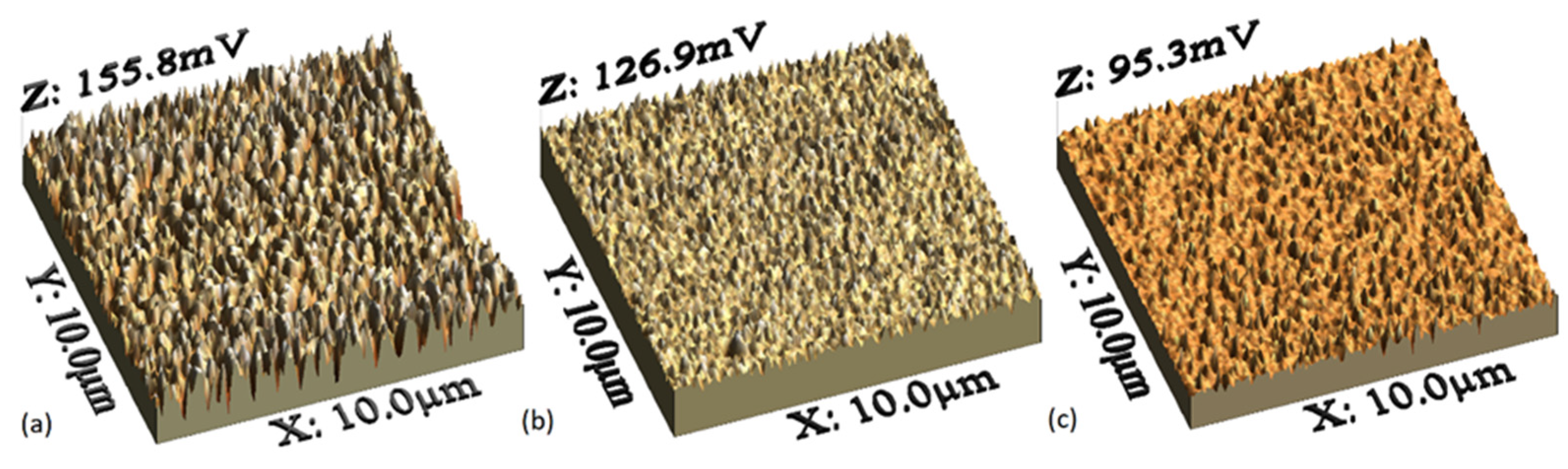
3.1. Structural and Morphological Characterization
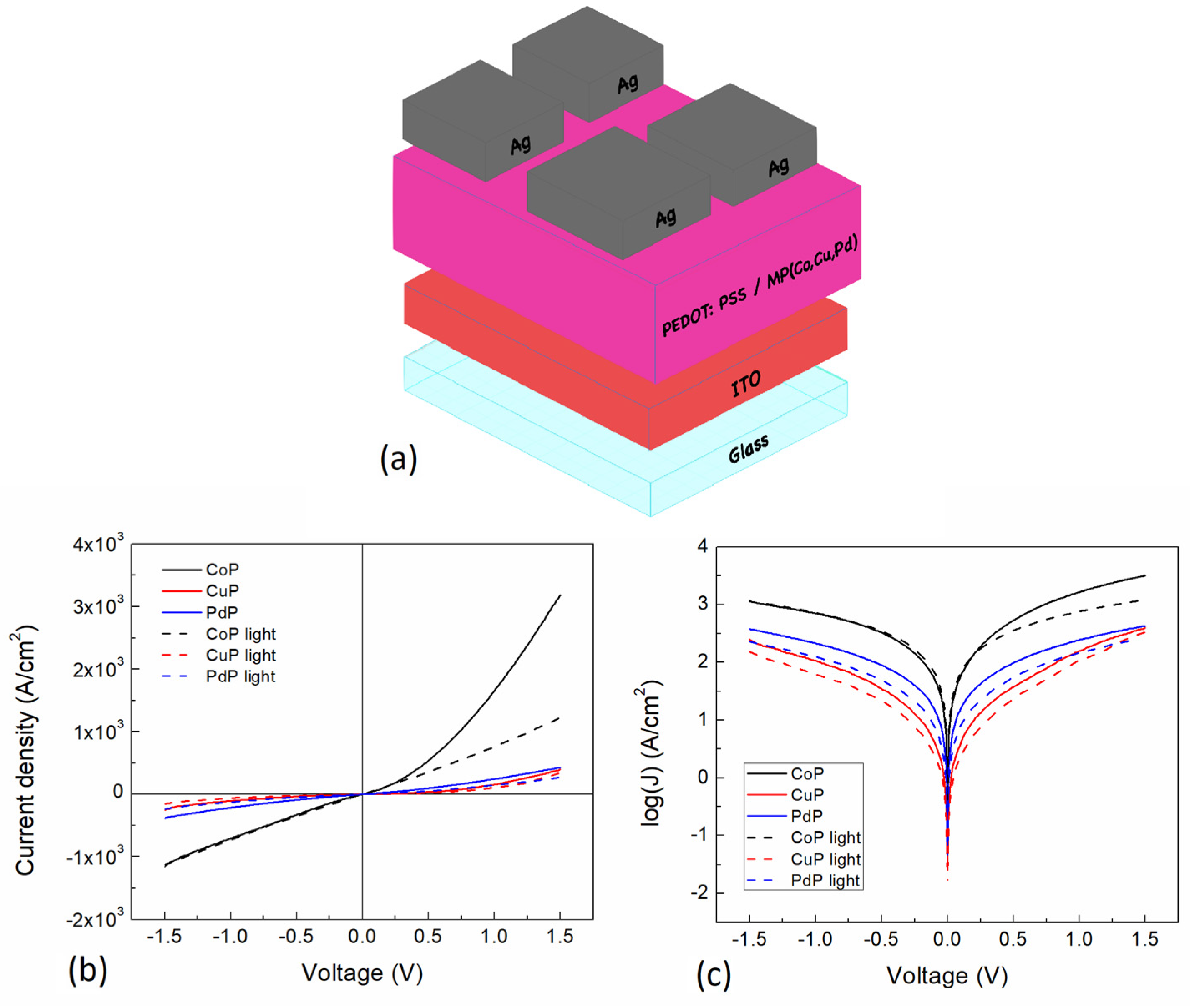
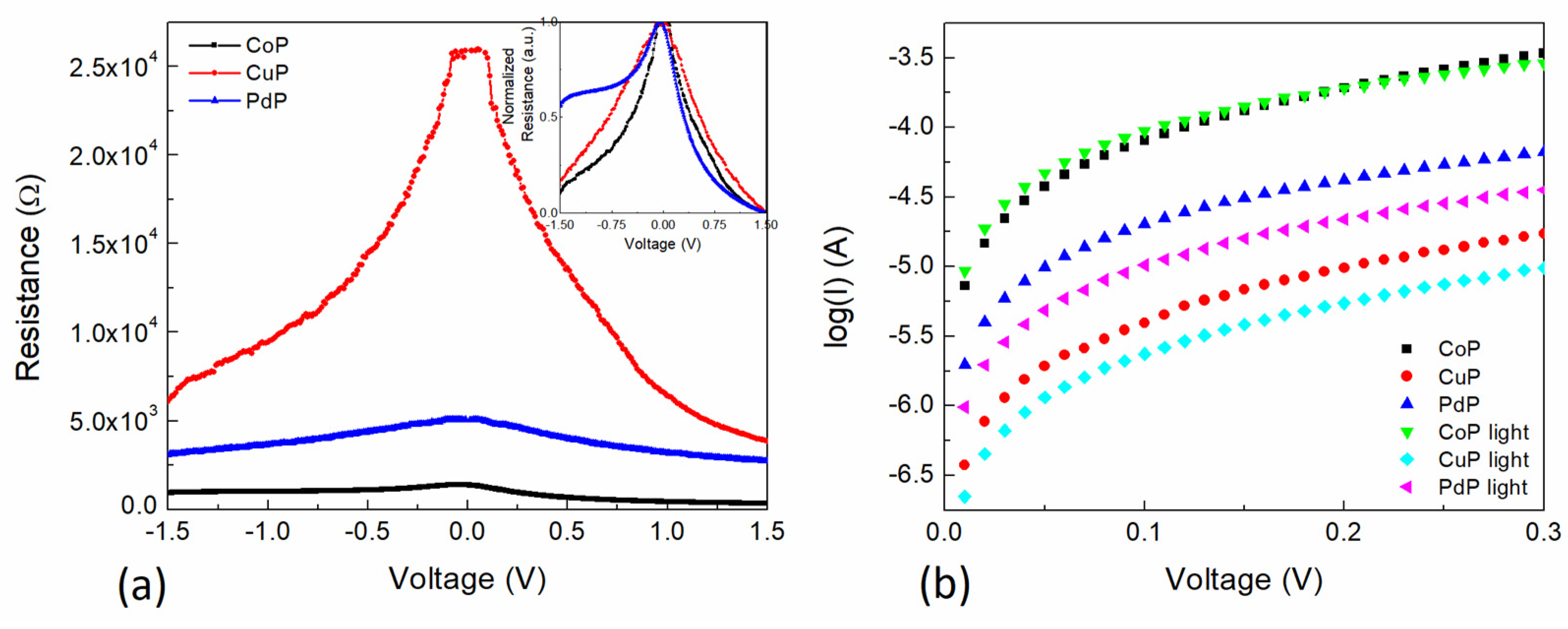
3.2. Electrical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puigdollers, J.; Voz, C.; Orpella, A.; Martin, I.; Vetter, M.; Alcubilla, R. Pentacene thin-films obtained by thermal evaporation in high vacuum. Thin Solid Films 2003, 427, 367–370. [Google Scholar] [CrossRef]
- Gebeyehu, D.; Maenning, B.; Drechsel, J.; Leo, K.; Pfeiffer, M. Bulk-heterojunction photovoltaic devices based on donor-acceptor organic small molecule blends. Sol. Energy Mater. Sol. Cells 2003, 79, 81–92. [Google Scholar] [CrossRef]
- Tonezzer, M.; Quaranta, A.; Maggioni, G.; Carturan, S.; Della Mea, G. Optical sensing responses of tetraphenyl porphyrins toward alcohol vapours: A comparison between vacuum evaporated and spin-coated thin films. Sens. Actuators B Chem. 2007, 122, 620–626. [Google Scholar] [CrossRef]
- Gryko, D.T.; Clausen, C.; Lindsey, J.S. Thiol-Derivatized Porphyrins for Attachment to Electroactive Surfaces. J. Org. Chem. 1999, 64, 8635–8647. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Wu, H.M.; Wei, Y.; Cheng, Z.P.; Wu, X.J. Structure and conductivity anisotropy of a new porphyrin Langmuir-Blodgett film. Solid State Commun. 1995, 95, 99–101. [Google Scholar] [CrossRef]
- George, H.; Guo, Q. Thin films of porphyrin molecules imaged with the atomic force microscope. Thin Solid Films 2009, 517, 2651–2655. [Google Scholar] [CrossRef]
- Reid, I.; Zhang, Y.; Demasi, A.; Blueser, A.; Piper, L.; Downes, J.E.; Matsuura, A.; Hughes, G.; Smith, K.E. Electronic structure of the organic semiconductor copper tetraphenylporphyrin (CuTPP). Appl. Surf. Sci. 2009, 256, 720–725. [Google Scholar] [CrossRef]
