Sterilization Induced Changes in Polypropylene-Based Ffp2 Masks
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sterilization Protocols
2.3. Characterizations
2.3.1. Fourier Transform-Infrared Spectroscopy (FTIR)
2.3.2. Size Exclusion Chromatography (SEC)
- PP: ηTCB, 135 °C = 16.1 × 10−4 · Mw0.733;
- PS: ηTCB, 135 °C = 16.1 × 10−4 · Mw0.677.
2.3.3. Thermal-Desorption–Gas Chromatography–Mass Spectrometry (TD-GC-MS)
- Approximatively 10 mg of the surgery mask (one layer or four layers altogether) was introduced in a glass tube packed under helium atmosphere;
- Thermodesorption (TDS): 140 °C (10 min); helium 30 mL/min;
- Trapping: –30 °C; 40 °C/s to 180 °C; helium 30 mL/min;
- GC: 50 °C; 15 °C/min to 220 °C; helium 1.0 mL/min;
- MS: Transfer line temperature—200 °C; electron impact ionization—70 eV; scan range—10–300 m/z;
- Gases were concentrated on a cold trap and desorbed by a sudden rise in temperature. Only 2 vol% of the desorbed gases was sent to the GC-MS.
3. Results and Discussion
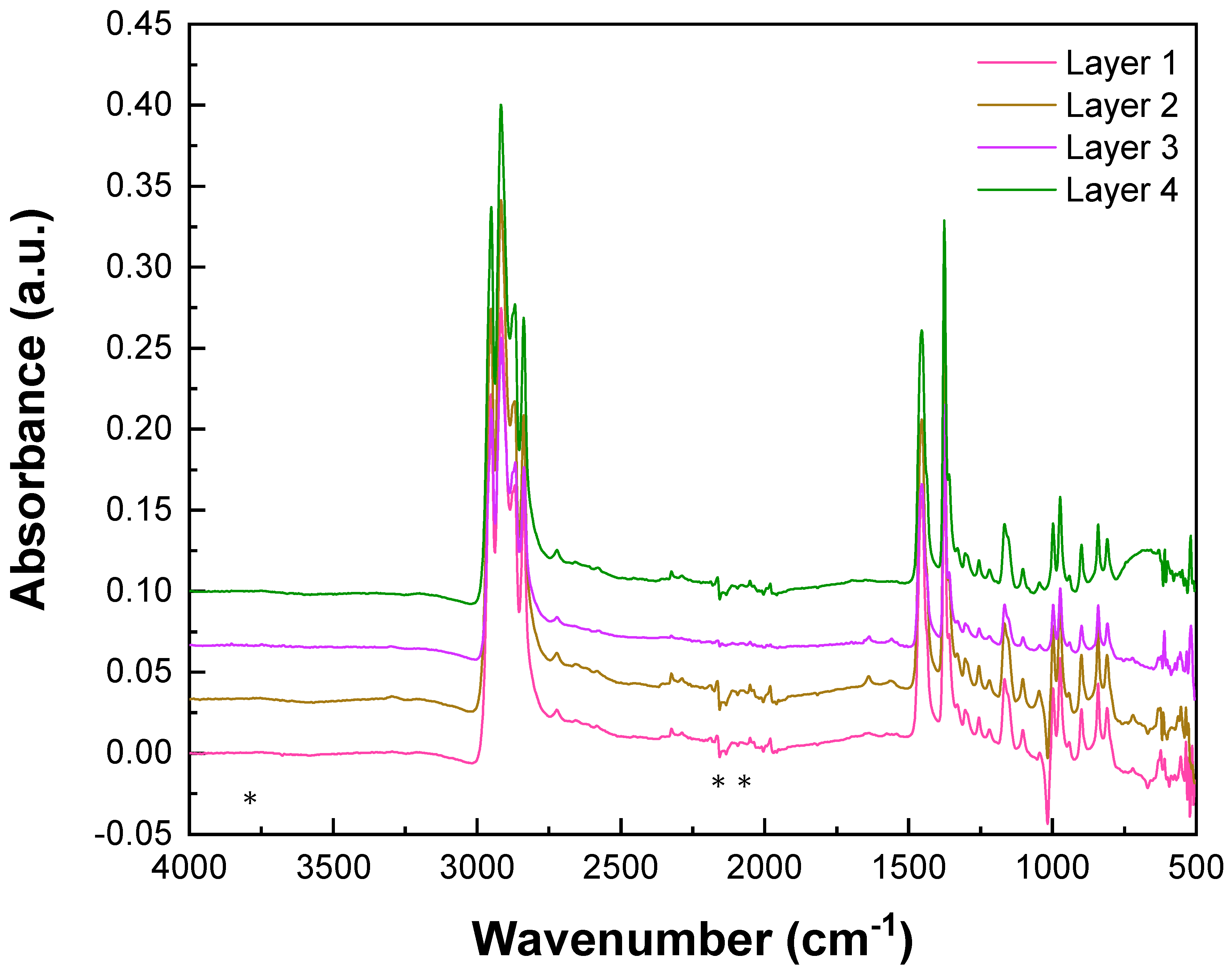
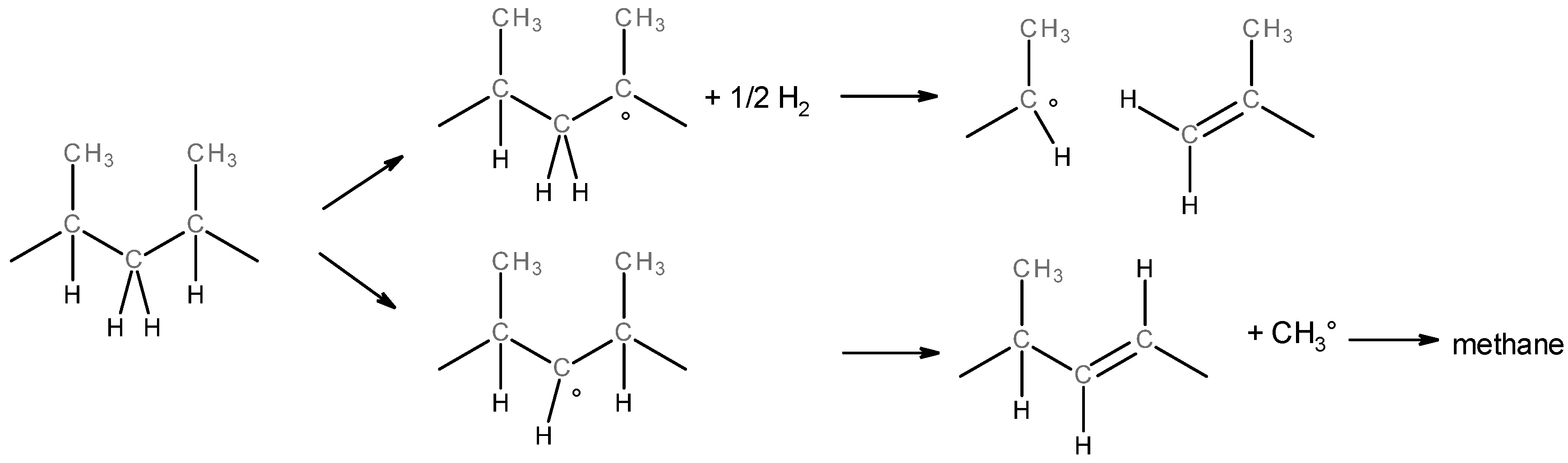
3.1. Characterization at the Molecular Scale
3.2. Characterization at the Macromolecular Scale
- -
- Layers 2 + 3: GS = 0.36 × 10−7 mol J−1 and GX = 0.15 × 10−7 mol J−1;
- -
- Layer 4: GS = 0.42 × 10−7 mol J−1 and GX = 0.05 × 10−7 mol J−1.
