Extraction of Polyhydroxyalkanoates from Purple Non-Sulfur Bacteria by Non-Chlorinated Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strain and Growth Conditions
2.3. Analytical Methods
2.4. Synthesis of Ionic Liquids
2.5. PHA Extraction with Chloroform
2.6. PHA Extraction with Cyclohexanone
2.7. PHA Extraction with Ionic Liquids
2.8. Extracted PHA Characterization
3. Results and Discussion
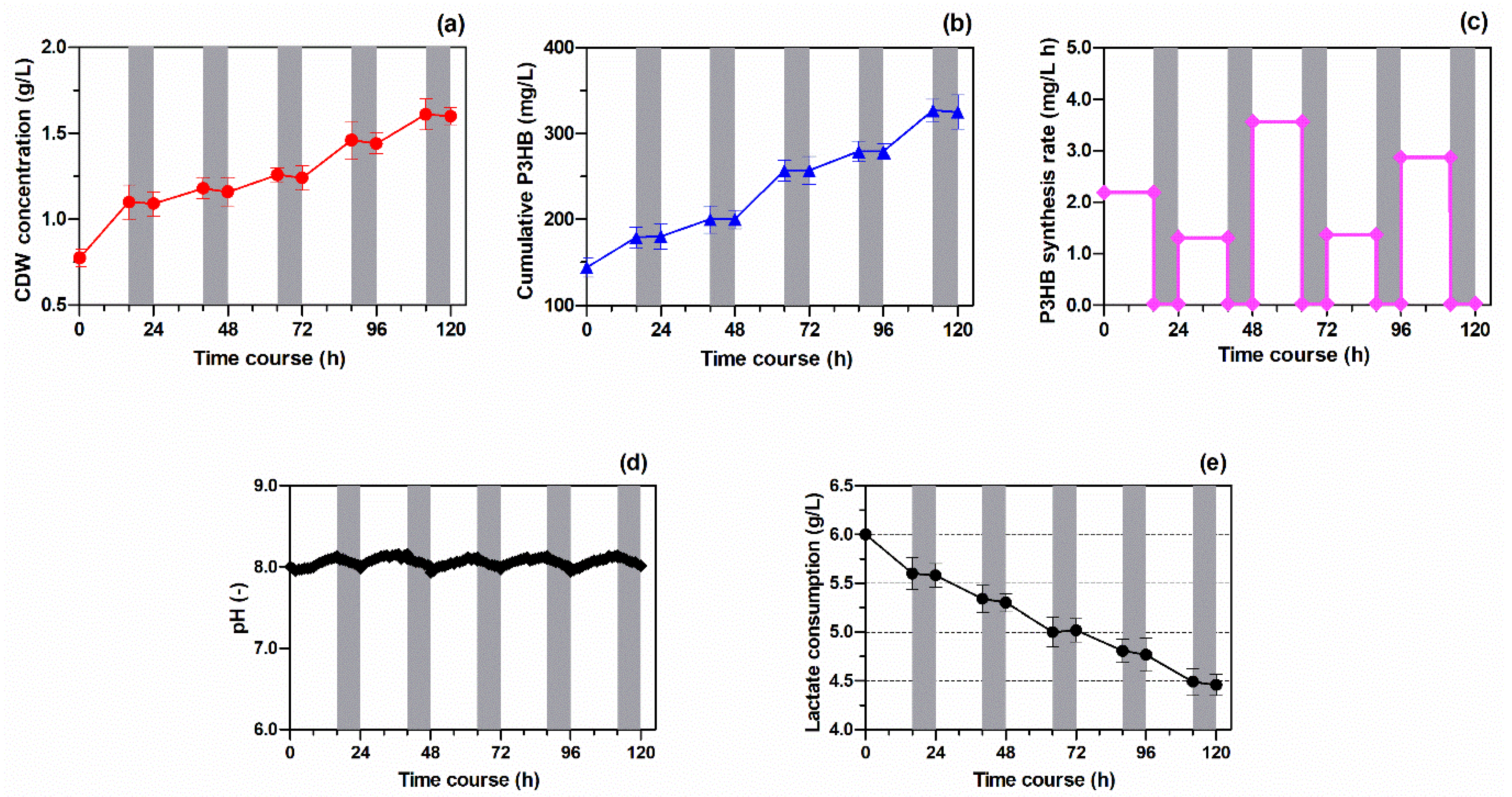
3.1. Production of PHA-Containing Bacterial Biomass
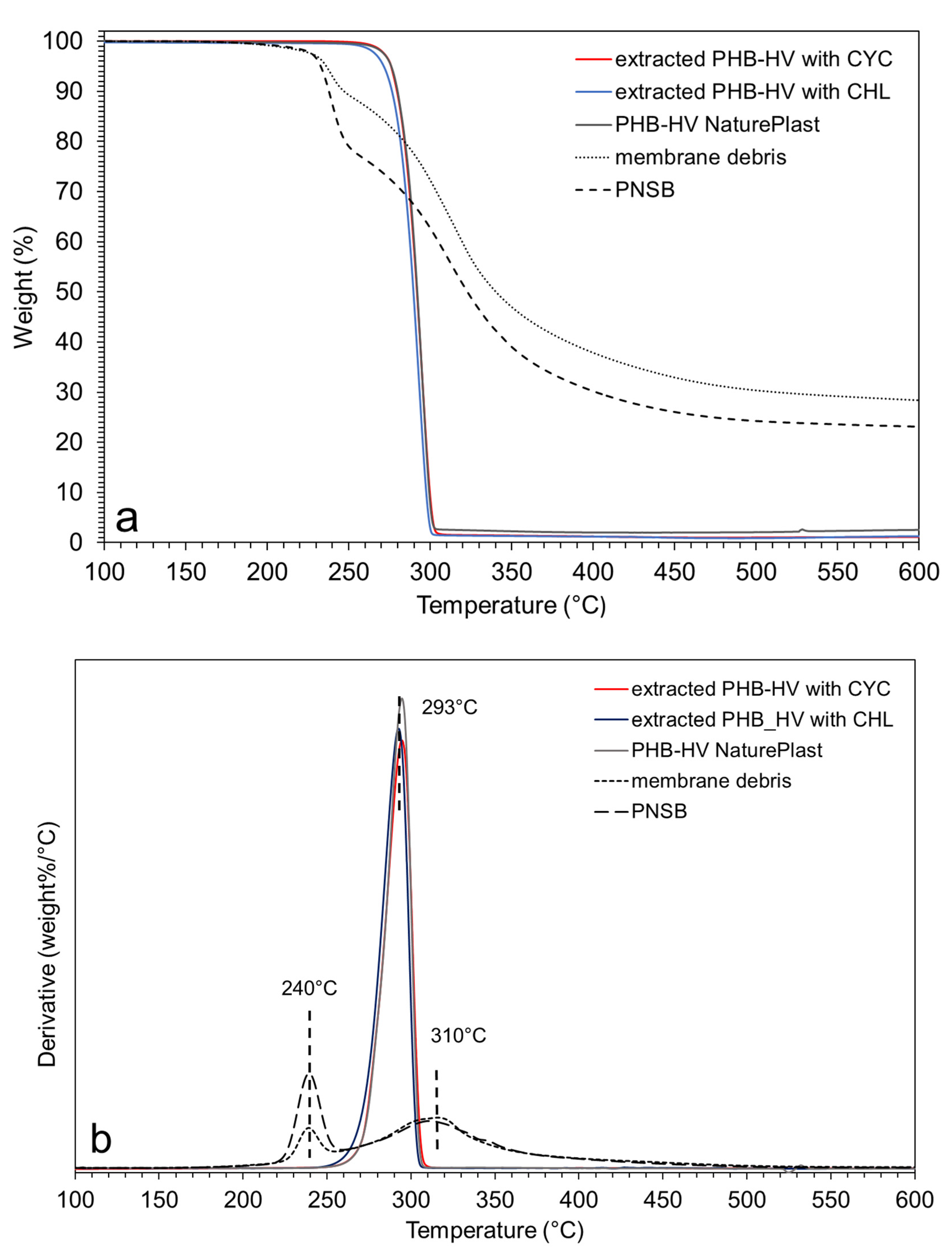
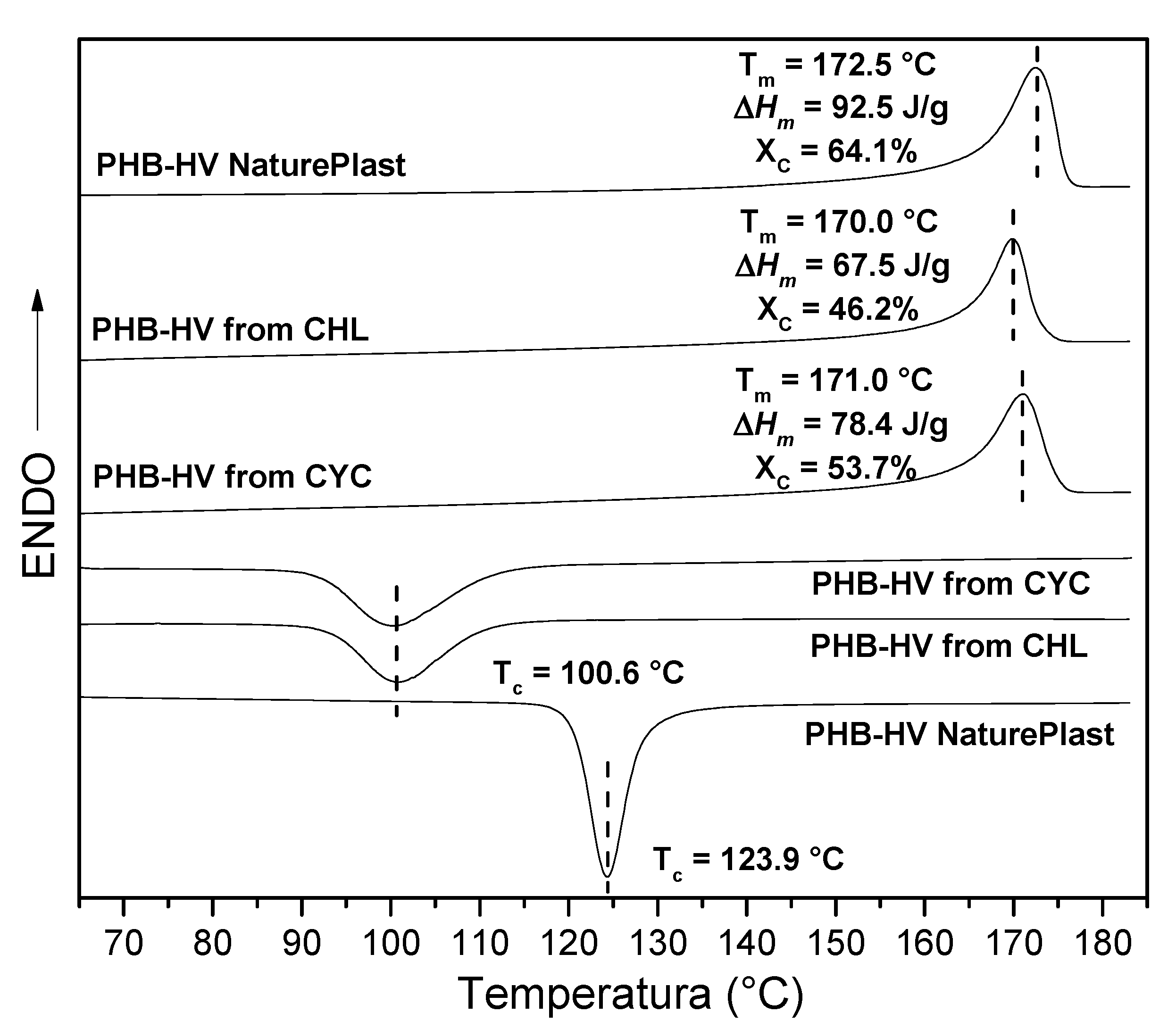
3.2. PHA Extraction with Chloroform and Cyclohexanone
3.3. PHA Extraction with Ionic Liquids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, Structure and Properties of Polyhydroxyalkanoates: Biological Polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Muthuraj, R.; Valerio, O.; Mekonnen, T.H. Recent Developments in Short- and Medium-Chain- Length Polyhydroxyalkanoates: Production, Properties, and Applications. Int. J. Biol. Macromol. 2021, 187, 422–440. [Google Scholar] [CrossRef]
- Yashavanth, P.R.; Meenakshi, D.; Soumen, K.M. Recent Progress and Challenges in Cyanobacterial Autotrophic Production of Polyhydroxybutyrate (PHB), a Bioplastic. J. Environ. Chem. Eng. 2021, 9, 105379. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.; Ugwu, C.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Zabed, H.M.; Qi, X. Recent Developments in Polyhydroxyalkanoates (PHAs) Production—A Review. Bioresour. Technol. 2020, 306, 123132. