What We Are Learning from COVID-19 for Respiratory Protection: Contemporary and Emerging Issues
Abstract
:1. Introduction
2. RPD Performance, Issues, and Challenges
2.1. Filtration Mechanisms and Efficiency
2.2. Filtration Efficiency Requirements and Testing Methods
| Tested Items | Test Condition | Results | Conclusions |
|---|---|---|---|
| N95 filter media, cloth masks, sweatshirts, T-shirts, towels, scarfs | Method: material testing Agent: NaCl aerosol Size: polydisperse particle median diameter 75 nm, 10 levels of monodisperse particles diameter 20–400 nm Flowrate: 33 L/min and 99 L/min Concentration: NA | In both flowrate conditions, N95 filter media had less than 4% penetration, while other tested items had 40–90% penetration for polydisperse particles and 9–98% penetration for various sizes of monodisperse particles | Common fabric material only provides marginal protection against small particles, filtration efficiency for different particle sizes varies significantly [95] |
| Surgical mask, T-shirt, scarf, tea towel, pillowcase, vacuum cleaner bag, cotton mix, linen, silk | Method: material testing Agent: bacterial aerosol (Batrophaeus), viral aerosol (Bacteriophage MS2) Size: 0.95–1.25 µm, 23 nm Flowrate: 30 L/min Concentration: 107 colony-forming units, 109 plaque-forming units | For bacterial aerosol, filtration efficiency ranged from 58% to 96%; for viral aerosol, filtration efficiency ranged from 51% to 90% | Surgical mask, double layer tea towel, and vacuum cleaner bag had similar filtration efficiencies (>94% for bacteria; >85% for viruses); double layer T-shirt did not offer any improvement over single layer [122] |
| N95 masks, surgical masks, cloth masks | Method: product testing with head manikin Agent: polystyrene latex, diluted diesel combustion particles Size: 5 levels of monodisperse particles 30 nm to 2.5 µm, diesel particle size < 500 nm Flowrate: 8 L/min and 19 L/min Concentration: 2.84 × 103 to 2.77 × 105 no./cm3; 4.13 × 102 to 2.66 × 104 no./cm3 | Cloth mask filtration efficiencies ranged from 15–57% for diesel particles and 39% to 65% for latex particles; disposable surgical masks had efficiencies of 78–94% for latex and 79% for diesel particles. | N95 masks were effective at removing most test particles; surgical masks were surprisingly effective for all test particles; cloth masks only had a marginal filtration efficiency [123] |
| N95 masks, surgical masks, various fabrics (cotton quilt, cotton 80 TPI, cotton 600 TPI, flannel, chiffon, natural silk, synthetic silk, satin, spandex, polyester) | Method: material testing Agent: NaCl aerosol Size: polydisperse sizes ranging from 10 nm to 6 µm Flowrate: 35 L/min and 90 L/min Concentration: NA | At the lower flow rate, several fabrics achieved the same filtration efficiency as N95 and surgical masks (75–99%); at the higher flow rate, N95 maintains a high efficiency (>94%) while the other materials exhibit a significantly reduced efficiency (14–64%), especially for particles <300 nm | At the lower flow rate, fabric combinations such as cotton-silk, cotton-chiffon, and cotton-flannel had filtration efficiencies above 80% irrespective of the particle size; the number of fabric layers and the fabric density (i.e., threads per inch) both affected filtration efficiency [77] |
2.3. Fit Requirements and Test Methods
2.3.1. Fit and Inward Leakage Issues
2.3.2. Fit and Inward Leakage Issues
2.4. Discomfort Related to Breathing Resistance and Air Exchange
2.5. Thermal, Moisture, and Physical Discomfort
3. User Groups, Specifications, and Key Issues
3.1. Healthcare Workers
3.2. Atypical RPD-Users and the General Public
3.3. Protection of Vulnerable Populations: Children and the Elderly
4. Numerical Modeling and Simulation of RPD Infection Control
4.1. Simulation of Human Breathing, Talking, Sneezing, and Coughing
4.2. Simulation of Droplet Transportation and Air Flow
4.3. Simulation of Particle Transport through RPDs
4.4. Knowledge Gaps and Recommendations for Future Research Directions
| Research Focus | References | |
|---|---|---|
| Realistic simulation of expiratory events | Coughing | [67,117,118,291] |
| Sneezing | [116,293] | |
| Human activities | Head movement | [116,279] |
| Walking | [282] | |
| Accurate representation of the interaction between droplets and the RPD’s internal structure | [91,284,285,287,292] | |
| Leakage flows | [66,67,279,290,291,294] | |
| Representation of CO2 levels | [212,274] | |
| Validation with experimental data | [116,295] | |
| Effects of environmental conditions | Wind speed | [66,118,291] |
| Relatively humidity/particulate matters | [66,286] | |
| Room ventilation | [113,278,279,296] | |
5. Decontamination and Reuse of RPDs
5.1. Challenges in RPD Decontamination
| Decontamination Method | Disinfection Method | Anti-Pathogen and Performance Impact | Feasibility and Limitations |
|---|---|---|---|
| Energetic Various types of heating to induce structural changes in virus proteins and disrupt the specific structures needed to recognize and bind to host cells | Ultraviolet germicidal irradiation (UVGI) |
|
|
| Moist heat |
|
| |
| Chemical To induce cross-linkage, coagulation, and clumping thereby disrupting the structure and function, affecting or killing an organism via oxidation | Vaporized H2O2 (VHP) |
|
|
| Home bleach |
|
|
5.2. Surgical and Cloth Mask Decontamination
5.3. Current RPD Decontamination Regulations and Issues
6. New Materials for Future RPDs
6.1. Nanomaterials
6.2. Biodegradable Materials
6.3. Biocidal Materials
6.3.1. Metal Ions and Nanoparticles
6.3.2. Quaternary Ammonium Salts
6.3.3. Photo-Induced Biocides
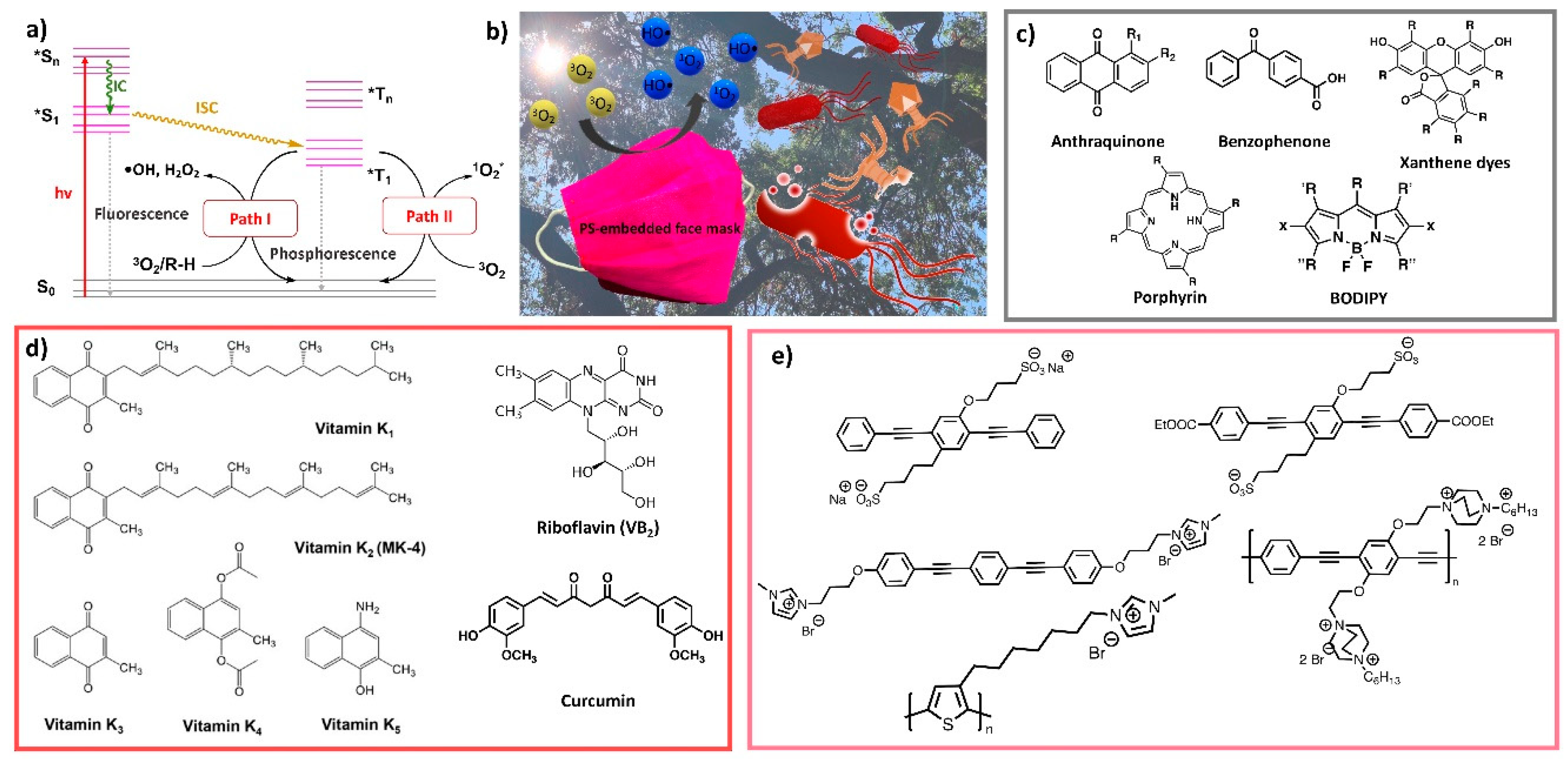
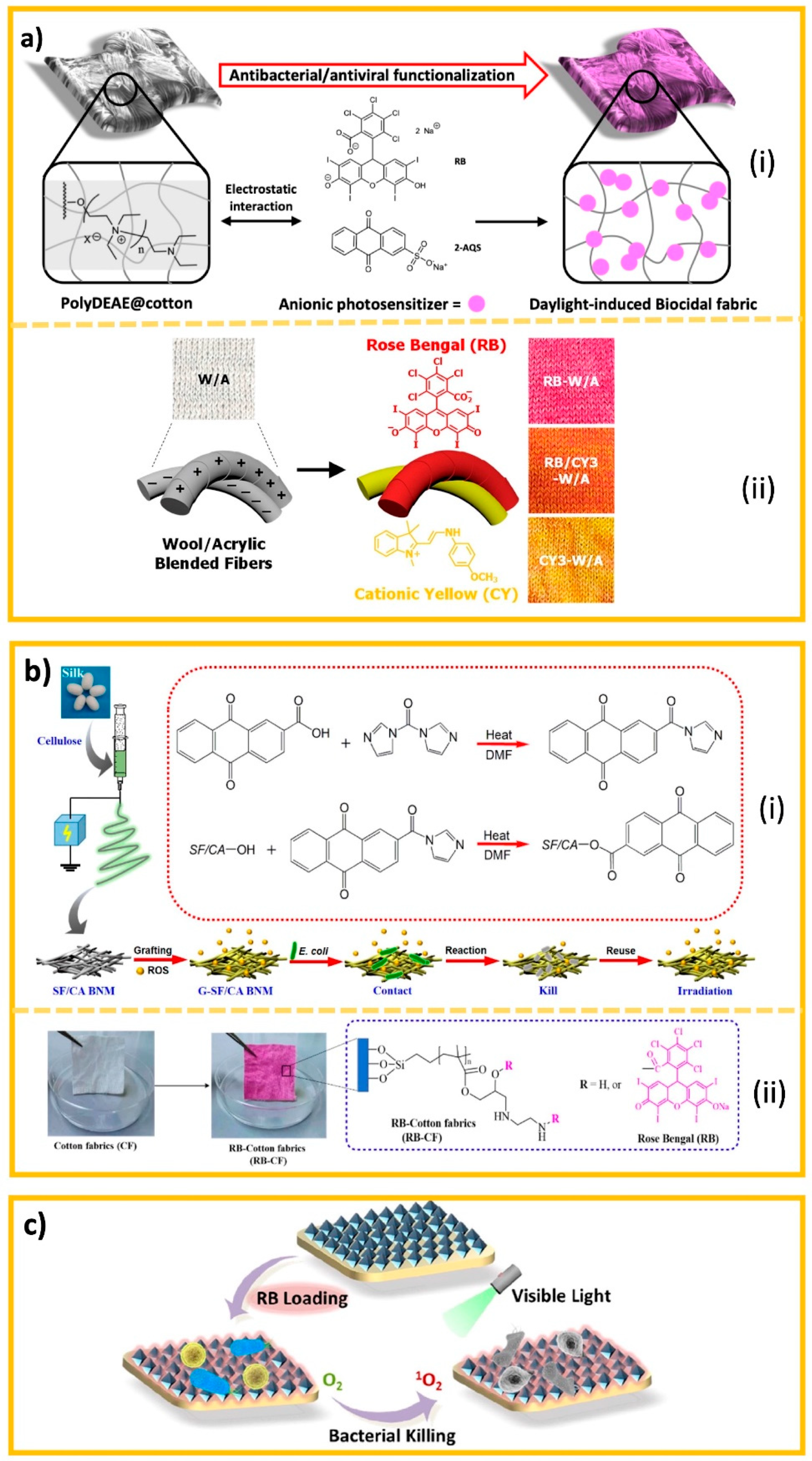
6.3.4. Halamine Biocides
7. Future Perspectives and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AATCC | American Association of Textile Chemists and Colorists |
| AGP | Aerosol-Generating Procedures |
| AI | Artificial Intelligence |
| ANSI | American National Standards Institute |
| ASTM | American Society for Testing and Materials |
| CDC | Centers for Disease Control and Prevention |
| CPR | Cardiopulmonary resuscitation |
| EUA | Emergency use authorization |
| FDA | Food and Drug Administration |
| FFP2 | Filtering facepiece 2 |
| FFR | Filtering facepiece respirators |
| HCW | Healthcare worker |
| MERS | Middle East Respiratory Syndrome |
| MOF | Metal organic framework |
| MPPS | Most penetrating particle size |
| NIOSH | National Institute for Occupational Safety & Health |
| NPPTL | National Personal Protective Technology Laboratory |
| OSHA | Occupational Safety and Health Administration |
| PAPR | Powered air purifying respirators |
| PM | Particulate matter |
| PP-DNS | Point particle direct numerical simulation |
| PPE | Personal protective equipment |
| PS | Photo-sensitizer |
| ROS | Reactive oxygen species |
| RPD | Respiratory protection devices |
| SARS | Severe acute respiratory syndrome |
| SMS | Spunbond-meltblown-spunbond |
| UVC | Ultraviolet C |
| UVGI | Ultraviolet germicidal irradiation |
| VHP | Vaporized Hydrogen Peroxide |
References
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.-D.; Novoselov, K.S. Sustainable personal protective clothing for healthcare applications: A review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2021. Available online: https://coronavirus.jhu.edu/map.html (accessed on 21 November 2021).
- CDC. SARS Basics Fact Sheet. 2017. Available online: https://www.cdc.gov/sars/about/fs-sars.html (accessed on 10 November 2021).
- CDC. 2009 H1N1 Pandemic (H1N1pdm09 Virus). 2019. Available online: https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html#:~:text=From%20April%2012%2C%202009%20to,the%20(H1N1)pdm09%20virus (accessed on 8 November 2021).
- ECDC. MERS-CoV Worldwide Overview. 2021. Available online: https://www.ecdc.europa.eu/en/middle-east-respiratory-syndrome-coronavirus-mers-cov-situation-update (accessed on 15 October 2021).
- WHO. Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. 2020. Available online: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (accessed on 15 October 2021).
- REMARS. Webinar: How to Operate and Use Building Services during the COVID-19 Crisis. 2020. Available online: https://remars.co.uk/webinar-how-to-operate-and-use-building-services-during-the-covid-19-crisis/ (accessed on 17 October 2021).