- Wróbel, D.; Siejak, A.; Siejak, P. Photovoltaic and spectroscopic studies of selected halogenated porphyrins for their application in organic solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 492–500. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Mele, G.; Duan, M.; Lu, X.; Palmisano, L.; Vasapollo, G.; Zhang, F. The photocatalytic activity of novel substituted porphyrin/TiO2-based composites. Dyes Pigments 2010, 84, 183–189. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting polymers: The Third Generation. Chem. Soc. Rev. 2010, 39, 2354–2371. [Google Scholar] [CrossRef]
- Heeger, A.J. 25th Anniversary Article: Bulk Heterojunction Solar Cells: Understanding the Mechanism of Operation. Adv. Mater. 2013, 26, 10–28. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, H.; Meng, Q.; Gong, X.; Hu, W. Organic photoresponse materials and devices. Chem. Soc. Rev. 2012, 41, 1754–1808. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Low Band Gap Conjugated Semiconducting Polymers. Adv. Mater. Technol. 2021, 6, 2000857. [Google Scholar] [CrossRef]
- Bulumulla, C.; Gunawardhana, R.; Gamage, P.L.; Miller, J.T.; Kularatne, R.N.; Biewer, M.C.; Stefan, M.C. Pyrrole-Containing Semiconducting Materials: Synthesis and Applications in Organic Photovoltaics and Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2020, 12, 32209–32232. [Google Scholar] [CrossRef] [PubMed]
- Lattante, S. Electron and Hole Transport Layers: Their use in Inverted Bulk Heterojunction Polymer Solar Cells. Electronics 2014, 3, 132–164. [Google Scholar] [CrossRef]
- Hilal, M.; Han, J.I. Improving the conductivity of PEDOT: PSS to nearly 1 million S/m with graphene on an ITO-glass substrate. Synth. Met. 2018, 245, 276–285. [Google Scholar] [CrossRef]
- Hilal, M.; Han, J.I. Study of interface chemistry between the carrier-transporting layers and their influences on the stability and performance of organic solar cells. Appl. Nanosci. 2018, 8, 1325–1341. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive PEDOT: PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Yousefi, M.H.; Fallahzadeh, A.; Saghaei, J.; Davoudi-Darareh, M. Fabrication of flexible ITO-free OLED using vapor-treated PEDOT: PSS thin film as anode. Disp. Technol. 2016, 12, 1647–1651. [Google Scholar] [CrossRef]