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of Synthesis, Characteristics, Processing and Potential Applications in Packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Salehizadeh, H.; Van Loosdrecht, M.C.M. Production of Polyhydroxyalkanoates by Mixed Culture: Recent Trends and Biotechnological Importance. Biotechnol. Adv. 2004, 22, 261–279. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; López-Gómez, J.P.; Roy, I. A Sustainable Approach for the Downstream Processing of Bacterial Polyhydroxyalkanoates: State-of-the-Art and Latest Developments. Biochem. Eng. J. 2019, 150, 107283. [Google Scholar] [CrossRef]
- Narodoslawsky, M. LCA of PHA Production—Identifying the Ecological Potential of Bio-Plastic. Chem. Biochem. Eng. Q. 2015, 29, 299–305. [Google Scholar] [CrossRef]
- Holmes, P.A.; Wright, L.F.; Alderson, B.; Senior, P.J. A Process for the Extraction of Poly-3-Hydroxy-Butyric Acid from Microbial Cells European Patant. No. EP0015123A1. 1980. Available online: https://patentimages.storage.googleapis.com/4d/68/b0/ef3bd1590af616/EP0015123A1.pdf (accessed on 28 November 2021).
- Koller, M.; Niebelschütz, H.; Braunegg, G. Strategies for Recovery and Purification of Poly[(R)-3-Hydroxyalkanoates] (PHA) Biopolyesters from Surrounding Biomass. Eng. Life Sci. 2013, 13, 549–562. [Google Scholar] [CrossRef]
- Information Resouces Management Association (Ed.) Public Health and Welfare: Concepts, Methodologies, Tools, and Applications; IGI Global: Hershey, PA, USA, 2017; ISBN 978-1-5225-1674-3. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer, Ed.; IARC: Lyon, France, 1999; Volume 73, ISBN 978-92-832-1273-7. [Google Scholar]
- Jiang, G.; Johnston, B.; Townrow, D.; Radecka, I.; Koller, M.; Chaber, P.; Adamus, G.; Kowalczuk, M. Biomass Extraction Using Non-Chlorinated Solvents for Biocompatibility Improvement of Polyhydroxyalkanoates. Polymers 2018, 10, 731. [Google Scholar] [CrossRef] [Green Version]