- Li, Y.; Qian, H.; Hang, J.; Chen, X.; Cheng, P.; Ling, H.; Wang, S.; Liang, P.; Li, J.; Xiao, S. Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build. Environ. 2021, 196, 107788. [Google Scholar] [CrossRef]
- Prather, K.A.; Wang, C.C.; Schooley, R.T. Reducing transmission of SARS-CoV-2. Science 2020, 368, 1422–1424. [Google Scholar] [CrossRef]
- Huang, H.; Fan, C.; Li, M.; Nie, H.-L.; Wang, F.-B.; Wang, H.; Wang, R.; Xia, J.; Zheng, X.; Zuo, X. COVID-19: A call for physical scientists and engineers. ACS Nano 2020, 14, 3747–3754. [Google Scholar] [CrossRef] [Green Version]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef] [Green Version]
- CDC. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html (accessed on 15 October 2021).
- CDC. Clinical Questions about COVID-19: Questions and Answers. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html#Transmission (accessed on 16 October 2021).
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef]
- Bian, L.; Gao, Q.; Gao, F.; Wang, Q.; He, Q.; Wu, X.; Mao, Q.; Xu, M.; Liang, Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 1201–1209. [Google Scholar] [CrossRef]
- Mahase, E. Delta variant: What is happening with transmission, hospital admissions, and restrictions? BMJ 2021, 373, n1513. [Google Scholar] [CrossRef]
- O’Dowd, A. Covid-19: Cases of delta variant rise by 79%, but rate of growth slows. BMJ 2021, 373, n1596. [Google Scholar] [CrossRef]
- Kupferschmidt, K.; Wadman, M. Delta variant triggers new phase in the pandemic. Science 2021, 372, 1375–1376. [Google Scholar] [CrossRef]
- Duong, D. Alpha, Beta, Delta, Gamma: What’s important to know about SARS-CoV-2 variants of concern? CMAJ Can. Med. Assoc. J. 2021, 193, E1059. [Google Scholar] [CrossRef]
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance 2021, 26, 2100509. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, J.; Deng, A.; Zhang, Y.; Zhuang, Y.; Hu, T.; Li, J.; Tu, H.; Li, B.; Zhou, Y. Transmission dynamics of an outbreak of the COVID-19 Delta variant B. 1.617. 2—Guangdong Province, China, May–June 2021. China CDC Wkly. 2021, 3, 584. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Threat Assessment Brief: Rapid Increase of a SARS-CoV-2 Variant with Multiple Spike Protein Mutations Observed in the UK. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/threat-assessment-brief-rapid-increase-sars-cov-2-variant-united-kingdom (accessed on 17 October 2021).
- Nature. COVID Research: A Year of Scientific Milestones. 2021. Available online: https://www.nature.com/articles/d41586-020-00502-w (accessed on 16 October 2021).
- Picheta, R. Europe is Learning a Crucial Lesson—Vaccines Work, but They Alone Won’t Stop Covid Now. 2021. Available online: https://www.cnn.com/2021/11/19/europe/europe-covid-vaccination-rates-fourth-wave-cmd-intl/index.html (accessed on 16 October 2021).
- Gandhi, M. Can People Spread the Coronavirus if They Don’t Have Symptoms? 5 Questions Answered about Asymptomatic COVID-19. 2020. Available online: https://theconversation.com/can-people-spread-the-coronavirus-if-they-dont-have-symptoms-5-questions-answered-about-asymptomatic-covid-19-140531 (accessed on 18 October 2021).
- Furukawa, N.W.; Brooks, J.T.; Sobel, J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg. Infect. Dis. 2020, 26, e201595. [Google Scholar] [CrossRef]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in health-care workers: A living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef]
- Pan, X.; Chen, D.; Xia, Y.; Wu, X.; Li, T.; Ou, X.; Zhou, L.; Liu, J. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect. Dis. 2020, 20, 410–411. [Google Scholar] [CrossRef]
- Qian, G.; Yang, N.; Ma, A.H.Y.; Wang, L.; Li, G.; Chen, X.; Chen, X. COVID-19 transmission within a family cluster by presymptomatic carriers in China. Clin. Infect. Dis. 2020, 71, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lee, H.P. The perspective of fluid flow behavior of respiratory droplets and aerosols through the facemasks in context of SARS-CoV-2. Phys. Fluids 2020, 32, 111301. [Google Scholar] [CrossRef]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, L.; Wymant, C.; Kendall, M.; Zhao, L.; Nurtay, A.; Abeler-Dörner, L.; Parker, M.; Bonsall, D.; Fraser, C. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020, 368, 6491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhamamsy, S.; DeVone, F.; Bayer, T.; Halladay, C.; Cadieux, M.; McConeghy, K.; Rajan, A.; Sachar, M.; Mujahid, N.; Nanda, A.; et al. Can we clinically identify pre-symptomatic and asymptomatic COVID-19? medRxiv 2021. [Google Scholar]
- Qiu, X.; Nergiz, A.I.; Maraolo, A.E.; Bogoch, I.I.; Low, N.; Cevik, M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission—A living systematic review. Clin. Microbiol. Infect. 2021, 27, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Tan, J.; Jiang, Y.; Wang, X.; Zhang, H. Evaluate the risk of resumption of business for the states of New York, New Jersey and Connecticut via a pre-symptomatic and asymptomatic transmission model of COVID-19. J. Data Sci. 2020, 1, 178–196. [Google Scholar] [CrossRef]
- Snider, B.; Patel, B.; McBean, E. Asymptomatic Cases, the Hidden Challenge in Predicting COVID-19 Caseload Increases. Infect. Dis. Rep. 2021, 13, 340–347. [Google Scholar] [CrossRef]
- Morawska, L.; Milton, D.K. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2311–2313. [Google Scholar] [CrossRef]
- CDC. CDC Calls on Americans to Wear Masks to Prevent COVID-19 Spread. 2020. Available online: https://www.cdc.gov/media/releases/2020/p0714-americans-to-wear-masks.html (accessed on 2 October 2021).
- Wong, S.Y.; Tan, B.H. Megatrends in infectious diseases: The next 10 to 15 years. Ann. Acad. Med. Singap. 2019, 48, 188–194. [Google Scholar]
- Casey, J.D.C. COVID-19 Outbreaks at Tyson Foods Plants Sicken Nearly 5000 Workers. 2020. Available online: https://www.expertinstitute.com/resources/insights/covid-19-outbreaks-at-tyson-foods-plants-sicken-nearly-5000-workers/ (accessed on 12 October 2021).
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on the Use of Elastomeric Respirators in Health Care. Reusable Elastomeric Respirators in Health Care: Considerations for Routine and Surge Use; Liverman, C.T., Yost, O.C., Rogers, B.M.E., Clever, L.H., Eds.; National Academies Press (US): Washington, DC, USA, 2018. [Google Scholar]
- Vanapalli, K.R.; Sharma, H.B.; Ranjan, V.P.; Samal, B.; Bhattacharya, J.; Dubey, B.K.; Goel, S. Challenges and strategies for effective plastic waste management during and post COVID-19 pandemic. Sci. Total Environ. 2021, 750, 141514. [Google Scholar] [CrossRef]
- Gorrasi, G.; Sorrentino, A.; Lichtfouse, E. Back to plastic pollution in COVID times. Environ. Chem. Lett. 2021, 19, 1–4. [Google Scholar] [CrossRef]
- CDC. Implementing Filtering Facepiece Respirator (FFR) Reuse, Including Reuse after Decontamination, when there Are Known Shortages of N95 Respirators. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html (accessed on 21 May 2021).
- Fischer, R.J.; Morris, D.H.; van Doremalen, N.; Sarchette, S.; Matson, M.J.; Bushmaker, T.; Yinda, C.K.; Seifert, S.N.; Gamble, A.; Williamson, B.N. Effectiveness of N95 respirator decontamination and reuse against SARS-CoV-2 virus. Emerg. Infect. Dis. 2020, 26, 2253. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Kasloff, S.B.; Leung, A.; Strong, J.E.; Funk, D.; Cutts, T. Stability of SARS-CoV-2 on critical personal protective equipment. Sci. Rep. 2021, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.M.; Richardson, A.W.; Harpest, S.D.; Hofacre, K.C.; Shaffer, R.E. Reaerosolization of MS2 bacteriophage from an N95 filtering facepiece respirator by simulated coughing. Ann. Occup. Hyg. 2012, 56, 315–325. [Google Scholar] [CrossRef]
- Qian, Y.; Willeke, K.; Grinshpun, S.A.; Donnelly, J. Performance of N95 respirators: Reaerosolization of bacteria and solid particles. Am. Ind. Hyg. Assoc. J. 1997, 58, 876–880. [Google Scholar] [CrossRef]
- Gosch, M.E.; Shaffer, R.E.; Eagan, A.E.; Roberge, R.J.; Davey, V.J.; Radonovich Jr, L.J. B95: A new respirator for health care personnel. Am. J. Infect. Control 2013, 41, 1224–1230. [Google Scholar] [CrossRef]
- Racz, L.; Yamamoto, D.P.; Eninger, R.M. Handbook of Respiratory Protection: Safeguarding against Current and Emerging Hazards; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- CDC. 42 CFR Part 84 Respiratory Protective Devices. 1997. Available online: https://www.cdc.gov/niosh/npptl/topics/respirators/pt84abs2.html (accessed on 10 October 2021).
- FDA. Face Masks, Including Surgical Masks, and Respirators for COVID-19. 2020. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/face-masks-including-surgical-masks-and-respirators-covid-19 (accessed on 12 October 2021).
- National Academies of Sciences, Engineering, and Medicine. Current Issues in the Assessment of Respiratory Protective Devices for Occupational and Non-Occupational Uses: Proceedings of a Workshop; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face masks in the new COVID-19 normal: Materials, testing, and perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef]
- Blachere, F.M.; Lemons, A.R.; Coyle, J.P.; Derk, R.C.; Lindsley, W.G.; Beezhold, D.H.; Woodfork, K.; Duling, M.G.; Boutin, B.; Boots, T. Face mask fit modifications that improve source control performance. medRxiv 2021. [Google Scholar] [CrossRef]
- Patel, R.B.; Skaria, S.D.; Mansour, M.M.; Smaldone, G.C. Respiratory source control using a surgical mask: An in vitro study. J. Occup. Environ. Hyg. 2016, 13, 569–576. [Google Scholar] [CrossRef] [Green Version]
- O’Dowd, K.; Nair, K.M.; Forouzandeh, P.; Mathew, S.; Grant, J.; Moran, R.; Bartlett, J.; Bird, J.; Pillai, S.C. Face masks and respirators in the fight against the COVID-19 pandemic: A review of current materials, advances and future perspectives. Materials 2020, 13, 3363. [Google Scholar] [CrossRef]
- AATCC. AATCC M14-2020 Guidance and Considerations for General Purpose Textile Face Coverings: Adult; AATCC: Research Triangle, NC, USA, 2020. [Google Scholar]
- ASTM. ASTM F3502-21 Standard Specification for Barrier Face Coverings; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- He, D.; Zhao, S.; Lin, Q.; Zhuang, Z.; Cao, P.; Wang, M.H.; Yang, L. The relative transmissibility of asymptomatic COVID-19 infections among close contacts. Int. J. Infect. Dis. 2020, 94, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Yokoe, D.S.; Havlir, D.V. Asymptomatic Transmission, the Achilles’ Heel of Current Strategies to Control COVID-19. In The COVID-19 Reader; Routledge: London, UK, 2020; pp. 36–39. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef]
- Feng, Y.; Marchal, T.; Sperry, T.; Yi, H. Influence of wind and relative humidity on the social distancing effectiveness to prevent COVID-19 airborne transmission: A numerical study. J. Aerosol Sci. 2020, 147, 105585. [Google Scholar] [CrossRef]
- Dbouk, T.; Drikakis, D. On respiratory droplets and face masks. Phys. Fluids 2020, 32, 063303. [Google Scholar] [CrossRef]
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Perrone, M.G.; Borelli, M.; Palmisani, J.; Di Gilio, A.; Piscitelli, P.; Miani, A. Airborne transmission route of COVID-19: Why 2 meters/6 feet of inter-personal distance could not be enough. Int. J. Environ. Res. Public Health 2020, 17, 2932. [Google Scholar] [CrossRef] [Green Version]
- Howard, J.; Huang, A.; Li, Z.; Tufekci, Z.; Zdimal, V.; van der Westhuizen, H.-M.; von Delft, A.; Price, A.; Fridman, L.; Tang, L.-H.; et al. An evidence review of face masks against COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2014564118. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Gao, L.; Cheng, C.; Zhou, Q.; Uy, J.P.; Heiner, K.; Sun, C. Efficacy of face mask in preventing respiratory virus transmission: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 36, 101751. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, N.; Witt, C.; Rapp, S.; Wild, P.S.; Andreae, M.O.; Pöschl, U.; Su, H. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science 2021, eabg6296. [Google Scholar] [CrossRef] [PubMed]
- Tcharkhtchi, A.; Abbasnezhad, N.; Seydani, M.Z.; Zirak, N.; Farzaneh, S.; Shirinbayan, M. An overview of filtration efficiency through the masks: Mechanisms of the aerosols penetration. Bioact. Mater. 2021, 6, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Hinds, W.C. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Lee, K.; Liu, B. On the minimum efficiency and the most penetrating particle size for fibrous filters. J. Air Pollut. Control Assoc. 1980, 30, 377–381. [Google Scholar] [CrossRef]
- Fjeld, R.A.; Owens, T.M. The effect of particle charge on penetration in an electret filter. IEEE Trans. Ind. Appl. 1988, 24, 725–731. [Google Scholar] [CrossRef]
- Gras, J. Air filtration: An integrated approach to the theory and applications of fibrous filters [Book Review]. Clean Air J. Clean Air Soc. Aust. N. Zldn. 1994, 28, 42. [Google Scholar]
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano 2020, 14, 6339–6347. [Google Scholar] [CrossRef]
- Janssen, L. Principles of physiology and respirator performance. Occup. Health Saf. 2003, 72, 73–81. [Google Scholar] [PubMed]
- Mahdavi, A. Efficiency Measurement of N95 Filtering Facepiece Respirators against Ultrafine Particles under Cyclic and Constant Flows; Concordia University: Montreal, QC, Canada, 2013. [Google Scholar]
- Podgorski, A.; Bałazy, A.; Gradoń, L. Application of nanofibers to improve the filtration efficiency of the most penetrating aerosol particles in fibrous filters. Chem. Eng. Sci. 2006, 61, 6804–6815. [Google Scholar] [CrossRef]
- Kilic, A.; Russell, S.; Shim, E.; Pourdeyhimi, B. 4—The charging and stability of electret filters. In Fibrous Filter Media; Brown, P.J., Cox, C.L., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 95–121. [Google Scholar]
- Xu, J.; Xiao, X.; Zhang, W.; Xu, R.; Kim, S.C.; Cui, Y.; Howard, T.T.; Wu, E. Air-filtering masks for respiratory protection from PM2.5 and pandemic pathogens. One Earth 2020, 3, 574–589. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Richardson, A.W.; Eshbaugh, J.P.; Hofacre, K.C.; Gardner, P.D. Respirator Filter Efficiency Testing against Particulate and Biological Aerosols under Moderate to High Flow Rates; Battelle Memorial Inst.: Columbus, OH, USA, 2006. [Google Scholar]
- Eshbaugh, J.P.; Gardner, P.D.; Richardson, A.W.; Hofacre, K.C. N95 and P100 respirator filter efficiency under high constant and cyclic flow. J. Occup. Environ. Hyg. 2008, 6, 52–61. [Google Scholar] [CrossRef]
- Qian, Y.; Willeke, K.; Grinshpun, S.A.; Donnelly, J.; Coffey, C.C. Performance of N95 respirators: Filtration efficiency for airborne microbial and inert particles. Am. Ind. Hyg. Assoc. J. 1998, 59, 128–132. [Google Scholar] [CrossRef] [PubMed]
- McCullough, N.; Brosseau, L.; Vesley, D. Collection of three bacterial aerosols by respirator and surgical mask filters under varying conditions of flow and relative humidity. Ann. Occup. Hyg. 1997, 41, 677–690. [Google Scholar] [CrossRef]
- He, X.; Grinshpun, S.A.; Reponen, T.; McKay, R.; Bergman, M.S.; Zhuang, Z. Effects of breathing frequency and flow rate on the total inward leakage of an elastomeric half-mask donned on an advanced manikin headform. Ann. Occup. Hyg. 2014, 58, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Givehchi, R.; Tan, Z. The effect of capillary force on airborne nanoparticle filtration. J. Aerosol Sci. 2015, 83, 12–24. [Google Scholar] [CrossRef]
- Xiao, X.; Qian, L. Investigation of humidity-dependent capillary force. Langmuir 2000, 16, 8153–8158. [Google Scholar] [CrossRef]
- Yi, L.; Fengzhi, L.; Qingyong, Z. Numerical simulation of virus diffusion in facemask during breathing cycles. Int. J. Heat Mass Transf. 2005, 48, 4229–4242. [Google Scholar] [CrossRef]
- Mahdavi, A.; Haghighat, F.; Bahloul, A.; Brochot, C.; Ostiguy, C. Particle loading time and humidity effects on the efficiency of an N95 filtering facepiece respirator model under constant and inhalation cyclic flows. Ann. Occup. Hyg. 2015, 59, 629–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guha, S.; McCaffrey, B.; Hariharan, P.; Myers, M.R. Quantification of leakage of sub-micron aerosols through surgical masks and facemasks for pediatric use. J. Occup. Environ. Hyg. 2017, 14, 214–223. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Clouter, A.; Renwick, L.; MacNee, W. Ultrafine particles. Occup. Environ. Med. 2001, 58, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengasamy, S.; Eimer, B.; Shaffer, R.E. Simple respiratory protection—Evaluation of the filtration performance of cloth masks and common fabric materials against 20–1000 nm size particles. Ann. Occup. Hyg. 2010, 54, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wong, T.; Chung, A.J.; Guo, Y.; Hu, J.; Guan, Y.; Yao, L.; Song, Q.; Newton, E. In vivo protective performance of N95 respirator and surgical facemask. Am. J. Ind. Med. 2006, 49, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- OSHA 29 CFR 1910.134. Personal Protective Equipment: Respiratory Protection; OSHA: Washington, DC, USA, 2006. [Google Scholar]
- CEN EN 149:2001+A1:2009. Respiratory Protective Devices—Filtering Half Masks to Protect against Particles—Requirements, Testing, Marking; CEN: Brussels, Belgium, 2009. [Google Scholar]
- CEN EN 13274 Series. Respiratory Protective Devices—Methods of Test Part1–Part8; CEN: Brussels, Belgium, 2019. [Google Scholar]
- ISO 10993-1:2018. Biological Evaluation of Medical Devices; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- OSHA 29 CFR 1910.1030. Personal Protective Equipment: Bloodborne Pathogens; OSHA: Washington, DC, USA, 1992. [Google Scholar]
- FDA. 510(k) Premarket Notification. 2021. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm (accessed on 25 July 2021).