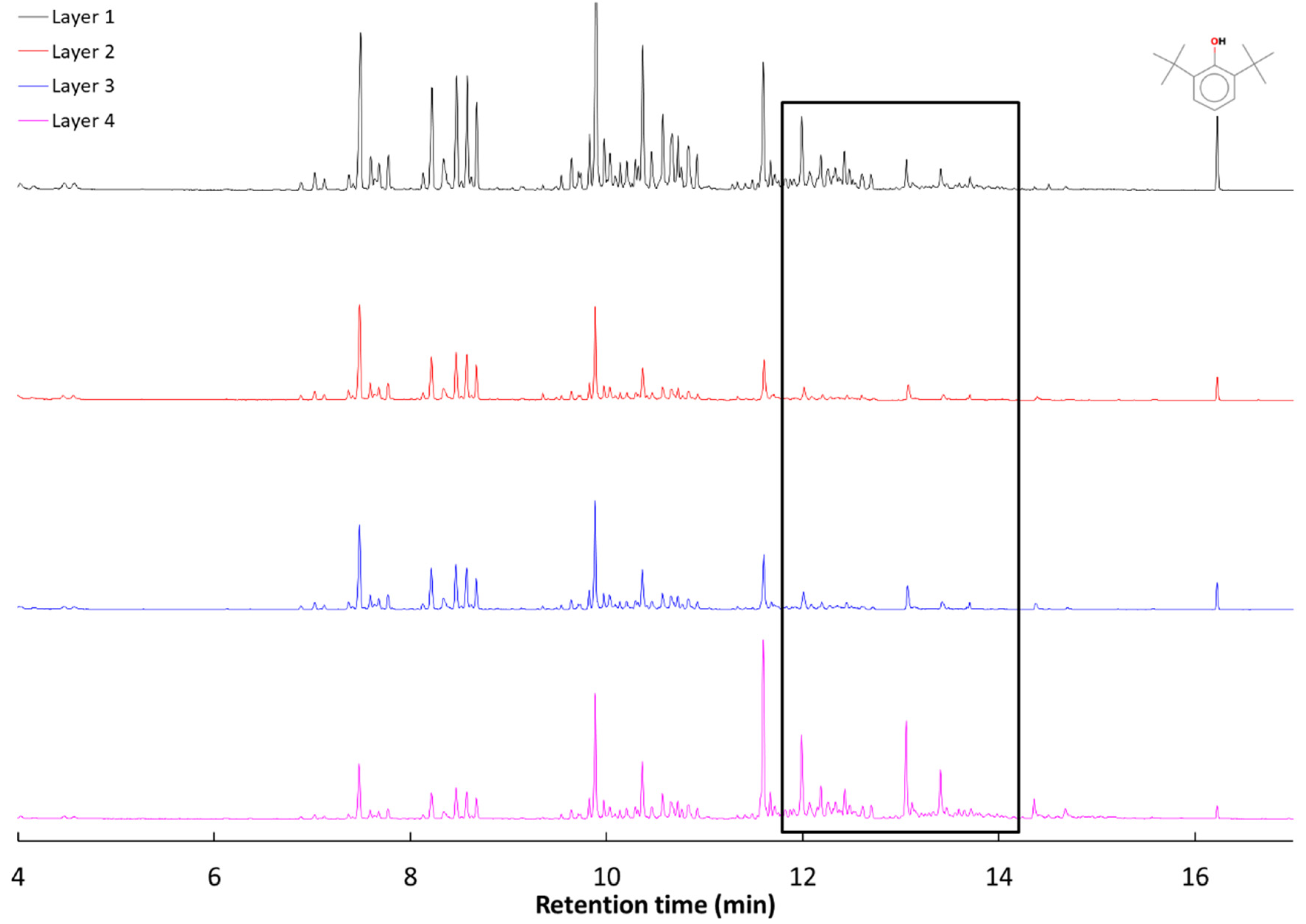
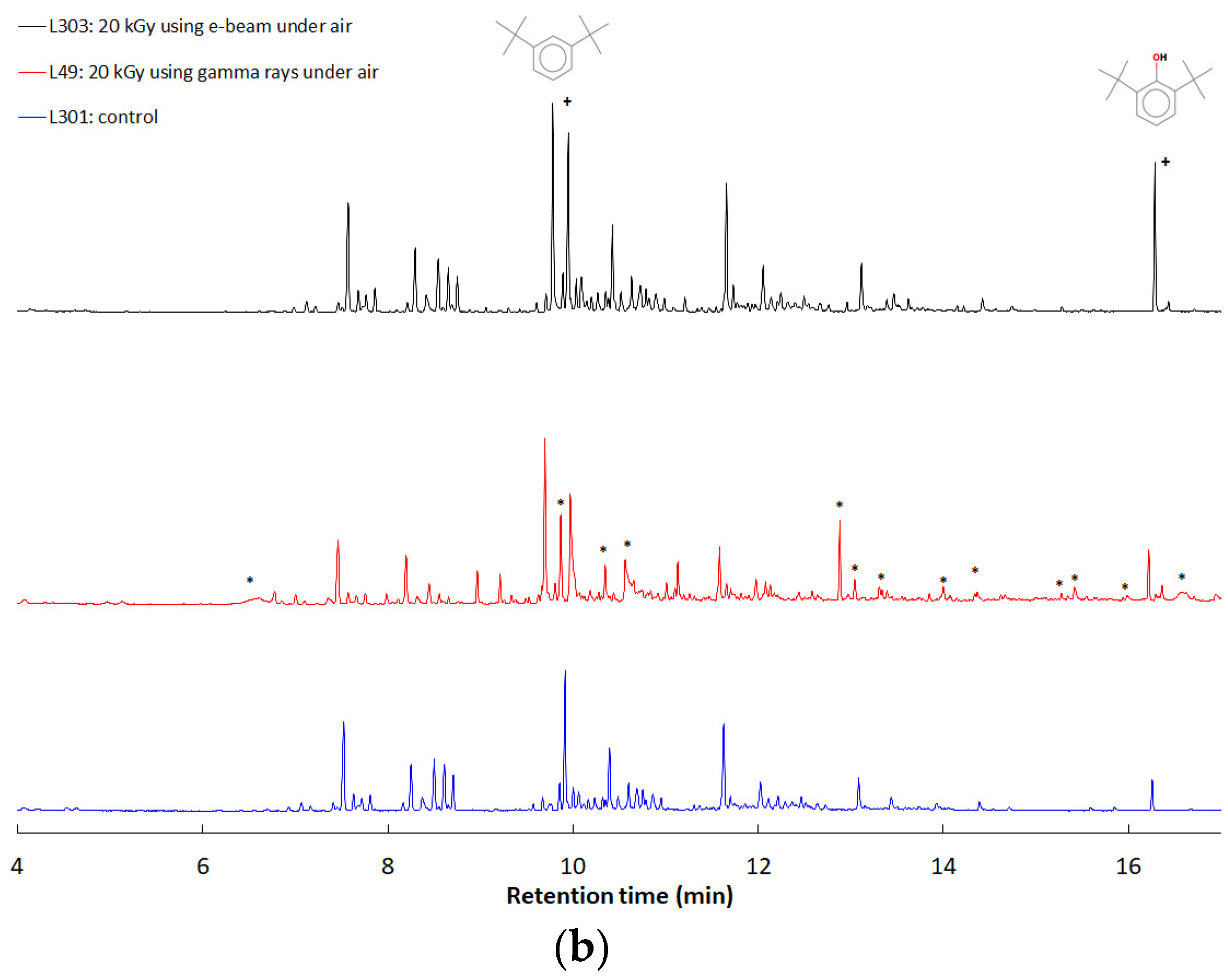
3.3. Characterization of the Volatiles Trapped in the Mask
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bernard, L.; Desoubeaux, G.; Bodier-Montagutelli, E.; Pardessus, J.; Brea, D.; Allimonnier, L.; Eymieux, S.; Raynal, P.-I.; Vasseur, V.; Vecellio, L.; et al. Controlled heat and humidity-based treatment for the reuse of personal protective equipment: A pragmatic proof-of-concept to address the mass shortage of surgical masks and N95/FFP2 respirators and to prevent the SARS-CoV2 transmission. Front. Med. 2020, 7, 584036. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.B.; Agostini, G.; Mitchell, J.C. A scoping review of surgical masks and N95 filtering facepiece respirators: Learning from the past to guide the future of dentistry. Saf. Sci. 2020, 131, 104920. [Google Scholar] [CrossRef]
- Celina, M.C.; Martinez, E.; Omana, M.A.; Sanchez, A.; Wiemann, D.; Tezak, M.; Dargaville, T.R. Extended use of face masks during the COVID-19 pandemic—Thermal conditioning and spray-on sur-face disinfection. Polym. Degrad. Stab. 2020, 179, 109251. [Google Scholar] [CrossRef]
- Song, W.; Pan, B.; Kan, H. Evaluation of the heat inactivation of virus contamination on medical mask. J. Microbes Infect. 2020, 15, 31–35. [Google Scholar]
- Aymes-Chodur, C.; Betz, N.; Legendre, B.; Yagoubi, N. Structural and physico-chemical studies on modification of polypropylene and its polyphenolic antioxidant by electron beam irradiation. Polym. Degrad. Stab. 2006, 91, 649–662. [Google Scholar] [CrossRef]
- Alariqi, S.A.; Kumar, A.P.; Rao, B.; Singh, R. Biodegradation of γ-sterilised biomedical polyolefins under composting and fungal culture environments. Polym. Degrad. Stab. 2006, 91, 1105–1116. [Google Scholar] [CrossRef]