- Rosengart, A.; Cesário, M.T.; de Almeida, M.C.M.D.; Raposo, R.S.; Espert, A.; de Apodaca, E.D.; da Fonseca, M.M.R. Efficient P(3HB) Extraction from Burkholderia Sacchari Cells Using Non-Chlorinated Solvents. Biochem. Eng. J. 2015, 103, 39–46. [Google Scholar] [CrossRef] [Green Version]
- López-Abelairas, M.; García-Torreiro, M.; Lú-Chau, T.; Lema, J.M.; Steinbüchel, A. Comparison of Several Methods for the Separation of Poly(3-Hydroxybutyrate) from Cupriavidus Necator H16 Cultures. Biochem. Eng. J. 2015, 93, 250–259. [Google Scholar] [CrossRef]
- Jiang, Y.; Mikova, G.; Kleerebezem, R.; van der Wielen, L.A.; Cuellar, M.C. Feasibility Study of an Alkaline-Based Chemical Treatment for the Purification of Polyhydroxybutyrate Produced by a Mixed Enriched Culture. AMB Express 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pospisilova, A.; Novackova, I.; Prikryl, R. Isolation of Poly(3-Hydroxybutyrate) from Bacterial Biomass Using Soap Made of Waste Cooking Oil. Bioresour. Technol. 2021, 326, 124683. [Google Scholar] [CrossRef] [PubMed]
- Schindl, A.; Hagen, M.L.; Muzammal, S.; Gunasekera, H.A.D.; Croft, A.K. Proteins in Ionic Liquids: Reactions, Applications, and Futures. Front. Chem. 2019, 7, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzetta, A.; Becherini, S.; Pretti, C.; Monni, G.; Casu, V.; Chiappe, C.; Guazzelli, L. Insights into the Levulinate-Based Ionic Liquid Class: Synthesis, Cellulose Dissolution Evaluation and Ecotoxicity Assessment. New J. Chem. 2019, 43, 13010–13019. [Google Scholar] [CrossRef]