- Kepić, D.P.; Marković, Z.M.; Jovanović, S.P.; Peruško, D.B.; Budimir, M.D.; Holclajtner-Antunović, I.D.; Pavlović, V.B.; Todorović Marković, B.M. Preparation of PEDOT: PSS thin films doped with graphene and graphene quantum dots. Synt. Met. 2014, 198, 150–154. [Google Scholar] [CrossRef]
- Sarkhan, N.A.; Rahman, Z.A.; Zakaria, A.; Ali, A.M.M. Enhanced electrical properties of poly(3,4-ethylenedioxythiophene:poly(4-styrenesulfonate) using graphene oxide. Mater. Today Proc. 2019, 17, 484–489. [Google Scholar] [CrossRef]
- Stevens, D.L.; Parra, A.; Grunlan, J.C. Thermoelectric Performance Improvement of Polymer Nanocomposites by Selective Thermal Degradation. ACS Appl. Energy Mater. 2019, 2, 5975–5982. [Google Scholar] [CrossRef]
- Yeo, J.S.; Yun, J.M.; Kim, D.Y.; Park, S.; Kim, S.S.; Yoon, M.H.; Kim, T.W.; Na, S.I. Significant Vertical Phase Separation in Solvent-Vapor-Annealed Poly(3,4-ethylenedioxythiophene): Poly(styrene sulfonate) Composite Films Leading to Better Conductivity and Work Function for High-Performance Indium Tin Oxide-Free Optoelectronics. ACS Appl. Mater. Interfaces 2012, 4, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Davis, M. Porphyrin (Porphine)—A neglected parent compound with potential. J. Porphyr. Phthalocyanines 2010, 14, 556–557. [Google Scholar] [CrossRef] [Green Version]
- Fischer, H.; Gleim, W. Synthese des Porphins: Justus Liebigs. Ann. Chem. 1936, 521, 157–160. [Google Scholar] [CrossRef]
- Rothemund, P. A New Porphyrin Synthesis. The Synthesis of Porphin. J. Am. Chem. Soc. 1936, 58, 625–627. [Google Scholar] [CrossRef]
- Basiuk, V.A. Interaction of Porphine and Its Metal Complexes with C 60 Fullerene: A DFT Study. J. Phys. Chem. A 2005, 109, 3704–3710. [Google Scholar] [CrossRef]