- Guignot, C.; Betz, N.; Legendre, B.; Le Moel, A.; Yagoubi, N. Degradation of segmented poly (ether-urethane) Tecoflex® induced by electron beam irradiation: Characterization and evaluation. Nucl. Instr. Meth. Phys. Res. B Beam Inter. Mat. Atoms 2001, 185, 100–107. [Google Scholar] [CrossRef]
- Alcaraz, J.-P.; Le Coq, L.; Pourchez, J.; Thomas, D.; Chazelet, S.; Boudry, I.; Barbado, M.; Silvent, S.; Dessale, C.; Antoine, F.; et al. Reuse of medical face masks in domestic and community settings without sacrificing safety: Ecological and economical lessons from the COVID-19 pandemic. Chemosphere 2022, 288, 132364. [Google Scholar] [CrossRef]
- Geymer, D.O. The effects of ionizing radiation on the molecular weight of crystalline polypropylene. Makromol. Chem. 1966, 99, 152–159. [Google Scholar] [CrossRef]
- Chang, Z.; LaVerne, J.A. Hydrogen production in the heavy ion radiolysis of polymers. 1. Polyethylene, polypropylene, poly(methyl methacrylate), and polystyrene. J. Phys. Chem. B 2000, 104, 10557–10562. [Google Scholar] [CrossRef]
- Viscusi, D.J.; Bergman, M.S.; Eimer, B.C.; Shaffer, R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann. Occup. Hyg. 2009, 53, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Bergman, M.S.; Viscusi, D.J.; Heimbuch, B.K.; Wander, J.; Sambol, A.R.; Shaffer, R.E. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J. Eng. Fibers Fabr. 2010, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-H.; Chen, C.-C.; Huang, S.-H.; Kuo, C.-W.; Lai, C.-Y.; Lin, W.-Y. Filter quality of electret masks in filtering 14.6–594 nm aerosol particles: Effects of five decontamination methods. PLoS ONE 2017, 12, e0186217. [Google Scholar] [CrossRef] [Green Version]
- Pirker, L.; Krajnc, A.P.; Malec, J.; Radulović, V.; Gradišek, A.; Jelen, A.; Remškar, M.; Mekjavić, I.B.; Kovač, J.; Mozetič, M.; et al. Sterilization of polypropylene membranes of facepiece respirators by ionizing radiation. J. Membr. Sci. 2021, 619, 118756. [Google Scholar] [CrossRef] [PubMed]