- Zheng, S.; Nie, Y.; Zhang, S.; Zhang, X.; Wang, L. Highly Efficient Dissolution of Wool Keratin by Dimethylphosphate Ionic Liquids. ACS Sustain. Chem. Eng. 2015, 3, 2925–2932. [Google Scholar] [CrossRef]
- Hecht, S.E.; Niehoff, R.L.; Narasimhan, K.; Neal, C.W.; Forshey, P.A.; Phan, D.V.; Brooker, A.D.M.; Combs, K.H. Extracting Biopolymers from a Biomass Using Ionic Liquids. U.S. Patent No. 7,763,715 B2, 27 July 2010. [Google Scholar]
- Kobayashi, D.; Fujita, K.; Nakamura, N.; Ohno, H. A Simple Recovery Process for Biodegradable Plastics Accumulated in Cyanobacteria Treated with Ionic Liquids. Appl. Microbiol. Biotechnol. 2015, 99, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Bharmoria, P.; Gehlot, P.S.; Agrawal, V.; Kumar, A.; Mishra, S. 1-Ethyl-3-Methylimidazolium Diethylphosphate Based Extraction of Bioplastic “Polyhydroxyalkanoates” from Bacteria: Green and Sustainable Approach. ACS Sustain. Chem. Eng. 2018, 6, 766–773. [Google Scholar] [CrossRef]
- Cho, C.-W.; Pham, T.P.T.; Zhao, Y.; Stolte, S.; Yun, Y.-S. Review of the Toxic Effects of Ionic Liquids. Sci. Total Environ. 2021, 786, 147309. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Carlozzi, P.; Di Lorenzo, T.; Ghanotakis, D.F.; Touloupakis, E. Effects of PH, Temperature and Salinity on P3HB Synthesis Culturing the Marine Rhodovulum Sulfidophilum DSM-1374. Appl. Microbiol. Biotechnol. 2020, 104, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Padovani, G.; Emiliani, G.; Giovanelli, A.; Traversi, M.L.; Carlozzi, P. Assessment of Glycerol Usage by Five Different Purple Non-Sulfur Bacterial Strains for Bioplastic Production. J. Environ. Chem. Eng. 2018, 6, 616–622. [Google Scholar] [CrossRef]