- ASTM F2100-20. Standard Specification for Performance of Materials Used in Medical Face Masks; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM F2299-03. Standard Test Method for Determining the Initial Efficiency of Materials Used in Medical Face Masks to Penetration by Particulates Using Latex Spheres; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM F2101-19. Standard Test Method for Evaluating the Bacterial Filtration Efficiency (BFE) of Medical Face Mask Materials, Using a Biological Aerosol of Staphylococcus aureus; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM F1862-17. Standard Test Method for Resistance of Medical Face Masks to Penetration by Synthetic Blood (Horizontal Projection of Fixed Volume at a Known Velocity); ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ISO 11737-1. Sterilization of Health Care Products—Microbiological Methods—Part 1: Determination of a Population of Microorganisms on Products; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- ISO 22609. Clothing for Protection against Infectious Agents—Medical Face Masks—Test Method for Resistance against Penetration by Synthetic Blood (Fixed Volume, Horizontally Projected); ISO: Geneva, Switzerland, 2004. [Google Scholar]
- CEN EN 14683:2019+AC:2019. Medical Face Masks—Requirements and Test Methods; CEN: Brussels, Belgium, 2019. [Google Scholar]
- Rengasamy, S.; Shaffer, R.; Williams, B.; Smit, S. A comparison of facemask and respirator filtration test methods. J. Occup. Environ. Hyg. 2017, 14, 92–103. [Google Scholar] [CrossRef]
- CDC. NPPTL Respirator Assessments to Support the COVID-19 Response. 2021. Available online: https://www.cdc.gov/niosh/npptl/respirators/testing/NonNIOSHresults.html (accessed on 16 July 2021).
- Zhang, B.; Guo, G.; Zhu, C.; Ji, Z.; Lin, C.-H. Transport and trajectory of cough-induced bimodal aerosol in an air-conditioned space. Indoor Built Environ. 2020, 1420326X20941166. [Google Scholar] [CrossRef]
- Abuhegazy, M.; Talaat, K.; Anderoglu, O.; Poroseva, S.V. Numerical investigation of aerosol transport in a classroom with relevance to COVID-19. Phys. Fluids 2020, 32, 103311. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Lin, C.H.; Chen, Q. Risk assessment of airborne infectious diseases in aircraft cabins. Indoor Air 2012, 22, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef] [PubMed]
- Busco, G.; Yang, S.R.; Seo, J.; Hassan, Y.A. Sneezing and asymptomatic virus transmission. Phys. Fluids 2020, 32, 073309. [Google Scholar] [CrossRef]
- Diwan, S.S.; Ravichandran, S.; Govindarajan, R.; Narasimha, R. Understanding transmission dynamics of COVID-19-type infections by direct numerical simulations of cough/sneeze flows. Trans. Indian Natl. Acad. Eng. 2020, 5, 255–261. [Google Scholar] [CrossRef]
- Dbouk, T.; Drikakis, D. On coughing and airborne droplet transmission to humans. Phys. Fluids 2020, 32, 053310. [Google Scholar] [CrossRef]
- Bourouiba, L.; Dehandschoewercker, E.; Bush, J.W. Violent expiratory events: On coughing and sneezing. J. Fluid Mech. 2014, 745, 537–563. [Google Scholar] [CrossRef]
- Tang, J.W.; Nicolle, A.D.; Klettner, C.A.; Pantelic, J.; Wang, L.; Suhaimi, A.B.; Tan, A.Y.; Ong, G.W.; Su, R.; Sekhar, C. Airflow dynamics of human jets: Sneezing and breathing-potential sources of infectious aerosols. PLoS ONE 2013, 8, e59970. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, H.; Sakata, S.; Kaga, A. A new methodology for studying dynamics of aerosol particles in sneeze and cough using a digital high-vision, high-speed video system and vector analyses. PLoS ONE 2013, 8, e80244. [Google Scholar] [CrossRef]
- Davies, A.; Thompson, K.-A.; Giri, K.; Kafatos, G.; Walker, J.; Bennett, A. Testing the efficacy of homemade masks: Would they protect in an influenza pandemic? Disaster Med. Public Health Prep. 2013, 7, 413–418. [Google Scholar] [CrossRef]
- Shakya, K.M.; Noyes, A.; Kallin, R.; Peltier, R.E. Evaluating the efficacy of cloth facemasks in reducing particulate matter exposure. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.H.; Yim, W.; Garg, A.K.; Shah, S.H.; Jokerst, J.V.; Chao, D.L. Assessing the Physiological Relevance of Cough Simulators for Respiratory Droplet Dispersion. J. Clin. Med. 2020, 9, 3002. [Google Scholar] [CrossRef] [PubMed]
- Blocken, B.; Malizia, F.; Van Druenen, T.; Marchal, T. Towards aerodynamically equivalent COVID19 1.5 m social distancing for walking and running. Preprint 2020. Available online: https://www.euroga.org/system/1/user_files/files/000/045/111/45111/150d3060c/original/Social_Distancing_v20_White_Paper.pdf (accessed on 18 July 2021).
- Wang, Y.; Xu, G.; Huang, Y.-W. Modeling the load of SARS-CoV-2 virus in human expelled particles during coughing and speaking. PLoS ONE 2020, 15, e0241539. [Google Scholar] [CrossRef]
- Fontes, D.; Reyes, J.; Ahmed, K.; Kinzel, M. A study of fluid dynamics and human physiology factors driving droplet dispersion from a human sneeze. Phys. Fluids 2020, 32, 111904. [Google Scholar] [CrossRef]
- Das, S.K.; Alam, J.-E.; Plumari, S.; Greco, V. Transmission of airborne virus through sneezed and coughed droplets. Phys. Fluids 2020, 32, 097102. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Tu, J. Thermal effect of human body on cough droplets evaporation and dispersion in an enclosed space. Build. Environ. 2019, 148, 96–106. [Google Scholar] [CrossRef]
- Han, Z.; Weng, W.; Huang, Q. Characterizations of particle size distribution of the droplets exhaled by sneeze. J. R. Soc. Interface 2013, 10, 20130560. [Google Scholar] [CrossRef]
- Gralton, J.; Tovey, E.; McLaws, M.-L.; Rawlinson, W.D. The role of particle size in aerosolised pathogen transmission: A review. J. Infect. 2011, 62, 1–13. [Google Scholar] [CrossRef]
- Yang, S.; Lee, G.W.; Chen, C.-M.; Wu, C.-C.; Yu, K.-P. The size and concentration of droplets generated by coughing in human subjects. J. Aerosol Med. 2007, 20, 484–494. [Google Scholar] [CrossRef]
- Johnson, G.; Morawska, L.; Ristovski, Z.; Hargreaves, M.; Mengersen, K.; Chao, C.Y.H.; Wan, M.; Li, Y.; Xie, X.; Katoshevski, D. Modality of human expired aerosol size distributions. J. Aerosol Sci. 2011, 42, 839–851. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, C.; Ji, Z.; Lin, C.-H. Design and characterization of a cough simulator. J. Breath Res. 2017, 11, 016014. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Reynolds, J.S.; Szalajda, J.V.; Noti, J.D.; Beezhold, D.H. A cough aerosol simulator for the study of disease transmission by human cough-generated aerosols. Aerosol Sci. Technol. 2013, 47, 937–944. [Google Scholar] [CrossRef]
- Seepana, S.; Lai, A.C. Experimental and numerical investigation of interpersonal exposure of sneezing in a full-scale chamber. Aerosol Sci. Technol. 2012, 46, 485–493. [Google Scholar] [CrossRef]
- Morawska, L.; Johnson, G.; Ristovski, Z.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Chao, C.Y.H.; Li, Y.; Katoshevski, D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009, 40, 256–269. [Google Scholar] [CrossRef] [Green Version]
- Lindsley, W.G.; Blachere, F.M.; Law, B.F.; Beezhold, D.H.; Noti, J.D. Efficacy of face masks, neck gaiters and face shields for reducing the expulsion of simulated cough-generated aerosols. Aerosol Sci. Technol. 2020, 55, 457–499. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Noti, J.D.; Blachere, F.M.; Szalajda, J.V.; Beezhold, D.H. Efficacy of face shields against cough aerosol droplets from a cough simulator. J. Occup. Environ. Hyg. 2014, 11, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, W.G.; Blachere, F.M.; McClelland, T.L.; Neu, D.T.; Mnatsakanova, A.; Martin Jr, S.B.; Mead, K.R.; Noti, J.D. Efficacy of an ambulance ventilation system in reducing EMS worker exposure to airborne particles from a patient cough aerosol simulator. J. Occup. Environ. Hyg. 2019, 16, 804–816. [Google Scholar] [CrossRef]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.; Kumita, M.; Honda, T.; Kimura, K.; Nozaki, K.; Emi, H.; Otani, Y. Breathing simulator of workers for respirator performance test. Ind. Health 2015, 53, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Nikander, K.; Denyer, J.; Everard, M.; Smaldone, G. Validation of a new breathing simulator generating and measuring inhaled aerosol with adult breathing patterns. J. Aerosol Med. 2000, 13, 139–146. [Google Scholar] [CrossRef]
- Bergman, M.S.; Zhuang, Z.; Hanson, D.; Heimbuch, B.K.; McDonald, M.J.; Palmiero, A.J.; Shaffer, R.E.; Harnish, D.; Husband, M.; Wander, J.D. Development of an advanced respirator fit-test headform. J. Occup. Environ. Hyg. 2014, 11, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.S.; He, X.; Joseph, M.E.; Zhuang, Z.; Heimbuch, B.K.; Shaffer, R.E.; Choe, M.; Wander, J.D. Correlation of respirator fit measured on human subjects and a static advanced headform. J. Occup. Environ. Hyg. 2015, 12, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.; Yu, L.; Yang, W.; Hu, L.; Li, N.; Wang, J.; Li, J.; Lu, J.; Dong, X.; Yin, Z. Assessment the protection performance of different level personal respiratory protection masks against viral aerosol. Aerobiologia 2013, 29, 365–372. [Google Scholar] [CrossRef] [PubMed]
- NCSU. Dynamic Breathing Headform Assessment of Mask Performance and Face Seal. 2020. Available online: https://textiles.ncsu.edu/tpacc/personal-protective-equipment/mask-performance-and-face-seal/ (accessed on 5 May 2021).
- ASTM F3407-20. Standard Test Method for Respirator Fit Capability for Negative-Pressure Half-Facepiece Particulate Respirators; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Zhuang, Z.; Bergman, M.; Brochu, E.; Palmiero, A.; Niezgoda, G.; He, X.; Roberge, R.; Shaffer, R. Temporal changes in filtering-facepiece respirator fit. J. Occup. Environ. Hyg. 2016, 13, 265–274. [Google Scholar] [CrossRef]
- Zhuang, Z.; Bradtmiller, B.; Shaffer, R.E. New respirator fit test panels representing the current US civilian work force. J. Occup. Environ. Hyg. 2007, 4, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.J.; Pisaniello, D.; Ahmad, J.; Edwards, S. Evaluation of a large-scale quantitative respirator-fit testing program for healthcare workers: Survey results. Infect. Control Hosp. Epidemiol. 2010, 31, 918–925. [Google Scholar] [CrossRef]
- Grinshpun, S.A.; Haruta, H.; Eninger, R.M.; Reponen, T.; McKay, R.T.; Lee, S.-A. Performance of an N95 filtering facepiece particulate respirator and a surgical mask during human breathing: Two pathways for particle penetration. J. Occup. Environ. Hyg. 2009, 6, 593–603. [Google Scholar] [CrossRef]
- Lee, S.-A.; Grinshpun, S.A.; Reponen, T. Respiratory performance offered by N95 respirators and surgical masks: Human subject evaluation with NaCl aerosol representing bacterial and viral particle size range. Ann. Occup. Hyg. 2008, 52, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, R.B.; Duling, M.G.; Calvert, C.A.; Coffey, C.C. Comparison of performance of three different types of respiratory protection devices. J. Occup. Environ. Hyg. 2006, 3, 465–474. [Google Scholar] [CrossRef]
- OSHA. Appendix A to §1910.134—Fit Testing Procedures (Mandatory). 2014. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA (accessed on 21 August 2021).