- Berlein, H.P.; Müller, G.J. Applied Laser Medicine; Springer Science & Business Media: Luxembourg, 2003; pp. 275–276. [Google Scholar]
- Alharbi, S.R.; Darwish, A.A.A.; Al Garni, S.E.; El-Saeedy, H.I.; El-Rahman, K.F.A. Influence of thickness and annealing on linear and nonlinear optical properties of manganese (III) chloride tetraphenyl porphine (MnTPPCl) organic thin films. Infrared Phys. Technol. 2016, 78, 77–83. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; El-Nahass, M.M.; Oraby, A.H.; Meikhail, M.S.; Zeyada, H.M. Structural and optical characterization of thermally evaporated nanocrystalline 5,10,15,20-tetraphenyl-21H,23H-porphine manganese (III) chloride thin films. Optik 2018, 167, 204–217. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; El-Deeb, A.F.; Metwally, H.S.; Hassanien, A.M. Influence of annealing on the optical properties of 5,10,15,20-tetraphenyl-21H, 23H-porphine iron (III) chloride thin films. Mater. Chem. Phys. 2011, 125, 247–251. [Google Scholar] [CrossRef]
- Magdaong, N.C.M.; Taniguchi, M.; Diers, J.R.; Niedzwiedzki, D.M.; Kirmaier, C.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Photophysical Properties and Electronic Structure of Zinc (II) Porphyrins Bearing 0–4 meso-Phenyl Substituents: Zinc Porphine to Zinc Tetraphenylporphyrin (ZnTPP). J. Phys. Chem. A 2020, 124, 7776–7794. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Zgierski, M.Z. Porphine force field: In-plane normal modes of free-base porphine; comparison with metalloporphines and structural implications. Phys. Chem. 1991, 95, 4268–4287. [Google Scholar] [CrossRef]
- Cristescu, R.; Popescu, C.; Popescu, A.; Grigorescu, S.; Mihailescu, I.N.; Ciucu, A.A.; Iordache, S.; Andronie, A.; Stamatin, I.; Fagadar-Cosma, E.; et al. MAPLE deposition of Mn (III) metalloporphyrin thin films: Structural, topographical and electrochemical investigations. Appl. Surf. Sci. 2011, 257, 5293–5297. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Mirica, M.C.; Balcu, I.; Bucovicean, C.; Cretu, C.; Armeanu, I.; Fagadar-Cosma, G. Syntheses, spectroscopic and AFM characterization of some manganese porphyrins and their hybrid silica nanomaterials. Molecules 2009, 14, 1370–1388. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, M.S.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Khosropour, A.R. Manganese porphyrin immobilized on magnetite nanoparticles as a recoverable nanocatalyst for epoxidation of olefins. Mater. Chem. Phys. 2014, 146, 113–120. [Google Scholar] [CrossRef]
- Zhao, Q.; Jamal, R.; Zhang, L.; Wang, M.; Abdiryim, T. The structure and properties of PEDOT synthesized by template-free solution method. Nanoscale Res. Lett. 2014, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Yeon, C.; Kim, G.T.; Lim, J.W.; Yun, S.J. Highly conductive PEDOT: PSS treated by sodium dodecyl sulfate for stretchable fabric heaters. RSC Adv. 2017, 7, 5888. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.S.; Yun, Y.Y.; Nam, J.D.; Son, Y.; Lee, Y. Effect of magnetic field on electrochemical polymerization of EDOT. Synt. Met. 2008, 158, 1043–1046. [Google Scholar] [CrossRef]
- Corradi, R.; Armes, S. Chemical synthesis of poly(3,4-ethylenedioxythiophene). Synt. Met. 1997, 87, 453–454. [Google Scholar] [CrossRef]
- Pan, Z.; Zheng, Y.; Guo, F.; Niu, P.; Wang, X. Decorating CoP and Pt Nanoparticles on Graphitic Carbon Nitride Nanosheets to Promote Overall Water Splitting by Conjugated Polymers. ChemSusChem 2017, 10, 87–90. [Google Scholar] [CrossRef]
- Belykh, L.B.; Skripov, N.I.; Sterenchuk, T.P.; Gvozdovskaya, K.L.; Sanzhieva, S.B.; Schmidt, F.K. Pd-P nanoparticles as active catalyst for the hydrogenation of acetylenic compounds. Nanoparticle Res. 2019, 21, 9. [Google Scholar] [CrossRef]