- Carlozzi, P.; Touloupakis, E. Bioplastic Production by Feeding the Marine Rhodovulum Sulfidophilum DSM-1374 with Four Different Carbon Sources under Batch, Fed-Batch and Semi-Continuous Growth Regimes. New Biotechnol. 2021, 62, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Carlozzi, P.; Touloupakis, E.; Di Lorenzo, T.; Giovannelli, A.; Seggiani, M.; Cinelli, P.; Lazzeri, A. Whey and Molasses as Inexpensive Raw Materials for Parallel Production of Biohydrogen and Polyesters via a Two-Stage Bioprocess: New Routes towards a Circular Bioeconomy. J. Biotechnol. 2019, 303, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Carlozzi, P.; Giovannelli, A.; Traversi, M.L.; Touloupakis, E. Poly(3-Hydroxybutyrate) Bioproduction in a Two-Step Sequential Process Using Wastewater. J. Water Process. Eng. 2021, 39, 101700. [Google Scholar] [CrossRef]
- Carlozzi, P.; Giovannelli, A.; Traversi, M.L.; Touloupakis, E.; Di Lorenzo, T. Poly-3-Hydroxybutyrate and H2 Production by Rhodopseudomonas Sp. S16-VOGS3 Grown in a New Generation Photobioreactor under Single or Combined Nutrient Deficiency. Int. J. Biol. Macromol. 2019, 135, 821–828. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mezzetta, A.; Pomelli, C.S.; Chiappe, C.; Guazzelli, L. Evaluation of the Effect of the Dicationic Ionic Liquid Structure on the Cycloaddition of CO2 to Epoxides. J. CO2 Util. 2019, 34, 437–445. [Google Scholar] [CrossRef]
- Chiappe, C.; Margari, P.; Mezzetta, A.; Pomelli, C.S.; Koutsoumpos, S.; Papamichael, M.; Giannios, P.; Moutzouris, K. Temperature Effects on the Viscosity and the Wavelength-Dependent Refractive Index of Imidazolium-Based Ionic Liquids with a Phosphorus-Containing Anion. Phys. Chem. Chem. Phys. 2017, 19, 8201–8209. [Google Scholar] [CrossRef]