- Fayolle, B.; Richaud, E.; Verdu, J.; Farcas, F. Embrittlement of polypropylene fibre during thermal oxidation. J. Mater. Sci. 2007, 43, 1026–1032. [Google Scholar] [CrossRef]
- Gnatta, J.R.; Queiroz de Souza, R.; de Santana Lemos, C.; Oliveira, R.A.; Martins, L.R.; de Araújo Moriya, G.A.; de Brito Poveda, V. Review: Safety in the practice of decontaminating filtering facepiece respirators: A systematic review. Am. J. Inf. Control 2021, 49, 825–835. [Google Scholar] [CrossRef]
- Grubisic, Z.; Rempp, P.; Benoit, H. A universal calibration for gel permeation chromatography. J. Polym. Sci. Part B Polym. Lett. 1967, 5, 753–759. [Google Scholar] [CrossRef]
- DeRosa, C.; Auriemma, F. Structure and physical properties of syndiotactic polypropylene: A highly crystalline thermoplastic elastomer. Prog. Polym. Sci. 2006, 31, 145–237. [Google Scholar] [CrossRef]
- Richaud, E.; Fayolle, B.; Davies, P. Tensile properties of polypropylene fibers. In Handbook of Properties of Textile and Technical Fibres, 2nd ed.; Bunsell, A.R., Ed.; The Textile Institute Book Series; Elsevier: Amsterdam, The Netherlands, 2018; pp. 515–543. [Google Scholar]
- Cambon, S. Etude du Mécanisme de Dégradation Radiochimique d’un Élastomère de Type EPDM; Université Blaise Pascal: Clermont-Ferrand, France, 2001. [Google Scholar]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Harcourt Brace Jovanovich: San Diego, CA, USA, 1991. [Google Scholar]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless melt-electrospinning of polypropylene nanofibres. J. Nanomat. 2012, 2012, 382639. [Google Scholar] [CrossRef]
- Zweifel, H.; Maier, R.D.; Schiller, M. Plastics Additives Handbook; Hanser Publications: Cincinnati, OH, USA, 2009. [Google Scholar]
- Lifshutz, N.; Gahan, R.E.; Stevens, G.C. Charge Stabilized Electret Filter Media. U.S. Patent 5,645,627, 1997. [Google Scholar]