- Zhao, G.H.; Luo, X.Z.; Chen, G.; Zhao, Y.J. A long-term static immersion experiment on the leaching behavior of heavy metals from waste printed circuit boards. Environ. Sci. Process. Impacts 2014, 16, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Pasha, A.; Khasim, S.; Khan, F.A.; Dhananjaya, N. Fabrication of gas sensor device using poly (3,4-ethylenedioxythiophene)-poly (styrenesulfonate)-doped reduced graphene oxide organic thin films for detection of ammonia gas at room temperature. Iran. Polym. J. 2019, 28, 183–192. [Google Scholar] [CrossRef]
- Zabihi, F.; Xie, Y.; Gao, S.; Eslamian, M. Morphology, conductivity, and wetting characteristics of PEDOT:PSS thin films deposited by spin and spray coating. Appl. Surf. Sci. 2015, 338, 163–177. [Google Scholar] [CrossRef]
- Laukaitis, G.; Lindroos, S.; Tamulevičius, S.; Leskelä, M.; Račkaitis, M. SILAR deposition of CdxZn1-xS thin films. Appl. Surf. Sci. 2000, 161, 396–405. [Google Scholar] [CrossRef]
- Ouyang, J.; Chu, C.W.; Chen, F.C.; Xu, Q.; Yang, Y. High-Conductivity Poly(3,4-ethylenedioxythiophene): Poly(styrene sulfonate) Film and Its Application in Polymer Optoelectronic Devices. Adv. Funct. Mater. 2005, 15, 203–208. [Google Scholar] [CrossRef]
- Han, Y.K.; Chang, M.Y.; Huang, W.Y.; Pan, H.Y.; Ho, K.S.; Hsieh, T.H.; Pan, S.Y. Improved Performance of Polymer Solar Cells Featuring One-Dimensional PEDOT Nanorods in a Modified Buffer Layer. J. Electrochem. Soc. 2011, 158, K88–K93. [Google Scholar] [CrossRef] [Green Version]
- Akins, D.L.; Zhu, H.R.; Guo, C. Absorption and Raman Scattering by Aggregated meso-Tetrakis(p-sulfonatophenyl)porphine. J. Phys. Chem. 1994, 98, 3612–3618. [Google Scholar] [CrossRef]
- Makhlouf, M.M.; Shehata, M.M.; Abdelhady, K. Tuning of structural and optical properties of 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine thin films as a promising photovoltaic absorber material. Opt. Mater. 2019, 98, 109378. [Google Scholar] [CrossRef]
- Longuet-Higgins, H.C.; Rector, C.W.; Platt, J.R. Molecular Orbital Calculations on Porphine and Tetrahydroporphine. J. Chem. Phys. 1950, 18, 1174–1181. [Google Scholar] [CrossRef]