- Ramsay, J.A.; Berger, E.; Voyer, R.; Chavarie, C.; Ramsay, B.A. Extraction of Poly-3-Hydroxybutyrate Using Chlorinated Solvents. Biotechnol. Tech. 1994, 8, 589–594. [Google Scholar] [CrossRef]
- Rosengart, A. A Study to Improve the Extraction Yields of Poly 3-Hydroxybutyrate from Burkholderia Sacchari Cells Avoiding Chlorinated Solvents; Università degli Studi di Padova: Padova, Italy, 2013. [Google Scholar]
- Ghani, N.A.; Sairi, N.A.; Aroua, M.K.; Alias, Y.; Yusoff, R. Density, Surface Tension, and Viscosity of Ionic Liquids (1-Ethyl-3-Methylimidazolium Diethylphosphate and 1,3-Dimethylimidazolium Dimethylphosphate) Aqueous Ternary Mixtures with MDEA. J. Chem. Eng. Data 2014, 59, 1737–1746. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Nie, Y.; Wang, H.; Zheng, S.; Zhang, S. Effects of Water Content on the Dissolution Behavior of Wool Keratin Using 1-Ethyl-3-Methylimidazolium Dimethylphosphate. Sci. China Chem. 2017, 60, 934–941. [Google Scholar] [CrossRef]
- Chen, X.; Liang, S.; Wang, S.-W.; Colby, R.H. Linear Viscoelastic Response and Steady Shear Viscosity of Native Cellulose in 1-Ethyl-3-Methylimidazolium Methylphosphonate. J. Rheol. 2018, 62, 81–87. [Google Scholar] [CrossRef]
- Jurasek, L.; Marchessault, R.H. Polyhydroxyalkanoate (PHA) Granule Formation in Ralstonia Eutropha Cells: A Computer Simulation. Appl. Microbiol. Biotechnol. 2004, 64, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Golomysova, A.; Gomelsky, M.; Ivanov, P.S. Flux Balance Analysis of Photoheterotrophic Growth of Purple Nonsulfur Bacteria Relevant to Biohydrogen Production. Int. J. Hydrogen Energy 2010, 35, 12751–12760. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Harwood, C.S. Calvin Cycle Flux, Pathway Constraints, and Substrate Oxidation State Together Determine the H2 Biofuel Yield in Photoheterotrophic Bacteria. MBio 2011, 2, e00323-10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCully, A.L.; McKinlay, J.B. Disrupting Calvin Cycle Phosphoribulokinase Activity in Rhodopseudomonas Palustris Increases the H2 Yield and Specific Production Rate Proportionately. Int. J. Hydrogen Energy 2016, 41, 4143–4149. [Google Scholar] [CrossRef] [Green Version]
- Carlozzi, P.; Seggiani, M.; Cinelli, P.; Mallegni, N.; Lazzeri, A. Photofermentative Poly-3-Hydroxybutyrate Production by Rhodopseudomonas Sp. S16-VOGS3 in a Novel Outdoor 70-L Photobioreactor. Sustainability 2018, 10, 3133. [Google Scholar] [CrossRef] [Green Version]
- Van Walsem, J.; Zhong, L.; Shih, S.S. Polymer Extraction Methods 2010. U.S. Patent No. 7,713,720 B2, 7 August 2007. [Google Scholar]
- Masood, F.; Hasan, F.; Ahmed, S.; Hameed, A. Biosynthesis and Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) from Bacillus Cereus FA11 Isolated from TNT-Contaminated Soil. Ann. Microbiol. 2012, 62, 1377–1384. [Google Scholar] [CrossRef]
- Bujok, J.; Gąsior-Głogowska, M.; Marszałek, M.; Trochanowska-Pauk, N.; Zigo, F.; Pavľak, A.; Komorowska, M.; Walski, T. Applicability of FTIR-ATR Method to Measure Carbonyls in Blood Plasma after Physical and Mental Stress. BioMed Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunaratne, L.M.W.K.; Shanks, R.A. Multiple Melting Behaviour of Poly(3-Hydroxybutyrate-Co-Hydroxyvalerate) Using Step-Scan DSC. Eur. Polym. J. 2005, 41, 2980–2988. [Google Scholar] [CrossRef]