- Regli, A.; Sommerfield, A.; von Ungern-Sternberg, B. The role of fit testing N95/FFP2/FFP3 masks: A narrative review. Anaesthesia 2021, 76, 91–100. [Google Scholar] [CrossRef]
- Clayton, M.; Vaughan, N. Fit for purpose? The role of fit testing in respiratory protection. Ann. Occup. Hyg. 2005, 49, 545–548. [Google Scholar] [CrossRef] [Green Version]
- Yassi, A.; Moore, D.; Fitzgerald, J.M.; Bigelow, P.; Hon, C.Y.; Bryce, E. Research gaps in protecting healthcare workers from SARS and other respiratory pathogens: An interdisciplinary, multi-stakeholder, evidence-based approach. J. Occup. Environ. Med. 2005, 47, 41–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, E.; Wada, K.; Dufresne, A. Implementing fit testing for N95 filtering facepiece respirators: Practical information from a large cohort of hospital workers. Am. J. Infect. Control 2008, 36, 298–300. [Google Scholar] [CrossRef]
- Lam, S.; Lee, J.; Yau, S.; Charm, C. Sensitivity and specificity of the user-seal-check in determining the fit of N95 respirators. J. Hosp. Infect. 2011, 77, 252–256. [Google Scholar] [CrossRef]
- Han, D.-H.; Choi, K.-L. Facial dimensions and predictors of fit for half-mask respirators in Koreans. Aiha J. 2003, 64, 815–822. [Google Scholar] [CrossRef]
- Chughtai, A.A.; Seale, H.; Rawlinson, W.D.; Kunasekaran, M.; Macintyre, C.R. Selection and use of respiratory protection by healthcare workers to protect from infectious diseases in hospital settings. Ann. Work Expo. Health 2020, 64, 368–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.Y.; Yoon, H.; Yoon, A.; Kim, T.; Lee, G.; Jung, K.Y.; Park, J.H.; Shin, T.G.; Cha, W.C.; Sim, M.S. N95 filtering facepiece respirators do not reliably afford respiratory protection during chest compression: A simulation study. Am. J. Emerg. Med. 2020, 38, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Suen, L.K.; Yang, L.; Ho, S.S.; Fung, K.H.; Boost, M.V.; Wu, C.S.; Au-Yeung, C.H.; O’Donoghue, M. Reliability of N95 respirators for respiratory protection before, during, and after nursing procedures. Am. J. Infect. Control 2017, 45, 974–978. [Google Scholar] [CrossRef]
- Kim, H.; Baek, J.-E.; Seo, H.-K.; Lee, J.-E.; Myong, J.-P.; Lee, S.-J.; Lee, J.-H. Assessing real-time performances of N95 respirators for health care workers by simulated workplace protection factors. Ind. Health 2015, 53, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Suen, L.; Guo, Y.; Ho, S.; Au-Yeung, C.; Lam, S. Comparing mask fit and usability of traditional and nanofibre N95 filtering facepiece respirators before and after nursing procedures. J. Hosp. Infect. 2020, 104, 336–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, G.L.; Mashingaidze, G.L. Respirator leakage in the pharmaceutical industry of Northwest England. Ann. Occup. Hyg. 1999, 43, 513–517. [Google Scholar] [CrossRef]
- Koehler, R.H.; He, X.K.; Grinshpun, S.A. A novel face seal design for filtering facepiece respirators: Development and pilot testing in a hospital operating room. J. Int. Soc. Respir. Prot. 2014, 31, 116–127. [Google Scholar]
- Grinshpun, S.A.; Corey, J.; Yermakov, M.; Wu, B.; Strickland, K.T.; Bergman, M.; Zhuang, Z. New respirator performance monitor (RePM) for powered air-purifying respirators. J. Occup. Environ. Hyg. 2020, 17, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Corey, J.; Yermakov, M.; Liu, Y.; Grinshpun, S.A. Laboratory evaluation of a novel real-time respirator seal integrity monitor. Ann. Work Expo. Health 2018, 62, 742–753. [Google Scholar] [CrossRef]
- Liu, Y.; Corey, J.; Yermakov, M.V.; Wu, B.; Grinshpun, S.A. Preliminary development of a real-time respirator seal integrity monitor with low-cost particle sensor. IEEE Trans. Ind. Appl. 2018, 54, 3928–3933. [Google Scholar] [CrossRef]
- Mueller, A.V.; Eden, M.J.; Oakes, J.M.; Bellini, C.; Fernandez, L.A. Quantitative method for comparative assessment of particle removal efficiency of fabric masks as alternatives to standard surgical masks for ppe. Matter 2020, 3, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Van der Sande, M.; Teunis, P.; Sabel, R. Professional and home-made face masks reduce exposure to respiratory infections among the general population. PLoS ONE 2008, 3, e2618. [Google Scholar] [CrossRef] [Green Version]
- Adenaiye, O.O.; Lai, J.; Bueno de Mesquita, P.J.; Hong, F.; Youssefi, S.; German, J.; Tai, S.-H.S.; Albert, B.; Schanz, M.; Weston, S.; et al. Infectious Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Exhaled Aerosols and Efficacy of Masks During Early Mild Infection. Clin. Infect. Dis. 2021, Ciab797. [Google Scholar] [CrossRef] [PubMed]
- Green, C.F.; Davidson, C.S.; Panlilio, A.L.; Jensen, P.A.; Jin, Y.; Gibbs, S.G.; Scarpino, P.V. Effectiveness of selected surgical masks in arresting vegetative cells and endospores when worn by simulated contagious patients. Infect. Control Hosp. Epidemiol. 2012, 33, 487–494. [Google Scholar] [CrossRef]
- Leung, N.H.; Chu, D.K.; Shiu, E.Y.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.; Yen, H.-L.; Li, Y.; Ip, D.K.; Peiris, J.M. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milton, D.K.; Fabian, M.P.; Cowling, B.J.; Grantham, M.L.; McDevitt, J.J. Influenza virus aerosols in human exhaled breath: Particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013, 9, e1003205. [Google Scholar] [CrossRef] [Green Version]
- Asadi, S.; Cappa, C.D.; Barreda, S.; Wexler, A.S.; Bouvier, N.M.; Ristenpart, W.D. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci. Rep. 2020, 10, 15665. [Google Scholar] [CrossRef] [PubMed]
- Williams, W. Physiological response to alterations in [O2] and [CO2]: Relevance to respiratory protective devices. J. Int. Soc. Respir. Prot. 2010, 27, 27–51. [Google Scholar]
- Li, Y.; Tokura, H.; Guo, Y.; Wong, A.; Wong, T.; Chung, J.; Newton, E. Effects of wearing N95 and surgical facemasks on heart rate, thermal stress and subjective sensations. Int. Arch. Occup. Environ. Health 2005, 78, 501–509. [Google Scholar] [CrossRef]
- Sinkule, E.J.; Powell, J.B.; Goss, F.L. Evaluation of N95 respirator use with a surgical mask cover: Effects on breathing resistance and inhaled carbon dioxide. Ann. Occup. Hyg. 2013, 57, 384–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eninger, R.M.; Honda, T.; Adhikari, A.; Heinonen-Tanski, H.; Reponen, T.; Grinshpun, S.A. Filter performance of N99 and N95 facepiece respirators against viruses and ultrafine particles. Ann. Occup. Hyg. 2008, 52, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Wang, D.Y. Objective assessment of increase in breathing resistance of N95 respirators on human subjects. Ann. Occup. Hyg. 2011, 55, 917–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Lee, S.; Wang, D.; Lee, H.P. Evaluation of nasal functions while wearing N95 respirator and surgical facemask. J. Biosci. Med. 2014, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Yao, B.-G.; Wang, Y.-X.; Ye, X.-Y.; Zhang, F.; Peng, Y.-L. Impact of structural features on dynamic breathing resistance of healthcare face mask. Sci. Total Environ. 2019, 689, 743–753. [Google Scholar] [CrossRef]
- Roberge, R.J.; Bayer, E.; Powell, J.B.; Coca, A.; Roberge, M.R.; Benson, S.M. Effect of exhaled moisture on breathing resistance of N95 filtering facepiece respirators. Ann. Occup. Hyg. 2010, 54, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, J.B. Respiratory Physiology: The Essentials; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Vincent, J.H. Aerosol Science for Industrial Hygienists; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Louhevaara, V.A. Physiological effects associated with the use of respiratory protective devices: A review. Scand. J. Work. Environ. Health 1984, 10, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sinkule, E. Automated Breathing and Metabolic Simulator (ABMS) Evaluation of N95 Respirator Use with Surgical Masks; University of Pittsburgh: Pittsburgh, PA, USA, 2013. [Google Scholar]
- Caretti, D.; Coyne, K.M. Unmanned assessment of respirator carbon dioxide levels: Comparison of methods of measurement. J. Occup. Environ. Hyg. 2008, 5, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Whitelaw, J.L.; Davies, B. Carbon dioxide rebreathing in respiratory protective devices: Influence of speech and work rate in full-face masks. Ergonomics 2013, 56, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Roberge, R.J.; Coca, A.; Williams, W.J.; Powell, J.B.; Palmiero, A.J. Physiological impact of the N95 filtering facepiece respirator on healthcare workers. Respir. Care 2010, 55, 569–577. [Google Scholar] [PubMed]
- Scheid, J.L.; Lupien, S.P.; Ford, G.S.; West, S.L. Commentary: Physiological and psychological impact of face mask usage during the COVID-19 pandemic. Int. J. Environ. Res. Public Health 2020, 17, 6655. [Google Scholar] [CrossRef]
- Çiriş Yildiz, C.; Ulaşli Kaban, H.; Tanriverdi, F.Ş. COVID-19 pandemic and personal protective equipment: Evaluation of equipment comfort and user attitude. Arch. Environ. Occup. Health 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bahramian, Q.; Gibson, P.; Schreuder-Gibson, H.; Sun, G. Chemical and biological decontamination functions of nanofibrous membranes. J. Mater. Chem. 2012, 22, 8532–8540. [Google Scholar] [CrossRef]
- Roberge, R.J.; Kim, J.-H.; Coca, A. Protective facemask impact on human thermoregulation: An overview. Ann. Occup. Hyg. 2012, 56, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Roberge, R.J.; Kim, J.-H.; Benson, S. N95 filtering facepiece respirator deadspace temperature and humidity. J. Occup. Environ. Hyg. 2012, 9, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Roberge, R.; Benson, S.; Kim, J.-H. Thermal burden of N95 filtering facepiece respirators. Ann. Occup. Hyg. 2012, 56, 808–814. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Wu, T.; Powell, J.B.; Roberge, R.J. Physiologic and fit factor profiles of N95 and P100 filtering facepiece respirators for use in hot, humid environments. Am. J. Infect. Control 2016, 44, 194–198. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, C.-P. Thermoregulation and thermal sensation in response to wearing tight-fitting respirators and exercising in hot-and-humid indoor environment. Build. Environ. 2019, 160, 106158. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Facial skin temperature and discomfort when wearing protective face masks: Thermal infrared imaging evaluation and hands moving the mask. Int. J. Environ. Res. Public Health 2020, 17, 4624. [Google Scholar] [CrossRef] [PubMed]
- Roberge, R.J.; Kim, J.-H.; Benson, S.M. Absence of consequential changes in physiological, thermal and subjective responses from wearing a surgical mask. Respir. Physiol. Neurobiol. 2012, 181, 29–35. [Google Scholar] [CrossRef]
- Epstein, D.; Korytny, A.; Isenberg, Y.; Marcusohn, E.; Zukermann, R.; Bishop, B.; Minha, S.a.; Raz, A.; Miller, A. Return to training in the COVID-19 era: The physiological effects of face masks during exercise. Scand. J. Med. Sci. Sports 2021, 31, 70–75. [Google Scholar] [CrossRef]
- Raven, P.B.; Dodson, A.T.; Davis, T.O. The physiological consequences of wearing industrial respirators: A review. Am. Ind. Hyg. Assoc. J. 1979, 40, 517–534. [Google Scholar] [CrossRef]
- Johnson, A.T. Respirator masks protect health but impact performance: A review. J. Biol. Eng. 2016, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shenal, B.V.; Radonovich, L.J., Jr.; Cheng, J.; Hodgson, M.; Bender, B.S. Discomfort and exertion associated with prolonged wear of respiratory protection in a health care setting. J. Occup. Environ. Hyg. 2012, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-P.; Yip, J.; Kan, C.-W.; Chiou, J.-C.; Yung, K.-F. Reusable face masks as alternative for disposable medical masks: Factors that affect their wear-comfort. Int. J. Environ. Res. Public Health 2020, 17, 6623. [Google Scholar] [CrossRef]
- Hayashi, C.; Tokura, H. The effects of two kinds of mask (with or without exhaust valve) on clothing microclimates inside the mask in participants wearing protective clothing for spraying pesticides. Int. Arch. Occup. Environ. Health 2004, 77, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Yi, L.; Tokura, H.; Wong, T.K.S.; Chung, J.W.Y.; Gohel, M.D.I.; Leung, P.H.-m.; Newton, E. Evaluation on masks with exhaust valves and with exhaust holes from physiological and subjective responses. J. Physiol. Anthropol. 2008, 27, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Baig, A.S.; Knapp, C.; Eagan, A.E.; Radonovich Jr, L.J. Health care workers’ views about respirator use and features that should be included in the next generation of respirators. Am. J. Infect. Control 2010, 38, 18–25. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Shen, S.; Rao, Y.; Chen, F. An improved FFR design with a ventilation fan: CFD simulation and validation. PLoS ONE 2016, 11, e0159848. [Google Scholar] [CrossRef]
- Liu, G.; Nie, J.; Han, C.; Jiang, T.; Yang, Z.; Pang, Y.; Xu, L.; Guo, T.; Bu, T.; Zhang, C. Self-powered electrostatic adsorption face mask based on a triboelectric nanogenerator. ACS Appl. Mater. Interfaces 2018, 10, 7126–7133. [Google Scholar] [CrossRef]
- Dai, J.; Yang, J.J.; Zhuang, Z. Sensitivity analysis of important parameters affecting contact pressure between a respirator and a headform. Int. J. Ind. Ergon. 2011, 41, 268–279. [Google Scholar] [CrossRef]
- Lei, Z.; Yang, J.; Zhuang, Z. Headform and N95 filtering facepiece respirator interaction: Contact pressure simulation and validation. J. Occup. Environ. Hyg. 2012, 9, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Ji, X.; Li, N.; Yang, J.; Zhuang, Z.; Rottach, D. Simulated effects of head movement on contact pressures between headforms and N95 filtering facepiece respirators-part 1: Headform model and validation. Ann. Occup. Hyg. 2014, 58, 1175–1185. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Ji, X.; Li, N.; Yang, J.; Zhuang, Z.; Rottach, D. Simulated effects of head movement on contact pressures between headforms and N95 filtering facepiece respirators part 2: Simulation. Ann. Occup. Hyg. 2014, 58, 1186–1199. [Google Scholar] [CrossRef]
- Tan, K.T.; Greaves, M.W. N95 acne. Int. J. Dermatol. 2004, 43, 522–523. [Google Scholar] [CrossRef]
- Hu, K.; Fan, J.; Li, X.; Gou, X.; Li, X.; Zhou, X. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine 2020, 99, e20603. [Google Scholar] [CrossRef]
- Lim, E.; Seet, R.; Lee, K.H.; Wilder-Smith, E.; Chuah, B.; Ong, B. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol. Scand. 2006, 113, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.J.; Bharatendu, C.; Goh, Y.; Tang, J.Z.; Sooi, K.W.; Tan, Y.L.; Tan, B.Y.; Teoh, H.L.; Ong, S.T.; Allen, D.M. Headaches associated with personal protective equipment–A cross-sectional study among frontline healthcare workers during COVID-19. Headache J. Head Face Pain 2020, 60, 864–877. [Google Scholar] [CrossRef] [Green Version]
- Rebmann, T.; Carrico, R.; Wang, J. Physiologic and other effects and compliance with long-term respirator use among medical intensive care unit nurses. Am. J. Infect. Control 2013, 41, 1218–1223. [Google Scholar] [CrossRef]
- Chan-Yeung, M. Severe acute respiratory syndrome (SARS) and healthcare workers. Int. J. Occup. Environ. Health 2004, 10, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kuster, S.P.; Shah, P.S.; Coleman, B.L.; Lam, P.-P.; Tong, A.; Wormsbecker, A.; McGeer, A. Incidence of influenza in healthy adults and healthcare workers: A systematic review and meta-analysis. PLoS ONE 2011, 6, e26239. [Google Scholar] [CrossRef] [Green Version]
- Luckhaupt, S.E.; Sweeney, M.H.; Funk, R.; Calvert, G.M.; Nowell, M.; D’Mello, T.; Reingold, A.; Meek, J.; Yousey-Hindes, K.; Arnold, K.E. Influenza-associated hospitalizations by industry, 2009–10 influenza season, USA. Emerg. Infect. Dis. 2012, 18, 556–562. [Google Scholar] [CrossRef]
- WHO. Health Worker Ebola Infections in Guinea, Liberia and Sierra Leone; WHO: Geneva, Switzerland, 2015; p. 16. [Google Scholar]
- CDC. Cases & Deaths among Healthcare Personnel. 2021. Available online: https://covid.cdc.gov/covid-data-tracker/#health-care-personnel (accessed on 25 August 2021).