- Bardeen, J.; Blatt, F.; Hall, L. Proceedings of the Photoconductivity Conference, 1st ed; Wiley: New York, NY, USA, 1956; p. 146. [Google Scholar]
- ElMahmoudy, M.; Inal, S.; Charrier, A.; Uguz, I.; Malliaras, G.G.; Sanaur, S. Tailoring the Electrochemical and Mechanical Properties of PEDOT:PSS Films for Bioelectronics. Macromol. Mater. Eng. 2017, 302, 1600497. [Google Scholar] [CrossRef]
- Hao, H.; Jianyong, O. Enhancements in the Mechanical Stretchability and Thermoelectric Properties of PEDOT:PSS for Flexible Electronics Applications. Acc. Mater. Res. 2020, 1, 146–157. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, Y.; Du, D.; Ouyang, J. PEDOT: PSS Films with Metallic Conductivity through a Treatment with Common Organic Solutions of Organic Salts and Their Application as a Transparent Electrode of Polymer Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 11629–11638. [Google Scholar] [CrossRef]
- Hosseini, E.; Ozhukil, V.; Karan, K. The key mechanism of conductivity in PEDOT: PSS thin films exposed by anomalous conduction behaviour upon solvent-doping and sulfuric acid post-treatment. Mater. Chem. C 2020, 8, 3982–3990. [Google Scholar] [CrossRef]
- Otley, M.; Alhashmi, A.F.; Guo, Y.; Santana, J.; Eren, E.; Li, M.; Lombardi, J.; Sotzing, G. Phase segregation of PEDOT:PSS on textile to produce materials of >10 A mm−2 current carrying capacity. Macromol. Mater. Eng. 2016, 302, 1600348. [Google Scholar] [CrossRef]


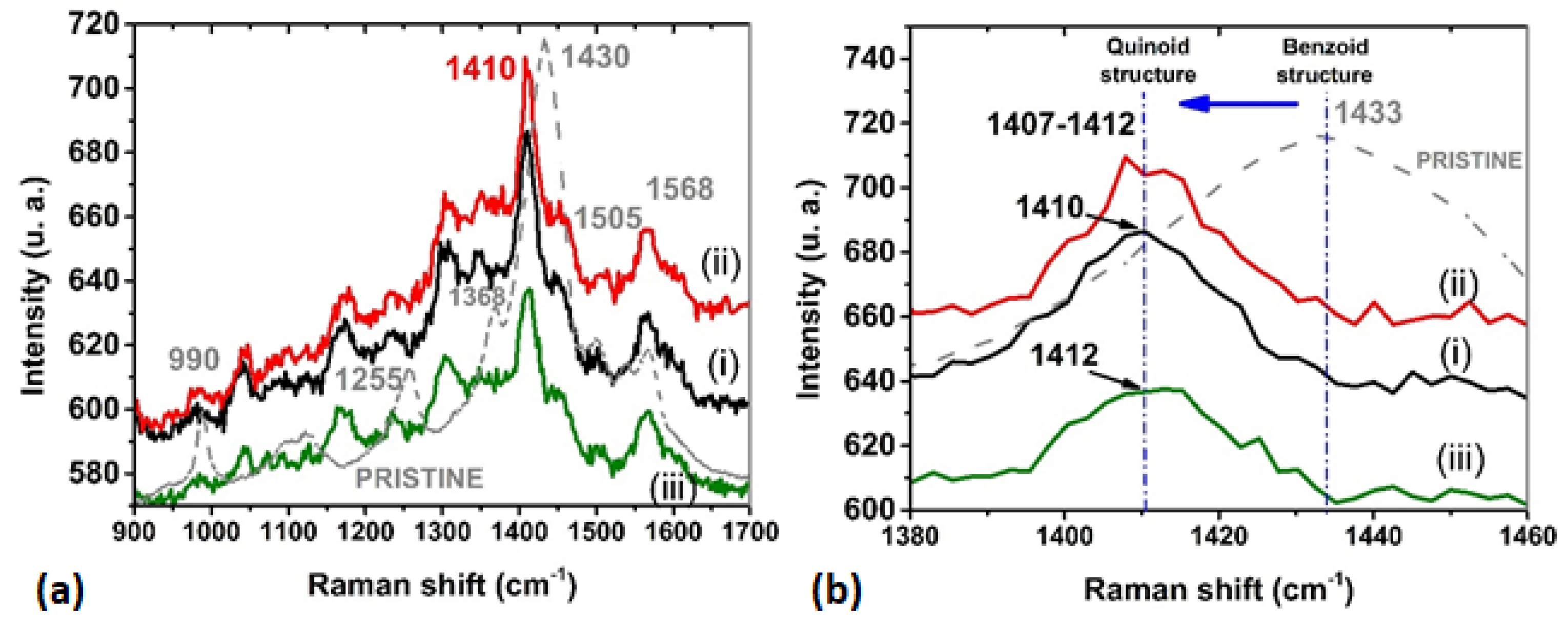


| CoP (cm−1) | CuP (cm−1) | PdP (cm−1) | Assignments * |
|---|---|---|---|
| 3442 | 3438 | 3442 | N–H pyrrole |
| 2929, 2858 | 2929, 2856 | 2929, 2856 | ν C–H Ph |
| 1484 | 1482 | 1484 | ν C=C Ph |
| 1457 | 1459 | 1457 | ν C=N |
| 1367 | 1367 | 1367 | ν C–N |
| 1228, 1153, 1056 | 1217, 1151, 1060 | 1229, 1151, 1058 | δ C–H Ph |
| 1020 | 1019 | 1017 | ν C–N pyrrole |
| 750, 701, 667 | 750, 700, 669 | 754, 692, 670 | γ C–H Ph |
| 558 | 556 | 560 | γ (pyr. fold) |
| 516 | 520 | 512 | δ C–C |
| 451, 409 | 451, 408 | 453, 415 | ν M–N |