- International Atomic Energy Agency (IAEA). Sterilization and Reprocessing of PPE, Including Respiratory Mask, by Ionizing Radiation; IAEA Technical Report; International Atomic Energy Agency (IAEA): Vienna, Austria, 2020. [Google Scholar]
- Verdu, J. Spectrométries infrarouge et Raman. Techniques de l’Ingénieur A3272 V1, 1982.
- Yakimets, I.; Lai, D.; Guignon, M. Effect of photo-oxidation cracks on behaviour of thick poly-propylene samples. Polym. Degrad. Stab. 2004, 86, 59–67. [Google Scholar] [CrossRef]
- Fayolle, B.; Audouin, L.; Verdu, J. A critical molar mass separating the ductile and brittle regimes as revealed by thermal oxidation in polypropylene. Polymer 2004, 45, 4323–4330. [Google Scholar] [CrossRef]
- Richaud, E.; Farcas, F.; Divet, L.; Benneton, J.P. Accelerated ageing of polypropylene geotextiles, the effect of temperature, oxygen pressure and aqueous media on fibers—Methodological aspects. Geotext. Geomembr. 2008, 26, 71–81. [Google Scholar] [CrossRef]
- Massey, S.; Adnot, A.; Roy, D. Hydrolytic aging of polypropylene studied by X-ray photoelectron spectroscopy. J. Appl. Polym. Sci. 2004, 92, 3830–3838. [Google Scholar] [CrossRef]
- Henry, J.L.; Ruaya, A.L.; Garton, A. The kinetics of polyolefin oxidation in aqueous media. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1693–1703. [Google Scholar] [CrossRef]
- Rostami, R.; Zarrebini, M.; Mandegari, M.; Sanginabadi, K.; Mostofinejad, D.; Abtahi, S.M.; Rostami, R. The effect of concrete alkalinity on behavior of reinforcing polyester and polypropylene fibers with similar properties. Cem. Concr. Compos. 2018, 97, 118–124. [Google Scholar] [CrossRef]
- Black, R.M.; Lyons, B.J. Effect of high-energy radiation on polypropylene. Nature 1957, 180, 1346–1347. [Google Scholar] [CrossRef]
- Kang, P.; Wu, P.; Jin, Y.; Shi, S.; Gao, D.; Chen, G.; Li, Q. Formation and emissions of volatile organic compounds from homo-PP and Co-PP resins during manufacturing process and accelerated photoaging degradation. Molecules 2020, 25, 2761. [Google Scholar] [CrossRef] [PubMed]