- Firew, T.; Sano, E.D.; Lee, J.W.; Flores, S.; Lang, K.; Salman, K.; Greene, M.C.; Chang, B.P. Protecting the front line: A cross-sectional survey analysis of the occupational factors contributing to healthcare workers’ infection and psychological distress during the COVID-19 pandemic in the USA. BMJ Open 2020, 10, e042752. [Google Scholar] [CrossRef]
- Heinzerling, A.; Stuckey, M.J.; Scheuer, T.; Xu, K.; Perkins, K.M.; Resseger, H.; Magill, S.; Verani, J.R.; Jain, S.; Acosta, M.; et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient—Solano County, California, February 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Tran, K.; Cimon, K.; Severn, M.; Pessoa-Silva, C.L.; Conly, J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PLoS ONE 2012, 7, e35797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee-Archer, P.; von Ungern-Sternberg, B.S. Pediatric anesthetic implications of COVID-19—A review of current literature. Pediatr. Anesth. 2020, 30, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Hunter, E.; Price, D.A.; Murphy, E.; van der Loeff, I.S.; Baker, K.F.; Lendrem, D.; Lendrem, C.; Schmid, M.L.; Pareja-Cebrian, L.; Welch, A. First experience of COVID-19 screening of health-care workers in England. Lancet 2020, 395, e77–e78. [Google Scholar] [CrossRef]
- Bolton, L.; Mills, C.; Wallace, S.; Brady, M.C.; Royal College of, S.; Language Therapists, C.-A.G. Aerosol generating procedures, dysphagia assessment and COVID-19: A rapid review. Int. J. Lang. Commun. Disord. 2020, 55, 629–636. [Google Scholar] [CrossRef]
- Thompson, K.; Hammond, N.; Eastwood, G.; Festa, M.; Glass, P.; Rajbhandari, D.; Seppelt, I.; Taylor, C.; Watts, N.; Myburgh, J. The Australian and New Zealand intensive care society clinical trials group point prevalence program, 2009-2016. Crit Care Resusc 2017, 19, 88–93. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, J.F. The COVID-19 pandemic, personal protective equipment and respirator: A narrative review. Int. J. Clin. Pract. 2020, 74, e13578. [Google Scholar] [CrossRef]
- Baka, A.; Cencirelli, O.; Finch, E.; Karki, T.; Kinross, P.; Plachouras, D. Infection Prevention and Control and Preparedness for COVID-19 in Healthcare Settings—Third Update. 2020. Available online: https://reliefweb.int/report/world/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings-third (accessed on 17 July 2021).
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.-H. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- CDC. Cluster of severe acute respiratory syndrome cases among protected health-care workers-Toronto, Canada, April 2003. MMWR: Morb. Mortal. Wkly. Rep. 2003, 52, 433–436. [Google Scholar] [PubMed]
- Christian, M.D.; Loutfy, M.; McDonald, L.C.; Martinez, K.F.; Ofner, M.; Wong, T.; Wallington, T.; Gold, W.L.; Mederski, B.; Green, K. Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg. Infect. Dis. 2004, 10, 287–293. [Google Scholar] [CrossRef] [Green Version]
- OSHA. Temporary Enforcement Guidance—Healthcare Respiratory Protection Annual Fit-Testing for N95 Filtering Facepieces During the COVID-19 Outbreak. 2020. Available online: https://www.osha.gov/memos/2020-03-14/temporary-enforcement-guidance-healthcare-respiratory-protection-annual-fit (accessed on 26 August 2021).
- The Guardian. NHS Hospitals Accused of Risking Staff Lives by Forgoing Mask Fit-Tests. 2020. Available online: https://www.theguardian.com/world/2020/apr/14/nhs-hospitals-accused-of-risking-staff-lives-by-abandoning-ppe-fit-tests-coronavirus (accessed on 30 August 2021).
- Lau, J.T.; Tsui, H.; Lau, M.; Yang, X. SARS transmission, risk factors, and prevention in Hong Kong. Emerg. Infect. Dis. 2004, 10, 587–592. [Google Scholar] [CrossRef]
- Kursumovic, E.; Lennane, S.; Cook, T.M. Deaths in healthcare workers due to COVID-19: The need for robust data and analysis. Anaesthesia 2020, 75, 989–992. [Google Scholar] [CrossRef]
- Papoutsi, E.; Giannakoulis, V.G.; Ntella, V.; Pappa, S.; Katsaounou, P. Global burden of COVID-19 pandemic on healthcare workers. ERJ Open Res. 2020, 6, 00195–2020. [Google Scholar] [CrossRef] [PubMed]
- Dyal, J.W.; Grant, M.P.; Broadwater, K.; Bjork, A.; Waltenburg, M.A.; Gibbins, J.D.; Hale, C.; Silver, M.; Fischer, M.; Steinberg, J.; et al. COVID-19 among workers in meat and poultry processing facilities―19 states, April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-H.; Sridhar, S.; Zhang, R.R.; Chu, H.; Fung, A.-F.; Chan, G.; Chan, J.-W.; To, K.-W.; Hung, I.-N.; Cheng, V.-C. Factors affecting stability and infectivity of SARS-CoV-2. J. Hosp. Infect. 2020, 106, 226–231. [Google Scholar] [CrossRef]
- Wines, M.; Harmon, A. What Happens When a Superspreader Event Keeps Spreading. New York Times. 11 December 2020. Available online: https://www.nytimes.com/2020/12/11/us/biogen-conference-covid-spread.html (accessed on 20 December 2020).
- Lau, M.S.; Grenfell, B.; Thomas, M.; Bryan, M.; Nelson, K.; Lopman, B. Characterizing superspreading events and age-specific infectiousness of SARS-CoV-2 transmission in Georgia, USA. Proc. Natl. Acad. Sci. USA 2020, 117, 22430–22435. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Centre for the Mathematical Modelling of Infectious Diseases; Abbott, S.; Kucharski, A.J.; Funk, S. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Res. 2020, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Wahl, B.; Dudala, S.R.; Gopal, K.; Neelima, S.; Reddy, K.J.; Radhakrishnan, J.; Lewnard, J.A. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science 2020, 370, 691–697. [Google Scholar] [CrossRef]
- Goh, D.Y.T.; Mun, M.W.; Lee, W.L.J.; Teoh, O.H.; Rajgor, D.D. A randomised clinical trial to evaluate the safety, fit, comfort of a novel N95 mask in children. Sci. Rep. 2019, 9, 18952. [Google Scholar] [CrossRef] [Green Version]
- AAP. Children and COVID-19: State-Level Data Report. 2021. Available online: https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ (accessed on 15 October 2021).
- Nikolopoulou, G.B.; Maltezou, H.C. COVID-19 in children: Where do we stand? Arch. Med Res. 2021, S0188-4409(0121)00148-X. [Google Scholar] [CrossRef]
- Dietert, R.R.; Etzel, R.A.; Chen, D.; Halonen, M.; Holladay, S.D.; Jarabek, A.M.; Landreth, K.; Peden, D.B.; Pinkerton, K.; Smialowicz, R.J. Workshop to identify critical windows of exposure for children’s health: Immune and respiratory systems work group summary. Environ. Health Perspect. 2000, 108 (Suppl. 3), 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landrigan, P.J.; Kimmel, C.A.; Correa, A.; Eskenazi, B. Children’s health and the environment: Public health issues and challenges for risk assessment. Environ. Health Perspect. 2004, 112, 257–265. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Miodovnik, A. Children’s health and the environment: An overview. Mt. Sinai J. Med. A J. Transl. Pers. Med. 2011, 78, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moya, J.; Bearer, C.F.; Etzel, R.A. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics 2004, 113 (Suppl. 3), 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Beaubien, J. Kids and Superspreaders Are Driving COVID-19 Cases in India, Huge Study Finds. 2020. Available online: https://www.npr.org/sections/goatsandsoda/2020/10/01/919237103/kids-and-superspreaders-are-driving-covid-19-cases-in-india-huge-study-finds (accessed on 25 October 2021).
- Guzelian, P.S.; Henry, C.J.; Olin, S.S. Similarities and Differences between Children and Adults; ILSI Press/International Life Sciences Institute: Washington, DC, USA, 1992. [Google Scholar]
- Calvo, C.; Alcolea, S.; Casas, I.; Pozo, F.; Iglesias, M.; Gonzalez-Esguevillas, M.; García-García, M.L. A 14-year prospective study of human coronavirus infections in hospitalized children: Comparison with other respiratory viruses. Pediatr. Infect. Dis. J. 2020, 39, 653–657. [Google Scholar] [CrossRef]
- CDC. COVID-19 Hospitalization and Death by Age. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html (accessed on 20 October 2021).
- CDC. Weekly Updates by Select Demographic and Geographic Characteristics. 2020. Available online: https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm (accessed on 24 October 2021).
- Bhattacharyya, S.; Dey, K.; Paul, A.R.; Biswas, R. A novel CFD analysis to minimize the spread of COVID-19 virus in hospital isolation room. Chaos Solitons Fractals 2020, 139, 110294. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, F.; Jabbari, M.; Esfahani, M.N.; Keshmiri, A. A computational simulation platform for designing real-time monitoring systems with application to COVID-19. Biosens. Bioelectron. 2021, 171, 112716. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Niu, J. Transient CFD simulation of the respiration process and inter-person exposure assessment. Build. Environ. 2006, 41, 1214–1222. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Uspal, W.E.; Wei, T. Airborne Transmission of COVID-19: Aerosol Dispersion, Lung Deposition, and Virus-Receptor Interactions. ACS Nano 2020, 14, 16502–16524. [Google Scholar] [CrossRef]
- Lv, D.; Zhu, M.; Jiang, Z.; Jiang, S.; Zhang, Q.; Xiong, R.; Huang, C. Green electrospun nanofibers and their application in air filtration. Macromol. Mater. Eng. 2018, 303, 1800336. [Google Scholar] [CrossRef]
- Bourouiba, L. The fluid dynamics of disease transmission. Annu. Rev. Fluid Mech. 2021, 53, 473–508. [Google Scholar] [CrossRef]
- Mittal, R.; Meneveau, C.; Wu, W. A mathematical framework for estimating risk of airborne transmission of COVID-19 with application to face mask use and social distancing. Phys. Fluids 2020, 32, 101903. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, A.; Girardi, M.; Bazzucchi, I.; Felici, F.; Sacchetti, M. Respiratory frequency and tidal volume during exercise: Differential control and unbalanced interdependence. Physiol. Rep. 2018, 6, e13908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, S.; Wexler, A.S.; Cappa, C.D.; Barreda, S.; Bouvier, N.M.; Ristenpart, W.D. Aerosol emission and superemission during human speech increase with voice loudness. Sci. Rep. 2019, 9, 2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhand, R.; Li, J. Coughs and sneezes: Their role in transmission of respiratory viral infections, including SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 202, 651–659. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Shen, S.; Cai, M. Investigation of the flow-field in the upper respiratory system when wearing N95 filtering facepiece respirator. J. Occup. Environ. Hyg. 2016, 13, 372–382. [Google Scholar] [CrossRef]
- Gupta, J.K.; Lin, C.H.; Chen, Q. Characterizing exhaled airflow from breathing and talking. Indoor Air 2010, 20, 31–39. [Google Scholar] [CrossRef]
- Alenezi, H.; Cam, M.E.; Edirisinghe, M. A novel reusable anti-COVID-19 transparent face respirator with optimized airflow. Bio-Des. Manuf. 2020, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Lin, C.H.; Chen, Q. Flow dynamics and characterization of a cough. Indoor Air 2009, 19, 517–525. [Google Scholar] [CrossRef]
- Mirikar, D.; Palanivel, S.; Arumuru, V. Droplet fate, efficacy of face mask, and transmission of virus-laden droplets inside a conference room. Phys. Fluids 2021, 33, 065108. [Google Scholar] [CrossRef] [PubMed]
- Pendar, M.-R.; Páscoa, J.C. Numerical modeling of the distribution of virus carrying saliva droplets during sneeze and cough. Phys. Fluids 2020, 32, 083305. [Google Scholar] [CrossRef]
- Bourouiba, L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA 2020, 323, 1837–1838. [Google Scholar] [CrossRef]
- Subramaniam, S. Lagrangian–Eulerian methods for multiphase flows. Prog. Energy Combust. Sci. 2013, 39, 215–245. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, H.; Zhang, X.; Wu, T.; Yang, X. Effects of space sizes on the dispersion of cough-generated droplets from a walking person. Phys. Fluids 2020, 32, 121705. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Chen, Q.; Liu, E. The role of computational fluid dynamics tools on investigation of pathogen transmission: Prevention and control. Sci. Total. Environ. 2020, 746, 142090. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Murallidharan, J.S.; Agrawal, A.; Bhardwaj, R. Why coronavirus survives longer on impermeable than porous surfaces. Phys. Fluids 2021, 33, 021701. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.D.; Bange, P.G.; Bhardwaj, R.; Sharma, A. Effects of substrate heating and wettability on evaporation dynamics and deposition patterns for a sessile water droplet containing colloidal particles. Langmuir 2016, 32, 11958–11972. [Google Scholar] [CrossRef] [Green Version]
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Perrone, M.G.; Borelli, M.; Palmisani, J.; Di Gilio, A.; Torboli, V.; Fontana, F.; et al. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ. Res. 2020, 188, 109754. [Google Scholar] [CrossRef]
- Willeke, K.; Qian, Y.; Donnelly, J.; Grinshpun, S.; Ulevicius, V. Penetration of airborne microorganisms through a surgical mask and a dust/mist respirator. Am. Ind. Hyg. Assoc. J. 1996, 57, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Ni, R.; Seo, J.-H. The flow physics of COVID-19. J. Fluid Mech. 2020, 894, F2. [Google Scholar] [CrossRef]
- Lei, Z.; Yang, J.; Zhuang, Z.; Roberge, R. Simulation and evaluation of respirator faceseal leaks using computational fluid dynamics and infrared imaging. Ann. Occup. Hyg. 2013, 57, 493–506. [Google Scholar] [CrossRef]
- Liu, B.Y.; Lee, J.-K.; Mullins, H.; Danisch, S.G. Respirator leak detection by ultrafine aerosols: A predictive model and experimental study. Aerosol Sci. Technol. 1993, 19, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Khosronejad, A.; Santoni, C.; Flora, K.; Zhang, Z.; Kang, S.; Payabvash, S.; Sotiropoulos, F. Fluid dynamics simulations show that facial masks can suppress the spread of COVID-19 in indoor environments. AIP Adv. 2020, 10, 125109. [Google Scholar] [CrossRef]
- Lee, H.R.; Liao, L.; Xiao, W.; Vailionis, A.; Ricco, A.J.; White, R.; Nishi, Y.; Chiu, W.; Chu, S.; Cui, Y. Three-dimensional analysis of particle distribution on filter layers inside N95 respirators by deep learning. Nano Lett. 2021, 21, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Akagi, F.; Haraga, I.; Inage, S.-I.; Akiyoshi, K. Effect of sneezing on the flow around a face shield. Phys. Fluids 2020, 32, 127105. [Google Scholar] [CrossRef] [PubMed]
- Perić, R.; Perić, M. Analytical and numerical investigation of the airflow in face masks used for protection against COVID-19 virus–Implications for mask design and usage. J. Appl. Fluid Mech. 2020, 13, 1911–1923. [Google Scholar] [CrossRef]
- Shafaghi, A.H.; Rokhsar Talabazar, F.; Koşar, A.; Ghorbani, M. On the effect of the respiratory droplet generation condition on COVID-19 transmission. Fluids 2020, 5, 113. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, T.; Yoo, K.H.; Capecelatro, J.; Boehman, A.L.; Maki, K. Disease transmission through expiratory aerosols on an urban bus. Phys. Fluids 2021, 33, 015116. [Google Scholar] [CrossRef]
- O’Reilly, K.B. Amid PPE Shortage, AMA Collaboration Offers Supplier for Doctors. 2020. Available online: https://www.ama-assn.org/delivering-care/public-health/amid-ppe-shortage-ama-collaboration-offers-supplier-doctors (accessed on 10 October 2021).