| Film | RMS (nm) | Bandgap No Treatment (eV) | Bandgap with IPA Treatment (eV) |
|---|---|---|---|
| PEDOT:PSS-CoP | 107.99 ± 7.7 | 2.18 ± 0.2 | 2.87 ± 0.4 |
| PEDOT:PSS-CuP | 43.175 ± 5.3 | 2.98 ± 0.1 | 2.75 ± 0.3 |
| PEDOT:PSS-PdP | 6.82 ± 1.11 | 2.97 ± 0.3 | 3.74 ± 0.1 |
| Porphine | σ | σ | Jo | Jsc | Vo | Voc | Rshunt | Rseries | Rseries |
|---|---|---|---|---|---|---|---|---|---|
| (Scm−1) | (Scm−1) | (A/cm2) | (A/cm2) | (V) | (V) | (Ω) | (Ω) | (Ω) | |
| Reverse | Forward | Darkness @0.2 V | Light @0.2 V | ||||||
| Co | 1.39 × 103 | 1.92 × 103 | −2.28 × 10−1 | −3.92 × 10−1 | 3.92 × 10−4 | 5.28 × 10−4 | 1.30 × 10−3 | 5.40 × 102 | 5.71 × 102 |
| Cu | 1.23 × 102 | 1.58 × 102 | −2.54 × 10−2 | −1.50 × 10−2 | 6.01 × 10−4 | 5.98 × 10−4 | 1.58 × 10−2 | 1.05 × 104 | 1.92 × 104 |
| Pd | 4.00 × 102 | 4.42 × 102 | −1.38 × 10−1 | −4.13 × 10−2 | 7.92 × 10−4 | 4.84 × 10−4 | 5.65 × 10−3 | 2.57 × 103 | 4.84 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Vergara, M.E.; Hamui, L.; González-Verdugo, D.; Cosme, I. Efficient Film Fabrication and Characterization of Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) (PEDOT:PSS)-Metalloporphine Nanocomposite and Its Application as Semiconductor Material. Polymers 2021, 13, 4008. https://doi.org/10.3390/polym13224008
Sánchez-Vergara ME, Hamui L, González-Verdugo D, Cosme I. Efficient Film Fabrication and Characterization of Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) (PEDOT:PSS)-Metalloporphine Nanocomposite and Its Application as Semiconductor Material. Polymers. 2021; 13(22):4008. https://doi.org/10.3390/polym13224008
Chicago/Turabian StyleSánchez-Vergara, María Elena, Leon Hamui, Daniela González-Verdugo, and Ismael Cosme. 2021. "Efficient Film Fabrication and Characterization of Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) (PEDOT:PSS)-Metalloporphine Nanocomposite and Its Application as Semiconductor Material" Polymers 13, no. 22: 4008. https://doi.org/10.3390/polym13224008