- Saito, O. On the effect of high energy radiation to polymers: I. Cross-linking and degradation. J. Phys. Soc. Jap. 1958, 13, 198–206. [Google Scholar] [CrossRef]
- Kiryukhin, V.P. Radiation crosslinking and chain scission of polymers. In Organic Radiation Chemistry Handbook; Milinchuk, V.K., Tupikov, V.I., Eds.; Ellis Horwood Limited: Chichester, UK, 1989. [Google Scholar]
- Schnabel, W. Degradation by high energy radiation. In Aspects of Polymer Degradation and Stabilisation; Jellinek, H.G., Ed.; Elsevier: Oxford, UK, 1978; pp. 149–190. [Google Scholar]
- Rivaton, A.; Cambon, S.; Gardette, J.-L. Radiochemical ageing of EPDM elastomers. 3. Mechanism of radiooxidation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2005, 227, 357–368. [Google Scholar] [CrossRef]
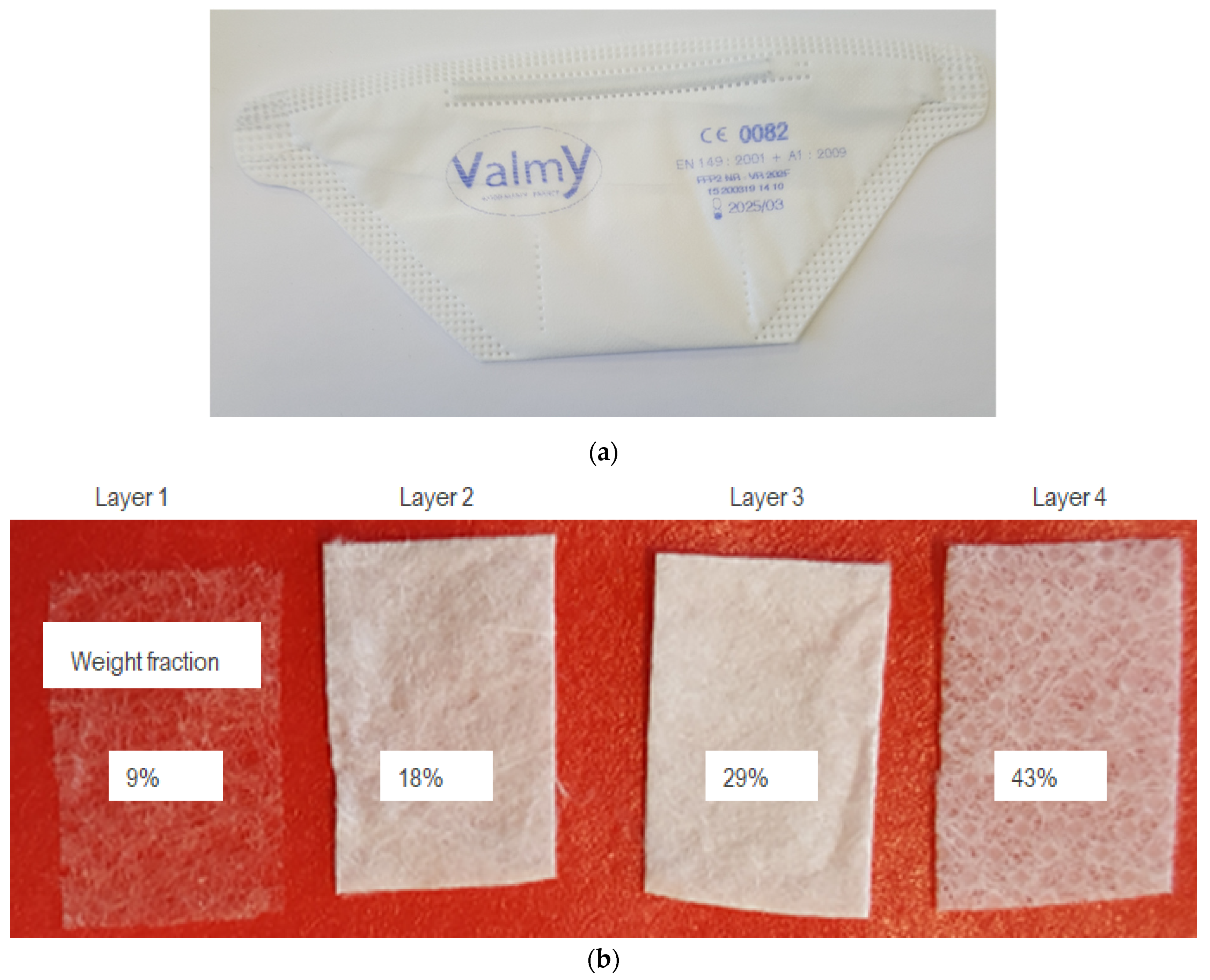



| Sample Number | Sterilization Treatment | Target Dose (kGy) | Controlled Dose (kGy) | Dosimetric Uncertainty (%) | Irradiator |
|---|---|---|---|---|---|
| L301 | Reference | 0 | - | IONISOS | |
| L302 | e-beam under air | 10 | 10.4 | 4.6 | |
| L303 | e-beam under air | 20 | 20.2 | 4.6 | |
| L305 | e-beam under vacuum | 20 | 20.2 | 4.6 | |
| L307 | e-beam under vacuum | 60 | 59.1 | 4.6 | |
| L308 | Washed with pure water | 0 | - | ||
| L310 | Washed with pure water + e-beam under vacuum | 20 | 19.7 | 4.6 | |
| L311 | Washed with detergent (Ultimate) | 0 | - | 4.6 | |
| L313 | Washed with detergent (Ultimate) + e-beam under vacuum | 20 | 19.7 | 4.6 | |
| L49 | γ-rays under air | 20 | 20.1 | 10 | ArcNucleart |
| L48 | γ-rays under vacuum | 20 | 20.1 | 10 |
| Absorption Area (cm−1) | Amide II Bonds’ Attribution | Bands Observed on Layers 2 and 3 of the Valmy FFP2 Mask |
|---|---|---|
| 3300–3050 | —NH-associated bond | 3295 |
| 1710–1660 | —C = O free bond | 1640 |
| 1570–1515 | 1560 |