- GUP. PPE Data on the Personal Protective Equipment Shortage across the U.S. 2021. Available online: https://getusppe.org/data/ (accessed on 19 November 2021).
- Dean, R. PPE: Polluting Planet Earth. Br. Dent. J. 2020, 229, 267. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 pandemic repercussions on the use and management of plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyang, W.; Barcelò, D.; Rocha-Santos, T. Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2020, 405, 126683. [Google Scholar] [CrossRef]
- Singh, N.; Tang, Y.; Zhang, Z.; Zheng, C. COVID-19 waste management: Effective and successful measures in Wuhan, China. Resour. Conserv. Recycl. 2020, 163, 105071. [Google Scholar] [CrossRef] [PubMed]
- Harhay, M.O.; Halpern, S.D.; Harhay, J.S.; Olliaro, P.L. Health care waste management: A neglected and growing public health problem worldwide. Trop. Med. Int. Health 2009, 14, 1414–1417. [Google Scholar] [CrossRef]
- WHO. Health-Care Waste. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/health-care-waste (accessed on 10 October 2021).
- Lopez, G.U.; Gerba, C.P.; Tamimi, A.H.; Kitajima, M.; Maxwell, S.L.; Rose, J.B. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl. Environ. Microbiol. 2013, 79, 5728–5734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, E.; Ylitalo, C.; Stepanova, N.; Shaffer, R. Assessing filtering facepiece respirator contamination during patient care in flu season: Experimental and modeling approaches. In Proceedings of the ISRP—Sixteenth International Conference: A Global View on Respiratory Protection, Boston, MA, USA, 24–27 September 2012. [Google Scholar]
- Birkner, J.S.; Fung, D.; Hinds, W.C.; Kennedy, N.J. Particle release from respirators, part I: Determination of the effect of particle size, drop height, and load. J. Occup. Environ. Hyg. 2011, 8, 1–9. [Google Scholar] [CrossRef]
- Coulliette, A.; Perry, K.; Edwards, J.; Noble-Wang, J. Persistence of the 2009 pandemic influenza A (H1N1) virus on N95 respirators. Appl. Environ. Microbiol. 2013, 79, 2148–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, E.; Shaffer, R. Survival of bacteriophage MS2 on filtering facepiece respirator coupons. Appl. Biosaf. 2010, 15, 71–76. [Google Scholar] [CrossRef]
- Duan, S.M.; Zhao, X.S.; Wen, R.F.; Huang, J.J.; Pi, G.H.; Zhang, S.X.; Han, J.; Bi, S.L.; Ruan, L.; Dong, X.P. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003, 16, 246–255. [Google Scholar] [PubMed]
- Hirose, R.; Ikegaya, H.; Naito, Y.; Watanabe, N.; Yoshida, T.; Bandou, R.; Daidoji, T.; Itoh, Y.; Nakaya, T. Survival of SARS-CoV-2 and influenza virus on the human skin: Importance of hand hygiene in COVID-19. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, ciaa1517. [Google Scholar] [CrossRef]
- Rabenau, H.; Cinatl, J.; Morgenstern, B.; Bauer, G.; Preiser, W.; Doerr, H. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005, 194, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chin, A.W.; Poon, L.L. Stability of SARS-CoV-2 in different environmental conditions–Authors’ reply. Lancet Microbe 2020, 1, e146. [Google Scholar] [CrossRef]
- Dehbandi, R.; Zazouli, M.A. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e145. [Google Scholar] [CrossRef]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. MSphere 2020, 5, e00441-20. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Y.; Cheng, P.K.; Lim, W.W. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005, 41, e67–e71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef]
- Chan, K.H.; Peiris, J.S.; Lam, S.Y.; Poon, L.L.; Yuen, K.Y.; Seto, W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011, 2011, 734690. [Google Scholar] [CrossRef]
- Morris, D.H.; Yinda, K.C.H.; Gamble, A.; Rossine, F.W.; Huang, Q.; Bushmaker, T.; Fischer, R.J.; Matson, M.J.; van Doremalen, N.; Vikesland, P.J. The effect of temperature and humidity on the stability of SARS-CoV-2 and other enveloped viruses. BioRxiv 2020. [Google Scholar] [CrossRef]
- Tsai, P. Performance of masks and discussion of the inactivation of SARS-CoV-2. Eng. Sci. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Jacobs, N.; Chan, K.; Leso, V.; D’Anna, A.; Hollins, D.; Iavicoli, I. A critical review of methods for decontaminating filtering facepiece respirators. Toxicol. Ind. Health 2020, 36, 654–680. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, C.E.; Sossa-Briceño, M.P.; Cortés, J.A. Decontamination and reuse of N95 filtering facemask respirators: A systematic review of the literature. Am. J. Infect. Control 2020, 48, 1520–1532. [Google Scholar] [CrossRef]
- Zorko, D.J.; Gertsman, S.; O’Hearn, K.; Timmerman, N.; Ambu-Ali, N.; Dinh, T.; Sampson, M.; Sikora, L.; McNally, J.D.; Choong, K. Decontamination interventions for the reuse of surgical mask personal protective equipment: A systematic review. J. Hosp. Infect. 2020, 106, 283–294. [Google Scholar] [CrossRef]
- Mun, C.H.; Tan, S.L.; Phua, S.Z.F.; Lim, W.Q.; Pang, S.W.; Novem, V.; Chee, G.; Soh, Y.H.S. Surgical Masks Decontamination for reuse by Members of the Public: Feasibility Study and Development of Home-Based Methods. 2020. Available online: https://doi.org/10.21203/rs.3.rs-72875/v1 (accessed on 10 October 2021).
- Heimbuch, B.K.; Wallace, W.H.; Kinney, K.; Lumley, A.E.; Wu, C.-Y.; Woo, M.-H.; Wander, J.D. A pandemic influenza preparedness study: Use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am. J. Infect. Control. 2011, 39, e1–e9. [Google Scholar] [CrossRef]
- Yang, H.; Hu, J.; Li, P.; Zhang, C. Ultraviolet germicidal irradiation for filtering facepiece respirators disinfection to facilitate reuse during COVID-19 pandemic: A review. Photodiagnosis Photodyn. Ther. 2020, 31, 101943. [Google Scholar] [CrossRef]
- Ozog, D.M.; Sexton, J.Z.; Narla, S.; Pretto-Kernahan, C.D.; Mirabelli, C.; Lim, H.W.; Hamzavi, I.H.; Tibbetts, R.J.; Mi, Q.-S. The effect of ultraviolet C radiation against different N95 respirators inoculated with SARS-CoV-2. Int. J. Infect. Dis. 2020, 100, 224–229. [Google Scholar] [CrossRef]
- Lindblad, M.; Tano, E.; Lindahl, C.; Huss, F. Ultraviolet-C decontamination of a hospital room: Amount of UV light needed. Burns 2020, 46, 842–849. [Google Scholar] [CrossRef]
- Lytle, C.D.; Sagripanti, J.-L. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J. Virol. 2005, 79, 14244–14252. [Google Scholar] [CrossRef] [Green Version]
- Wigginton, K.R.; Pecson, B.M.; Sigstam, T.r.; Bosshard, F.; Kohn, T. Virus inactivation mechanisms: Impact of disinfectants on virus function and structural integrity. Environ. Sci. Technol. 2012, 46, 12069–12078. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Kim, J.; Kim, J.Y.; Yoon, J.; Kim, J.H. Mechanisms of Escherichia coli inactivation by several disinfectants. Water Res. 2010, 44, 3410–3418. [Google Scholar] [CrossRef]
- Thompson, K.M. The Science of Disinfectants. 2012. Available online: https://www.cmmonline.com/articles/the-science-of-disinfectants (accessed on 24 October 2021).
- Viscusi, D.J.; King, W.P.; Shaffer, R.E. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J. Int. Soc. Respir. Prot. 2007, 24, 93. [Google Scholar]
- Smith, J.S.; Hanseler, H.; Welle, J.; Rattray, R.; Campbell, M.; Brotherton, T.; Moudgil, T.; Pack, T.F.; Wegmann, K.; Jensen, S. Effect of various decontamination procedures on disposable N95 mask integrity and SARS-CoV-2 infectivity. J. Clin. Transl. Sci. 2020, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, D.J.; Bergman, M.S.; Novak, D.A.; Faulkner, K.A.; Palmiero, A.; Powell, J.; Shaffer, R.E. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J. Occup. Environ. Hyg. 2011, 8, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Lore, M.B.; Heimbuch, B.K.; Brown, T.L.; Wander, J.D.; Hinrichs, S.H. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann. Occup. Hyg. 2012, 56, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Zulauf, K.E.; Green, A.B.; Ba, A.N.N.; Jagdish, T.; Reif, D.; Seeley, R.; Dale, A.; Kirby, J.E. Microwave-generated steam decontamination of N95 respirators utilizing universally accessible materials. MBio 2020, 11, e00997-20. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, D.J.; Bergman, M.S.; Eimer, B.C.; Shaffer, R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann. Occup. Hyg. 2009, 53, 815–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, M.S.; Viscusi, D.J.; Heimbuch, B.K.; Wander, J.D.; Sambol, A.R.; Shaffer, R.E. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J. Eng. Fibers Fabr. 2010, 5. [Google Scholar] [CrossRef] [Green Version]
- Lieu, A.; Mah, J.; Zanichelli, V.; Exantus, R.C.; Longtin, Y. Impact of extended use and decontamination with vaporized hydrogen peroxide on N95 respirator fit. Am. J. Infect. Control 2020, 48, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Daeschler, S.C.; Manson, N.; Joachim, K.; Chin, A.W.; Chan, K.; Chen, P.Z.; Tajdaran, K.; Mirmoeini, K.; Zhang, J.J.; Maynes, J.T. Effect of moist heat reprocessing of N95 respirators on SARS-CoV-2 inactivation and respirator function. CMAJ 2020, 192, E1189–E1197. [Google Scholar] [CrossRef]
- Liao, L.; Xiao, W.; Zhao, M.; Yu, X.; Wang, H.; Wang, Q.; Chu, S.; Cui, Y. Can N95 respirators be reused after disinfection? How many times? ACS Nano 2020, 14, 6348–6356. [Google Scholar] [CrossRef]
- CDC. Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. 2020. Available online: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html (accessed on 24 August 2021).
- O’Hearn, K.; Gertsman, S.; Sampson, M.; Webster, R.; Tsampalieros, A.; Ng, R.; Gibson, J.; Lobos, A.T.; Acharya, N.; Agarwal, A.; et al. Decontaminating N95 and SN95 masks with ultraviolet germicidal irradiation does not impair mask efficacy and safety. J. Hosp. Infect. 2020, 106, 163–175. [Google Scholar] [CrossRef]
- Fisher, E.M.; Williams, J.L.; Shaffer, R.E. Evaluation of microwave steam bags for the decontamination of filtering facepiece respirators. PLoS ONE 2011, 6, e18585. [Google Scholar] [CrossRef] [Green Version]
- Sickbert-Bennett, E.E.; Samet, J.M.; Clapp, P.W.; Chen, H.; Berntsen, J.; Zeman, K.L.; Tong, H.; Weber, D.J.; Bennett, W.D. Filtration efficiency of hospital face mask alternatives available for use during the COVID-19 pandemic. JAMA Intern. Med. 2020, 180, 1607–1612. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Martin Jr, S.B.; Thewlis, R.E.; Sarkisian, K.; Nwoko, J.O.; Mead, K.R.; Noti, J.D. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J. Occup. Environ. Hyg. 2015, 12, 509–517. [Google Scholar] [CrossRef]
- Fisher, E.M.; Shaffer, R.E. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J. Appl. Microbiol. 2011, 110, 287–295. [Google Scholar] [CrossRef]
- Boškoski, I.; Gallo, C.; Wallace, M.B.; Costamagna, G. COVID-19 pandemic and personal protective equipment shortage: Protective efficacy comparing masks and scientific methods for respirator reuse. Gastrointest. Endosc. 2020, 92, 519–523. [Google Scholar] [CrossRef]
- Mills, D.; Harnish, D.A.; Lawrence, C.; Sandoval-Powers, M.; Heimbuch, B.K. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am. J. Infect. Control 2018, 46, e49–e55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernez, D.; Save, J.; Oppliger, A.; Concha-Lozano, N.; Hopf, N.B.; Niculita-Hirzel, H.; Resch, G.; Michaud, V.; Dorange-Pattoret, L.; Charrière, N. Reusability of filtering facepiece respirators after decontamination through drying and germicidal UV irradiation. BMJ Global Health 2020, 5, e003110. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.; Stiegel, M.; Greeson, N.; Vogel, A.; Thomann, W.; Brown, M.; Sempowski, G.D.; Alderman, T.S.; Condreay, J.P.; Burch, J. Decontamination and reuse of N95 respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARS-CoV-2 (COVID-19) pandemic. Appl. Biosaf. 2020, 25, 67–70. [Google Scholar] [CrossRef]
- O’Hearn, K.; Gertsman, S.; Webster, R.; Tsampalieros, A.; Ng, R.; Gibson, J.; Sampson, M.; Sikora, L.; McNally, J.D. Efficacy and safety of disinfectants for decontamination of N95 and SN95 filtering facepiece respirators: A systematic review. J. Hosp. Infect. 2020, 106, 504–521. [Google Scholar] [CrossRef]
- Lowe, J.J.; Paladino, K.D.; Farke, J.D.; Boulter, K.; Cawcutt, K.; Emodi, M.; Gibbs, S.; Hankins, R.; Hinkle, L.; Micheels, T. N95 filtering facepiece respirator ultraviolet germicidal irradiation (UVGI) process for decontamination and reuse. Neb. Med. 2020. [Google Scholar]
- Kohli, I.; Lyons, A.B.; Golding, B.; Narla, S.; Torres, A.E.; Parks-Miller, A.; Ozog, D.; Lim, H.W.; Hamzavi, I.H. UVC germicidal units: Determination of dose received and parameters to be considered for N95 respirator decontamination and reuse. Photochem. Photobiol. 2020, 96, 1083–1087. [Google Scholar] [CrossRef]