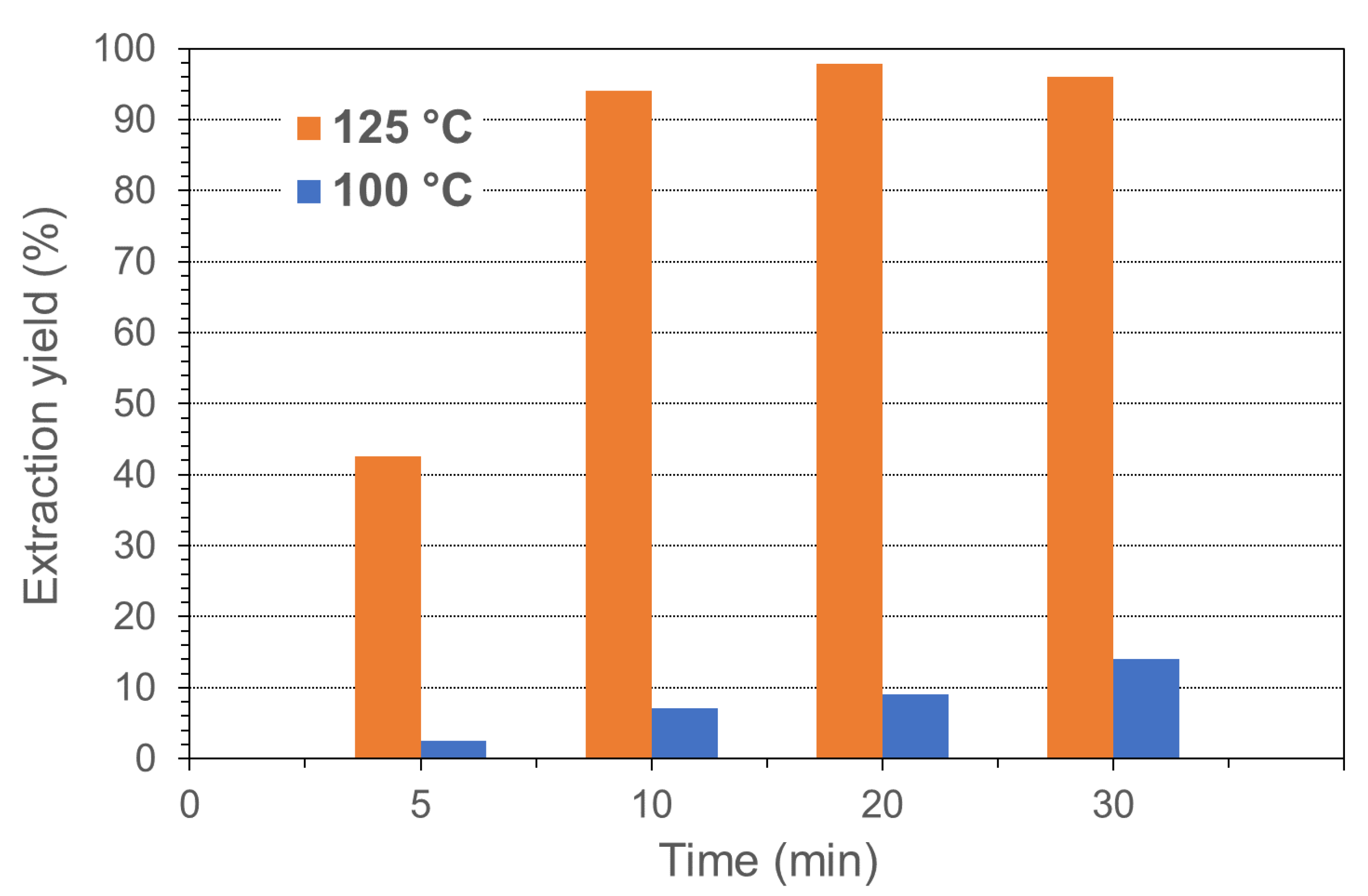


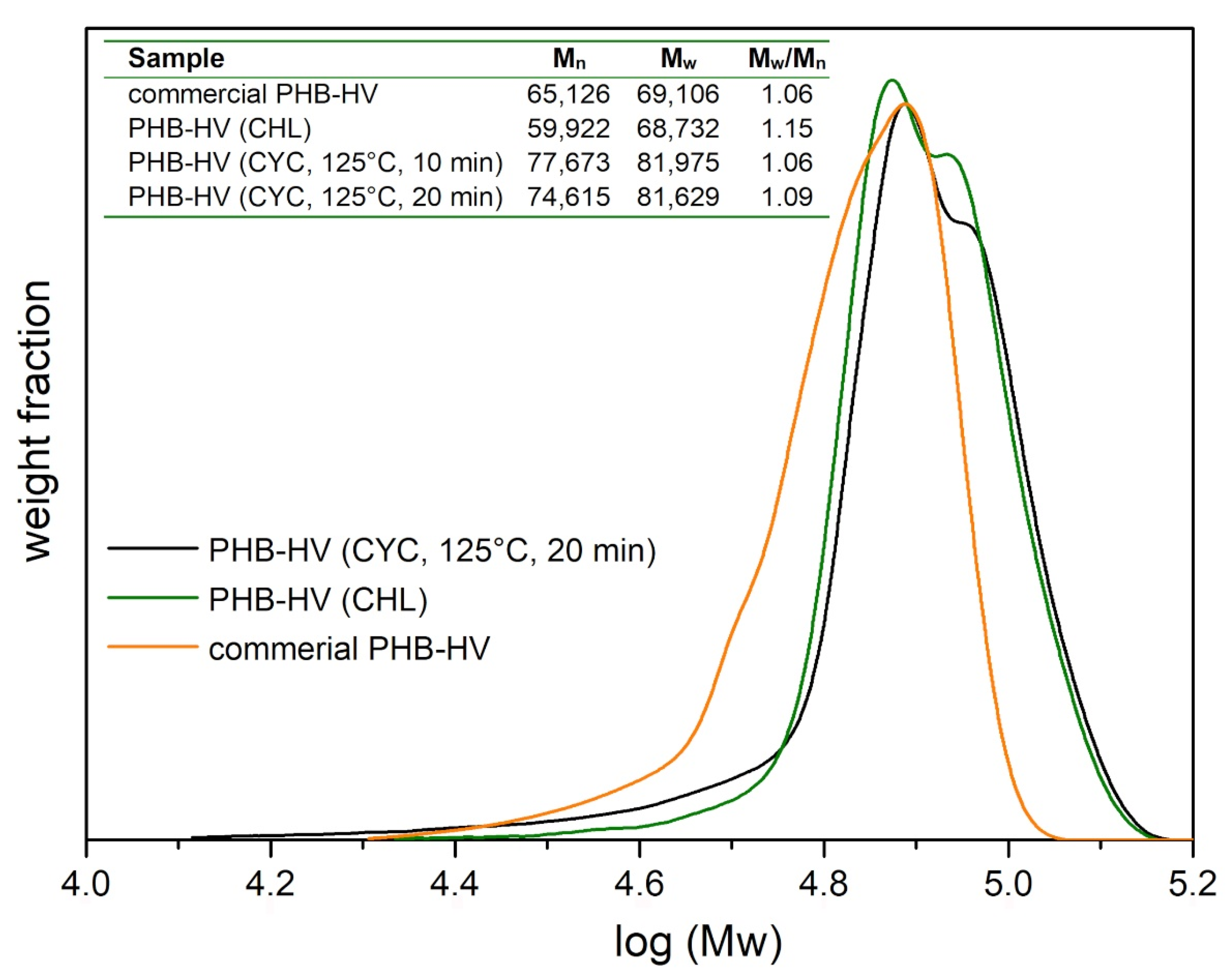
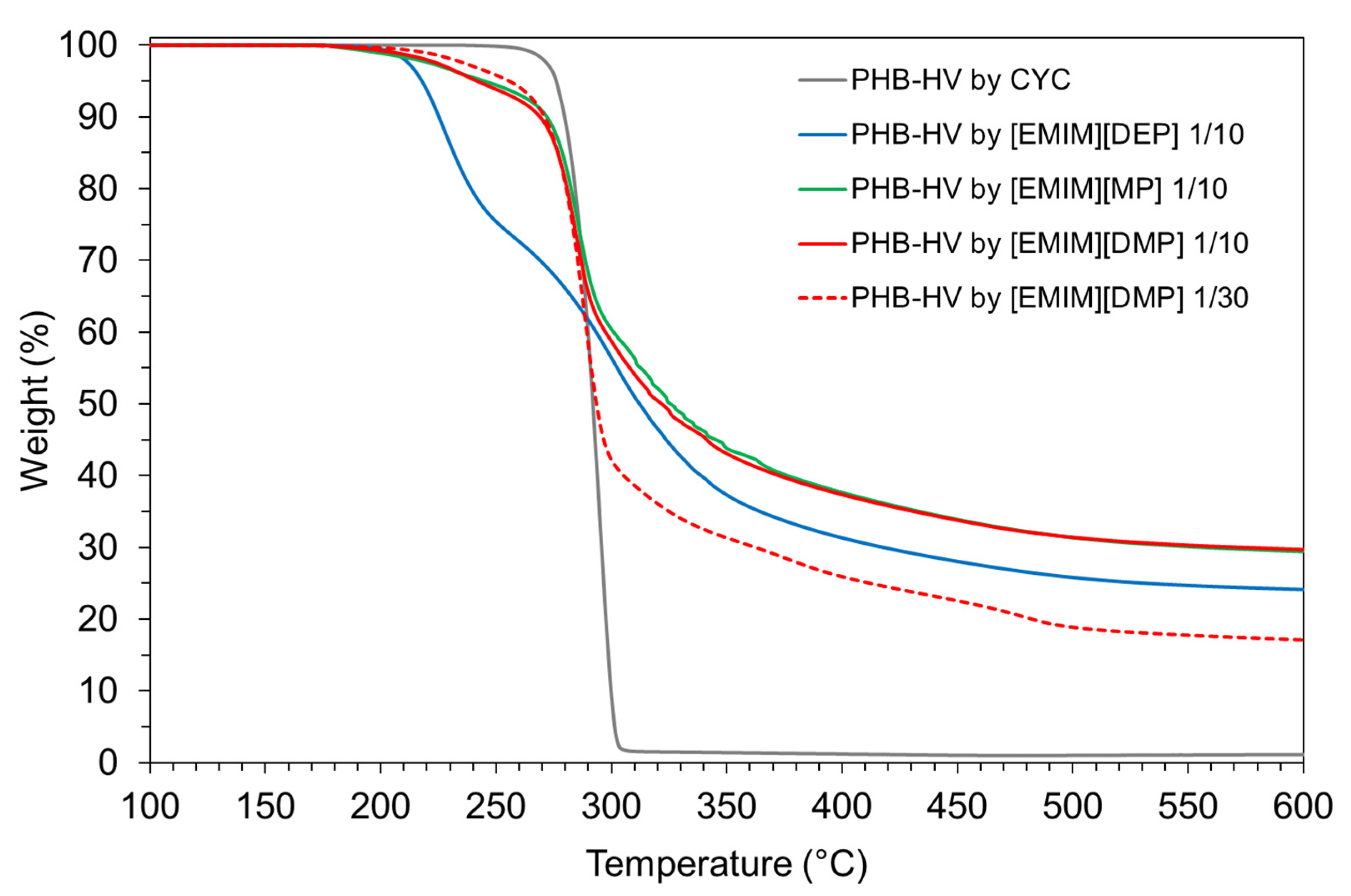
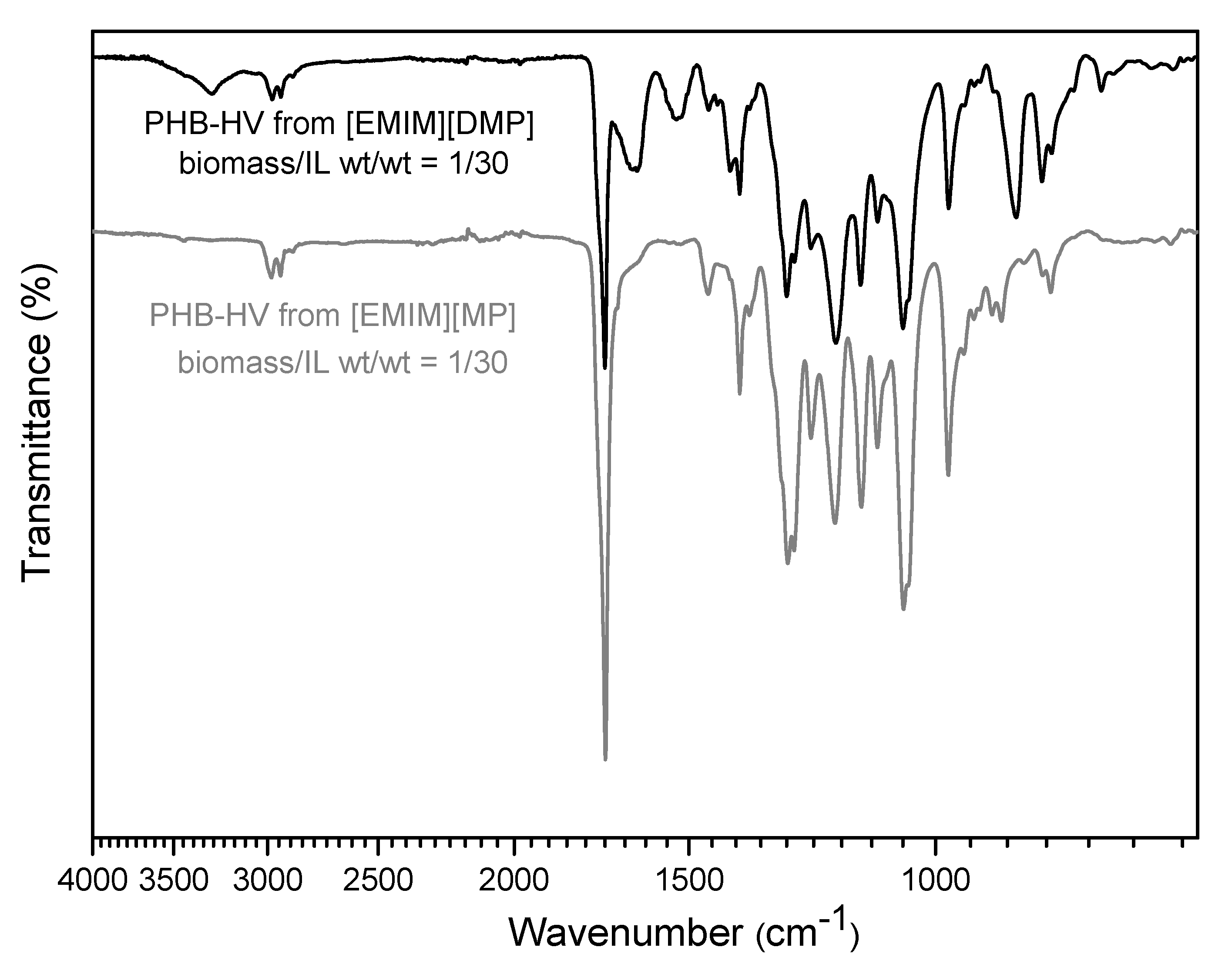
| Paper | Bacterium Strain | PHA Content (wt.%) | bacteria/CYC Ratio wt/v % (% g/mL) | Extraction Temperature (°C) | Extraction Time (min) | Extraction Yield (%) |
|---|---|---|---|---|---|---|
| this one | Rhodovulum Sulfidophilum DSM-1374 | 14.0 | 6.0 | 125 | 5 | 43 |
| 6.0 | 125 | 10 | 95 | |||
| 6.0 | 125 | 20 | 98 | |||
| [14] | Cupriavidus necator H16 | 82.3 | 2.0 | 80 | 1200 | 16 |
| 2.0 | 100 | 5 | 90 | |||
| 2.0 | 120 | 3 | 99 | |||
| [15] | Burkholderia sacchari DSM 17165 | 57.7 | 1.5 | 120–130 | 15 | 98 |
| 6.0 | 120–130 | 15 | 87 | |||
| 6.0 | 120–130 | 30 | 89 | |||
| [45] | Esch. coli | 80.0 | 7.0 | 90 | 35 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippi, S.; Cinelli, P.; Mezzetta, A.; Carlozzi, P.; Seggiani, M. Extraction of Polyhydroxyalkanoates from Purple Non-Sulfur Bacteria by Non-Chlorinated Solvents. Polymers 2021, 13, 4163. https://doi.org/10.3390/polym13234163
Filippi S, Cinelli P, Mezzetta A, Carlozzi P, Seggiani M. Extraction of Polyhydroxyalkanoates from Purple Non-Sulfur Bacteria by Non-Chlorinated Solvents. Polymers. 2021; 13(23):4163. https://doi.org/10.3390/polym13234163
Chicago/Turabian StyleFilippi, Sara, Patrizia Cinelli, Andrea Mezzetta, Pietro Carlozzi, and Maurizia Seggiani. 2021. "Extraction of Polyhydroxyalkanoates from Purple Non-Sulfur Bacteria by Non-Chlorinated Solvents" Polymers 13, no. 23: 4163. https://doi.org/10.3390/polym13234163