| Sample Number | Sterilization Treatment | Target Dose (kGy) | Layers 2 + 3 | Layer 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Mw | Mn | χc | Mw | Mn | Χc | |||
| L301 | Reference | 0 | 94.9–101.6 | 33.0–28.2 | 26.85 | 251.4–251.5 | 71.5–71.9 | 23.8 |
| L302 | e-beam under air | 10 | 32.7 | 29.5 | ||||
| L303 | e-beam under air | 20 | 71.4–107.9 | 26.7–28.1 | 30.5 | 154.0–140.0 | 50.8–48.1 | 26.6 |
| L305 | e-beam under vacuum | 20 | 88.5–105.1 | 27.0–28.9 | 26.7 | 166.5–170.7 | 49.8–54.9 | 27.1 |
| L307 | e-beam under vacuum | 60 | 112.1–90.7 | 23.3–31.3 | 30.6 | 155.9–156.6 | 49.2–47.9 | 23.8 |
| L308 | Washed with pure water | 0 | 91.9–95.9 | 24.0–29.2 | 32.3 | 235.0–242.1 | 69.0–70.2 | 26.7 |
| L310 | Washed with pure water + e-beam under vacuum | 20 | 90.2–98.6 | 24.0–25.9 | 33.1 | 172.3–182.6 | 54.9–64.9 | 33.1 |
| L311 | Washed with detergent (Ultimate) | 0 | 74.4–81.6 | 20.5–27.6 | 32.2 | 246.8 | 67.8–74.7 | 30.1 |
| L313 | Washed with detergent (Ultimate) + e-beam under vacuum | 20 | 80.2–82.3 | 23.0–24.0 | 30.8 | 180.4–186.0 | 55.4–56.0 | 26.8 |
| L49 | γ-rays under air | 20 | 46.0–43.6 | 15.1–15.4 | 31.8 | 64.0–64.8 | 22.6 | 30.3 |
| L48 | γ-rays under vacuum | 20 | 76.4–80.8 | 21.9–22.6 | 30.8 | 159.9–161.8 | 49.3–52.2 | 25.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richaud, E.; Ferry, M.; Carpentier, F.; Rouif, S.; Cortella, L.; Esnouf, S. Sterilization Induced Changes in Polypropylene-Based Ffp2 Masks. Polymers 2021, 13, 4107. https://doi.org/10.3390/polym13234107
Richaud E, Ferry M, Carpentier F, Rouif S, Cortella L, Esnouf S. Sterilization Induced Changes in Polypropylene-Based Ffp2 Masks. Polymers. 2021; 13(23):4107. https://doi.org/10.3390/polym13234107
Chicago/Turabian StyleRichaud, Emmanuel, Muriel Ferry, Floriane Carpentier, Sophie Rouif, Laurent Cortella, and Stéphane Esnouf. 2021. "Sterilization Induced Changes in Polypropylene-Based Ffp2 Masks" Polymers 13, no. 23: 4107. https://doi.org/10.3390/polym13234107