- Jatta, M.; Kiefer, C.; Patolia, H.; Pan, J.; Harb, C.; Marr, L.C.; Baffoe-Bonnie, A. N95 reprocessing by low temperature sterilization with 59% vaporized hydrogen peroxide during the 2020 COVID-19 pandemic. Am. J. Infect. Control 2021, 49, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Derr, T.H.; James, M.A.; Kuny, C.V.; Kandel, P.P.; Beckman, M.D.; Hockett, K.L.; Bates, M.A.; Szpara, M.L. Aerosolized Hydrogen Peroxide Decontamination of N95 Respirators, with Fit-Testing and Virologic Confirmation of Suitability for Re-Use During the COVID-19 Pandemic. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Toomey, E.; Conway, Y.; Burton, C.; Smith, S.; Smalle, M.; Chan, X.; Adisesh, A.; Tanveer, S.; Ross, L.; Thomson, I.; et al. Extended use or re-use of single-use surgical masks and filtering facepiece respirators during COVID-19: A rapid systematic review. Infect. Control. Hosp. Epidemiol. 2020, 42, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Seresirikachorn, K.; Phoophiboon, V.; Chobarporn, T.; Tiankanon, K.; Aeumjaturapat, S.; Chusakul, S.; Snidvongs, K. Decontamination and reuse of surgical masks and N95 filtering facepiece respirators during the COVID-19 pandemic: A systematic review. Infect. Control. Hosp. Epidemiol. 2020, 42, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Chen, C.-C.; Huang, S.-H.; Kuo, C.-W.; Lai, C.-Y.; Lin, W.-Y. Filter quality of electret masks in filtering 14.6–594 nm aerosol particles: Effects of five decontamination methods. PLoS ONE 2017, 12, e0186217. [Google Scholar] [CrossRef] [Green Version]
- MacIntyre, C.R.; Dung, T.C.; Chughtai, A.A.; Seale, H.; Rahman, B. Contamination and washing of cloth masks and risk of infection among hospital health workers in Vietnam: A post hoc analysis of a randomised controlled trial. BMJ Open 2020, 10, e042045. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bahl, P.; Chughtai, A.A.; MacIntyre, C.R. Last-resort strategies during mask shortages: Optimal design features of cloth masks and decontamination of disposable masks during the COVID-19 pandemic. BMJ Open Respir. Res. 2020, 7, e000698. [Google Scholar] [CrossRef]
- Chughtai, A.A.; Seale, H.; MacIntyre, C.R. Effectiveness of cloth masks for protection against severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020, 26, e200948. [Google Scholar] [CrossRef]
- Chughtai, A.A.; Seale, H.; MacIntyre, C.R. Use of cloth masks in the practice of infection control—evidence and policy gaps. Int. J. Infect. Control 2013, 9, 1–12. [Google Scholar] [CrossRef]
- Li, D.F.; Cadnum, J.L.; Redmond, S.N.; Jones, L.D.; Donskey, C.J. It’s not the heat, it’s the humidity: Effectiveness of a rice cooker-steamer for decontamination of cloth and surgical face masks and N95 respirators. Am. J. Infect. Control 2020, 48, 854–855. [Google Scholar] [CrossRef]
- Andreola, M.-L.; Becquart, F.; Jomaa, W.; Verhoeven, P.O.; Baldacchino, G.; Hemour, S.; consortium, D.-M. The properties of hot household hygroscopic materials and their potential use for non-medical facemask decontamination. PLoS ONE 2021, 16, e0255148. [Google Scholar] [CrossRef]
- OSHA. Enforcement Memos: Enforcement Guidance on Decontamination of Filtering Facepiece Respirators in Healthcare During the Coronavirus Disease 2019 (COVID-19) Pandemic; OSHA: Washington, DC, USA, 2020. [Google Scholar]
- FDA. Recommendations for Sponsors Requesting EUAs for Decontamination and Bioburden Reduction Systems for Surgical Masks and Respirators During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency: Guidance for Industry and Food and Drug Administration Staff; FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- Ludwig-Begall, L.F.; Wielick, C.; Dams, L.; Nauwynck, H.; Demeuldre, P.-F.; Napp, A.; Laperre, J.; Haubruge, E.; Thiry, E. The use of germicidal ultraviolet light, vaporized hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with a SARS-CoV-2 surrogate virus. J. Hosp. Infect. 2020, 106, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hsu, P.-C.; Lee, H.-W.; Ye, M.; Zheng, G.; Liu, N.; Li, W.; Cui, Y. Transparent air filter for high-efficiency PM 2.5 capture. Nat. Commun. 2015, 6, 7025. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Liu, C.; Hsu, P.-C.; Liu, K.; Zhang, R.; Liu, Y.; Cui, Y. Roll-to-roll transfer of electrospun nanofiber film for high-efficiency transparent air filter. Nano Lett. 2016, 16, 1270–1275. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Credibility of polymeric and ceramic membrane filtration in the removal of bacteria and virus from water: A review. J. Environ. Manag. 2020, 268, 110583. [Google Scholar] [CrossRef]
- Koh, E.; Park, H.M.; Lee, Y.T. Preparation and modification of an embossed nanofibrous materials for robust filtration performance of PM0. 2 removal. J. Ind. Eng. Chem. 2021, 93, 339–350. [Google Scholar] [CrossRef]
- De Cesare, F.; Di Mattia, E.; Zussman, E.; Macagnano, A. A study on the dependence of bacteria adhesion on the polymer nanofibre diameter. Environ. Sci. Nano 2019, 6, 778–797. [Google Scholar] [CrossRef]
- Tebyetekerwa, M.; Xu, Z.; Yang, S.; Ramakrishna, S. Electrospun nanofibers-based face masks. Adv. Fiber Mater. 2020, 2, 161–166. [Google Scholar] [CrossRef]
- Zangmeister, C.D.; Radney, J.G.; Vicenzi, E.P.; Weaver, J.L. Filtration efficiencies of nanoscale aerosol by cloth mask materials used to slow the spread of SARS-CoV-2. ACS Nano 2020, 14, 9188–9200. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Zhang, M.; Ji, B.; Yong, T.; Sun, G. Hierarchical Nucleophilic Nanofibrous Membranes for Fast, Durable, and Bare-Eye Visible Detoxification of Carcinogenic Alkylating Toxicants. Adv. Funct. Mater. 2019, 29, 1905990. [Google Scholar] [CrossRef]
- Song, J.; Zhang, B.; Lu, Z.; Xin, Z.; Liu, T.; Wei, W.; Zia, Q.; Pan, K.; Gong, R.H.; Bian, L.; et al. Hierarchical porous poly (l-lactic acid) nanofibrous membrane for ultrafine particulate aerosol filtration. ACS Appl. Mater. Interfaces 2019, 11, 46261–46268. [Google Scholar] [CrossRef]
- Bian, Y.; Chen, C.; Wang, R.; Wang, S.; Pan, Y.; Zhao, B.; Chen, C.; Zhang, L. Effective removal of particles down to 15 nm using scalable metal-organic framework-based nanofiber filters. Appl. Mater. Today 2020, 20, 100653. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Yu, J.; Luo, W.; Ding, B. Microwave structured polyamide-6 nanofiber/net membrane with embedded poly (m-phenylene isophthalamide) staple fibers for effective ultrafine particle filtration. J. Mater. Chem. A 2016, 4, 6149–6157. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.; Ge, J.; Si, Y.; Yu, J.; Yin, X.; Ding, B. Ultrathin Cellulose Voronoi-Nanonet Membranes Enable High-Flux and Energy-Saving Water Purification. ACS Appl. Mater. Interfaces 2020, 12, 31852–31862. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Wang, R.; Wang, S.; Yao, C.; Ren, W.; Chen, C.; Zhang, L. Metal–organic framework-based nanofiber filters for effective indoor air quality control. J. Mater. Chem. A 2018, 6, 15807–15814. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Ma, L.; Chen, J.; Fan, Y.; Zhang, S.; Shi, H.; Li, Z.; Luo, P. Hierarchically stabilized PAN/β-FeOOH nanofibrous membrane for efficient water purification with excellent antifouling performance and robust solvent resistance. ACS Appl. Mater. Interfaces 2019, 11, 34487–34496. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wang, X.; Yu, J.; Ding, B. Ultrahigh metal–organic framework loading and flexible nanofibrous membranes for efficient CO2 capture with long-term, ultrastable recyclability. ACS Appl. Mater. Interfaces 2018, 10, 34802–34810. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wu, W.; Zhou, S.; Xing, T.; Sun, G.; Chen, G. Polydopamine-induced growth of mineralized γ-FeOOH nanorods for construction of silk fabric with excellent superhydrophobicity, flame retardancy and UV resistance. Chem. Eng. J. 2020, 382, 122988. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Wang, X.; Si, Y.; Yu, J.; Ding, B. Thermoconductive, moisture-permeable, and superhydrophobic nanofibrous membranes with interpenetrated boron nitride network for personal cooling fabrics. ACS Appl. Mater. Interfaces 2020, 12, 32078–32089. [Google Scholar] [CrossRef] [PubMed]
- Atthi, N.; Sripumkhai, W.; Pattamang, P.; Thongsook, O.; Srihapat, A.; Meananeatra, R.; Supadech, J.; Klunngien, N.; Jeamsaksiri, W. Fabrication of robust PDMS micro-structure with hydrophobic and antifouling properties. Microelectron. Eng. 2020, 224, 111255. [Google Scholar] [CrossRef]
- Wu, Y.; Qi, H.; Li, B.; Zhanhua, H.; Li, W.; Liu, S. Novel hydrophobic cotton fibers adsorbent for the removal of nitrobenzene in aqueous solution. Carbohydr. Polym. 2017, 155, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Shirai, F.; Sageshima, M.; Ikeda, N.; Okamoto, Y.; Dohi, Y. Distinctive bacteria-binding property of cloth materials. Am. J. Infect. Control 2004, 32, 27–30. [Google Scholar] [CrossRef]
- Siedlecki, L.C.C.A. Bacterial Cell–Biomaterials Interactions. In Handbook of Biomaterials Biocompatibility; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Sinclair, T.; Robles, D.; Raza, B.; Van den Hengel, S.; Rutjes, S.; de Roda Husman, A.; de Grooth, J.; de Vos, W.; Roesink, H. Virus reduction through microfiltration membranes modified with a cationic polymer for drinking water applications. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 33–41. [Google Scholar] [CrossRef]
- Mi, X.; Albukhari, S.M.; Heldt, C.L.; Heiden, P.A. Virus and chlorine adsorption onto guanidine modified cellulose nanofibers using covalent and hydrogen bonding. Carbohydr. Res. 2020, 498, 108153. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Frey, M.W. Electrospun nanofibers for chemical separation. Nanomaterials 2020, 10, 982. [Google Scholar] [CrossRef]
- Nidzworski, D.; Siuzdak, K.; Niedziałkowski, P.; Bogdanowicz, R.; Sobaszek, M.; Ryl, J.; Weiher, P.; Sawczak, M.; Wnuk, E.; Goddard, W.A. A rapid-response ultrasensitive biosensor for influenza virus detection using antibody modified boron-doped diamond. Sci. Rep. 2017, 7, 15707. [Google Scholar] [CrossRef] [PubMed]
- Richter, Ł.; Janczuk-Richter, M.; Niedziółka-Jönsson, J.; Paczesny, J.; Hołyst, R. Recent advances in bacteriophage-based methods for bacteria detection. Drug Discov. Today 2018, 23, 448–455. [Google Scholar] [CrossRef]
- Memon, H.; Yasin, S.; Ali Khoso, N.; Hussain, M. Indoor decontamination textiles by photocatalytic oxidation: A review. J. Nanotechnol. 2015, 104142. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.H.; Krobjilowski, A. Meltblown fabrics from biodegradable polymers. Int. Nonwovens J. 2001, 1558925001os-1000106. [Google Scholar] [CrossRef] [Green Version]
- Mengistu Lemma, S.; Bossard, F.; Rinaudo, M. Preparation of pure and stable chitosan nanofibers by electrospinning in the presence of poly (ethylene oxide). Int. J. Mol. Sci. 2016, 17, 1790. [Google Scholar] [CrossRef] [Green Version]
- Kuntzler, S.G.; Costa, J.A.V.; de Morais, M.G. Development of electrospun nanofibers containing chitosan/PEO blend and phenolic compounds with antibacterial activity. Int. J. Biol. Macromol. 2018, 117, 800–806. [Google Scholar] [CrossRef]
- Wang, Q.; Ju, J.; Tan, Y.; Hao, L.; Ma, Y.; Wu, Y.; Zhang, H.; Xia, Y.; Sui, K. Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr. Polym. 2019, 205, 125–134. [Google Scholar] [CrossRef]
- Bonino, C.A.; Krebs, M.D.; Saquing, C.D.; Jeong, S.I.; Shearer, K.L.; Alsberg, E.; Khan, S.A. Electrospinning alginate-based nanofibers: From blends to crosslinked low molecular weight alginate-only systems. Carbohydr. Polym. 2011, 85, 111–119. [Google Scholar] [CrossRef]
- Min, K.; Kim, S.; Kim, S. Silk protein nanofibers for highly efficient, eco-friendly, optically translucent, and multifunctional air filters. Sci. Rep. 2018, 8, 9598. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, L.; Zhou, Z.; Wu, X.; Wang, Y. Preparation and properties of electrospun soy protein isolate/polyethylene oxide nanofiber membranes. ACS Appl. Mater. Interfaces 2012, 4, 4331–4337. [Google Scholar] [CrossRef] [PubMed]
- Sun, G. Antibacterial textile materials for medical applications. In Functional Textiles for Improved Performance, Protection and Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 360–375. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.; Patil, S.; Mullani, S.; Delekar, S. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Cantisani, M.; Ingle, A.; Galdiero, M.; Rai, M. Silver nanoparticles as novel antibacterial and antiviral agents. In Handbook of Nanobiomedical Research: Fundamentals, Applications and Recent Developments: Volume 1. Materials for Nanomedicine; World Scientific: Singapore, 2014; pp. 565–594. [Google Scholar] [CrossRef]
- Ogilvie, B.H.; Solis-Leal, A.; Lopez, J.B.; Poole, B.D.; Robison, R.A.; Berges, B.K. Alcohol-free hand sanitizer and other quaternary ammonium disinfectants quickly and effectively inactivate SARS-CoV-2. J. Hosp. Infect. 2021, 108, 142–145. [Google Scholar] [CrossRef]
- EPA. List N Tool: COVID-19 Disinfectants. 2020. Available online: https://cfpub.epa.gov/giwiz/disinfectants/index.cfm (accessed on 8 August 2021).
- Tang, P.; Zhang, Z.; El-Moghazy, A.Y.; Wisuthiphaet, N.; Nitin, N.; Sun, G. Daylight-Induced Antibacterial and Antiviral Cotton Cloth for Offensive Personal Protection. ACS Appl. Mater. Interfaces 2020, 12, 49442–49451. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, Z.; Wu, W.; Fu, Q.; Huang, K.; Nitin, N.; Ding, B.; Sun, G. Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci. Adv. 2018, 4, eaar5931. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; El-Moghazy, A.Y.; Wisuthiphaet, N.; Nitin, N.; Castillo, D.; Murphy, B.G.; Sun, G. Daylight-Induced Antibacterial and Antiviral Nanofibrous Membranes Containing Vitamin K Derivatives for Personal Protective Equipment. ACS Appl. Mater. Interfaces 2020, 12, 49416–49430. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, N.; Xu, W.; Sun, G. Layer-by-layer structured nanofiber membranes with photoinduced self-cleaning functions. J. Phys. Chem. C 2011, 115, 6825–6832. [Google Scholar] [CrossRef]
- Zhuo, J.; Sun, G. Antimicrobial functions on cellulose materials introduced by anthraquinone vat dyes. ACS Appl. Mater. Interfaces 2013, 5, 10830–10835. [Google Scholar] [CrossRef]
- Lu, A.; Ma, Z.; Zhuo, J.; Sun, G.; Zhang, G. Layer-by-layer structured gelatin nanofiber membranes with photoinduced antibacterial functions. J. Appl. Polym. Sci. 2013, 128, 970–975. [Google Scholar] [CrossRef]
- Zhu, Q.; Jin, Y.; Sun, G.; Yan, K.; Wang, D. AQC functionalized CNCs/PVA-co-PE composite nanofibrous membrane with flower-like microstructures for photo-induced multi-functional protective clothing. Cellulose 2018, 25, 4819–4830. [Google Scholar] [CrossRef]
- Yi, S.; Wu, Y.; Zhang, Y.; Zou, Y.; Dai, F.; Si, Y. Antibacterial Activity of Photoactive Silk Fibroin/Cellulose Acetate Blend Nanofibrous Membranes against Escherichia coli. ACS Sustain. Chem. Eng. 2020, 8, 16775–16780. [Google Scholar] [CrossRef]
- Hou, A.; Hu, L.; Zheng, C.; Xie, K.; Gao, A. Light-controllable antibacterial composite films based on modified waterborne polyurethane. Prog. Org. Coat. 2020, 149, 105940. [Google Scholar] [CrossRef]
- Hou, A.; Sun, G. Multifunctional finishing of cotton fabrics with 3, 3′, 4, 4′-benzophenone tetracarboxylic dianhydride: Reaction mechanism. Carbohydr. Polym. 2013, 95, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Sun, G. Multifunctional finishing of cotton with 3, 3′, 4, 4′-benzophenone tetracarboxylic acid: Functional performance. Carbohydr. Polym. 2013, 96, 435–439. [Google Scholar] [CrossRef]
- Hu, L.; Hou, A.; Xie, K.; Gao, A. Light-induced production of reactive oxygen species by a novel water-soluble benzophenone derivative containing quaternary ammonium groups and its assembly on the protein fiber surface. ACS Appl. Mater. Interfaces 2019, 11, 26500–26506. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Sun, S.; Fan, Y.; Zou, Y.; Dai, F.; Si, Y. Scalable fabrication of rechargeable photoactive cellulose nanofibrous membranes for efficient degradation of dyes. Cellulose 2020, 27, 5285–5296. [Google Scholar] [CrossRef]
- Vanerio, N.; Stijnen, M.; de Mol, B.A.; Kock, L.M. Biomedical applications of photo-and sono-activated Rose Bengal: A review. Photobiomodul. Photomed. Laser Surg. 2019, 37, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Chen, J.; Li, L.; Wang, X.; Wei, Q.; Ghiladi, R.A.; Wang, Q. Wool/acrylic blended fabrics as next-generation photodynamic antimicrobial materials. ACS Appl. Mater. Interfaces 2019, 11, 29557–29568. [Google Scholar] [CrossRef]
- Wang, T.; Chen, W.; Dong, T.; Lv, Z.; Zheng, S.; Cao, X.; Wei, Q.; Ghiladi, R.A.; Wang, Q. Color-Variable Photodynamic Antimicrobial Wool/Acrylic Blended Fabrics. Materials 2020, 13, 4141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, H.; Li, J.; Song, H.; Wang, S.; Zhang, Z.; Chen, S. Green light–triggered antimicrobial cotton fabric for wastewater disinfection. Mater. Today Phys. 2020, 15, 100254. [Google Scholar] [CrossRef]
- Hao, L.; Jiang, R.; Fan, Y.; Xu, J.-N.; Tian, L.; Zhao, J.; Ming, W.; Ren, L. Formation and antibacterial performance of metal–organic framework films via dopamine-mediated fast assembly under visible light. ACS Sustain. Chem. Eng. 2020, 8, 15834–15842. [Google Scholar] [CrossRef]
- Jiang, L.; Gan, C.R.R.; Gao, J.; Loh, X.J. A perspective on the trends and challenges facing porphyrin-based anti-microbial materials. Small 2016, 12, 3609–3644. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.L.; Scholle, F.; Zhu, J.; Lu, Y.; Zhang, X.; Situ, X.; Ghiladi, R.A. Photosensitizer-embedded polyacrylonitrile nanofibers as antimicrobial non-woven textile. Nanomaterials 2016, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wu, S.; Mensah, A.; Wang, Q.; Huang, F.; Li, D.; Wei, Q. Insight into light-driven antibacterial cotton fabrics decorated by in situ growth strategy. J. Colloid Interface Sci. 2020, 579, 233–242. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, H.; Gao, A.; Hou, A. Functional modification of cellulose fabrics with phthalocyanine derivatives and the UV light-induced antibacterial performance. Carbohydr. Polym. 2018, 201, 382–386. [Google Scholar] [CrossRef]
- Stoll, K.R.; Scholle, F.; Zhu, J.; Zhang, X.; Ghiladi, R.A. BODIPY-embedded electrospun materials in antimicrobial photodynamic inactivation. Photochem. Photobiol. Sci. 2019, 18, 1923–1932. [Google Scholar] [CrossRef]
- Rhodes, M.; Bucher, J.; Peckham, J.; Kissling, G.; Hejtmancik, M.; Chhabra, R. Carcinogenesis studies of benzophenone in rats and mice. Food Chem. Toxicol. 2007, 45, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tang, S.; Yuan, D.; Tang, J.; Zhang, C.; Li, N.; Rao, Y. Improved degradation of anthraquinone dye by electrochemical activation of PDS. Ecotoxicol. Environ. Saf. 2019, 177, 77–85. [Google Scholar] [CrossRef]
- Zhang, Z.; Si, Y.; Sun, G. Photoactivities of vitamin K derivatives and potential applications as daylight-activated antimicrobial agents. ACS Sustain. Chem. Eng. 2019, 7, 18493–18504. [Google Scholar] [CrossRef]
- Orsuwan, A.; Kwon, S.; Bumbudsanpharoke, N.; Ko, S. Novel LDPE-riboflavin composite film with dual function of broad-spectrum light barrier and antimicrobial activity. Food Control 2019, 100, 176–182. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, J.; Biswas, T.T.; Tang, R.-C.; Nierstrasz, V. Inkjet printing of curcumin-based ink for coloration and bioactivation of polyamide, silk, and wool fabrics. ACS Sustain. Chem. Eng. 2018, 7, 2073–2082. [Google Scholar] [CrossRef]
- Monge, F.A.; Jagadesan, P.; Bondu, V.; Donabedian, P.L.; Ista, L.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G.; Kell, A.M. Highly effective inactivation of SARS-CoV-2 by conjugated polymers and oligomers. ACS Appl. Mater. Interfaces 2020, 12, 55688–55695. [Google Scholar] [CrossRef]
- Liu, C.; Xu, W.Z.; Charpentier, P.A. Synthesis and Photocatalytic Antibacterial Properties of Poly [2,11′-thiopheneethylenethiophene-alt-2, 5-(3-carboxyl) thiophene]. ACS Appl. Polym. Mater. 2020, 2, 1886–1896. [Google Scholar] [CrossRef]
- Sun, G.; Xu, X.; Bickett, J.R.; Williams, J.F. Durable and regenerable antibacterial finishing of fabrics with a new hydantoin derivative. Ind. Eng. Chem. Res. 2001, 40, 1016–1021. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Si, Y.; Huang, K.; Nitin, N.; Sun, G. Rechargeable antibacterial N-halamine films with antifouling function for food packaging applications. ACS Appl. Mater. Interfaces 2019, 11, 17814–17822. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Li, J.; Zhao, C.; Deng, Y.; Ma, Y.; Wang, D.; Sun, G. Biocidal and rechargeable N-halamine nanofibrous membranes for highly efficient water disinfection. ACS Biomater. Sci. Eng. 2017, 3, 854–862. [Google Scholar] [CrossRef]
- Qian, L.; Sun, G. Durable and regenerable antimicrobial textiles: Synthesis and applications of 3-methylol-2, 2, 5, 5-tetramethyl-imidazolidin-4-one (MTMIO). J. Appl. Polym. Sci. 2003, 89, 2418–2425. [Google Scholar] [CrossRef]
- Cherrie, J.W.; Wang, S.; Mueller, W.; Wendelboe-Nelson, C.; Loh, M. In-mask temperature and humidity can validate respirator wear-time and indicate lung health status. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 578–583. [Google Scholar] [CrossRef] [PubMed]
- University of California Davis. Smart Mask Project. 2020. Available online: https://lepsucd.com/smart-mask-project/ (accessed on 25 October 2021).
- Eisenbrey, J.; Daecher, A. Temperature sensitive surgical face mask for identifying at risk patients and reducing viral infection. Google Patents No. 16/092, 2019. [Google Scholar]
- Courtney, J.M.S.B.; Matthew, N.; Jennings, J. A Wearable Air Purifier; Air-Ring: Delaware, PA, USA, 2018. [Google Scholar]
- Afroj, S.; Karim, N.; Wang, Z.; Tan, S.; He, P.; Holwill, M.; Ghazaryan, D.; Fernando, A.; Novoselov, K.S. Engineering graphene flakes for wearable textile sensors via highly scalable and ultrafast yarn dyeing technique. ACS Nano 2019, 13, 3847–3857. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wang, C.; Zhong, J.; Lin, S.; Xiao, Y.; Zhong, Q.; Jiang, H.; Wu, N.; Li, W.; Chen, S. Electrospun polyetherimide electret nonwoven for bi-functional smart face mask. Nano Energy 2017, 34, 562–569. [Google Scholar] [CrossRef]
- Lam, I.T.W.; Hsu, A. Face Mask Having Transparent Plastic Piece. Google Patents No. 16/115, 2019. [Google Scholar]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M. Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendix, A. Harvard and MIT Researchers are Developing a Face Mask that Lights Up when It Detects the Coronavirus. 2020. Available online: https://www.businessinsider.com/coronavirus-face-mask-light-up-screening-tool-test-2020-5 (accessed on 23 October 2021).
- Javaid, M.; Haleem, A.; Vaishya, R.; Bahl, S.; Suman, R.; Vaish, A. Industry 4.0 technologies and their applications in fighting COVID-19 pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 419–422. [Google Scholar] [CrossRef]
- Ishack, S.; Lipner, S.R. Applications of 3D printing technology to address COVID-19–related supply shortages. Am. J. Med. 2020, 133, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Swennen, G.R.; Pottel, L.; Haers, P.E. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int. J. Oral Maxillofac. Surg. 2020, 49, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, D.; Rao, Y.J.; Mitic, K.; Obaid, S.; Pierce, D.; Huckenpahler, J.; Berger, J.; Goyal, S.; Loew, M. Rapid Prototyping of Reusable 3D-Printed N95 Equivalent Respirators at the George Washington University. 2020. Available online: Preprints.org (accessed on 23 October 2021). [CrossRef] [Green Version]
- Liu, D.; Koo, T.; Wong, J.; Wong, Y.; Fung, K.; Chan, Y.; Lim, H. Adapting re-usable elastomeric respirators to utilise anaesthesia circuit filters using a 3D-printed adaptor-a potential alternative to address N95 shortages during the COVID-19 pandemic. Anaesthesia 2020, 75, 1022–1027. [Google Scholar] [CrossRef]
- Cai, M.; Li, H.; Shen, S.; Wang, Y.; Yang, Q. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J. Occup. Environ. Hyg. 2018, 15, 226–234. [Google Scholar] [CrossRef]
- Rao, A.S.S.; Vazquez, J.A. Identification of COVID-19 can be quicker through artificial intelligence framework using a mobile phone–based survey when cities and towns are under quarantine. Infect. Control Hosp. Epidemiol. 2020, 41, 826–830. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T. Artificial intelligence in the battle against coronavirus (COVID-19): A survey and future research directions. arXiv 2020, arXiv:2008.07343. [Google Scholar]
- Kamel Boulos, M.N.; Geraghty, E.M. Geographical tracking and mapping of coronavirus disease COVID-19/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic and associated events around the world: How 21st century GIS technologies are supporting the global fight against outbreaks and epidemics. Int. J. Health Geogr. 2020, 19, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCall, B. COVID-19 and artificial intelligence: Protecting health-care workers and curbing the spread. Lancet Digit. Health 2020, 2, e166–e167. [Google Scholar] [CrossRef]
- Abbass, H.A.; Sarker, R.; Newton, C. PDE: A Pareto-frontier differential evolution approach for multi-objective optimization problems. In Proceedings of the 2001 Congress on Evolutionary Computation (IEEE Cat. No.01TH8546), Soeul, Korea, 27–30 May 2001. [Google Scholar]
- Dunford, R.; Su, Q.; Tamang, E. The Pareto Principle. 2014. Available online: https://pearl.plymouth.ac.uk/bitstream/handle/10026.1/14054/TPSS-2014-Vol7n1_140-148Dunford.pdf?sequence=1&isAllowed=y (accessed on 21 August 2021).
- Singh, D.; Kumar, V.; Kaur, M. Classification of COVID-19 patients from chest CT images using multi-objective differential evolution–based convolutional neural networks. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Libotte, G.B.; Lobato, F.S.; Platt, G.M.; Neto, A.J.S. Determination of an optimal control strategy for vaccine administration in COVID-19 pandemic treatment. Comput. Methods Programs Biomed. 2020, 196, 105664. [Google Scholar] [CrossRef]






| Influencing Factors | Filtration Efficiency Change | Reference |
|---|---|---|
| Particle size | With decreasing particle size, the filtration efficiency starts to decrease before increasing again (Figure 3) | [93,94] |
| Airflow rate/face velocity | The higher the airflow rate, the lower the filtration efficiency | [77,84,95] |
| Breathing pattern | Unsteady breathing pattern reduces filtration efficiency | [79,84,87] |
| Respiration frequency | Higher respiration frequency reduces filtration efficiency | [79,86] |
| Humidity | Higher humidity reduces filtration efficiency | [84,90,96] |
| Loading time | Longer loading time increases filtration efficiency | [79,89] |
| Specified Performance | United States | European Union | ||
|---|---|---|---|---|
| Regulation/Guidance: OSHA 29 CFR 1910.134 NIOSH 42 CFR 84 | Regulation/Guidance: EU 2016/425 PPE Regulation EU 2017/745 Medical Device Regulation | |||
| Requirements: NIOSH 42 CFR 84 | Test Methods | Requirements: EN 149 +A1 | Test Methods | |
| Particulates filtration efficiency (%) | N95 R95 P95 ≥ 95 N99 R99 P99 ≥ 99 N100 R100 P100 ≥ 99.97 | TEB-APR-STP-0051 to 0059 Challenge with NaCl for N series; Dioctyl phthalate for R and P series; Flow rate 85 L/min; 0.075 µm CMD with GSD < 1.86; <200 mg/m3 | FFP1 ≥ 80 FFP2 ≥ 94 FFP3 ≥ 99 | EN 149 +A1 EN 13274-7 Challenge with NaCl and paraffin oil; Flow rate 95 L/min; 0.06–0.1 µm CMD with GSD 2–3; 8 ± 4 mg/m3 |
| Total inward leakage (TIL, %) | NA | NA | FFP1 ≤ 22 FFP2 ≤ 8 FFP3 ≤ 2 | EN 149 +A1 EN 13274-1 Challenge with NaCl on human subjects |
| Breathing resistance (inhalation) | All N, R, P series ≤ 35 mm H2O (343 Pa) | TEB-APR-STP-0007 Head form manikin test; Flow rate 85 L/min | FFP1 ≤ 0.6 (mbar, 60 Pa) and 2.1 (210) FFP2 ≤ 0.7 (70) and 2.4 (240) FFP3 ≤ 1 (100) and 3 (300) | EN 149 +A1 EN 13274-3 Head form manikin test; Flow rate 30 L/min and 95 L/min |
| Breathing resistance (exhalation) | All N, R, P series ≤ 25 mm H2O (245 Pa) | TEB-APR-STP-0003 Head form manikin test; Flow rate 85 L/min | FFP 1, 2, 3 ≤ 3 mbar (300 Pa) | EN 149 +A1 EN 13274-3 Head form manikin test; Flow rate 160 L/min |
| Exhalation valve leakage | Leakage ≤ 30 mL/min | TEB-APR-STP-0004 At −22 mm H2O (−245 pa) | NA | NA |
| CO2 content requirement (%) | NA | NA | FFP 1, 2, 3 ≤ 1 | EN 149 +A1 EN 13274-6 Head form manikin test |
| Flammability | NA | NA | Pass | EN 149 +A1 EN 13274-4 |
| Biocompatibility | NA | NA | Pass | ISO 10993-1 ISO 10993-5 ISO 10993-10 |
| Specified Performance | United States | European Union | ||
|---|---|---|---|---|
| Regulation/Guidance: OSHA 29 CFR 1910.134 OSHA 29 CFR 1910.1030 FDA 510(k) | Regulation/Guidance: EU 2016/425 PPE Regulation EU 2017/745 Medical Device Regulation | |||
| Requirements: ASTM F2100 | Test Methods | Requirements: EN 14,683 + AC | Test Methods | |
| Sub-micron particulates filtration efficiency, 0.1 µm (%) | Level 1 ≥ 95 Level 2 ≥ 98 Level 3 ≥ 98 | ASTM F2299 Challenge with Latex spheres; face velocity 0.5–25 cm/sec; 107–108 particles/m3 with dilution; 1–5 min test | NA | NA |
| Bacterial filtration efficiency, 3 µm (%) | Level 1 ≥ 95 Level 2 ≥ 98 Level 3 ≥ 98 | ASTM F2101 Challenge with Staphylococcus aureus at 28.3 L/min; 2200 ± 500 particle per test; 2 min test | Type I ≥ 95 Type II ≥ 98 Type IIR ≥ 98 | EN 14,683 + AC Annex B Challenge with Staphylococcus aureus at 28.3 L/min; 1700–3000 colony forming units per test; 2 min test |
| Differential pressure, Pa/cm2 (mmH2O/cm2) | Level 1 < 50 (5) Level 2 < 60 (6) Level 3 < 60 (6) | EN 14,683 + AC Annex B Flow rate 8 L/min | Type I < 40 (4) Type II < 40 (4) Type IIR < 60 (6) | EN 14,683 + AC Annex B Flow rate 8 L/min |
| Synthetic blood/splash resistance, mmHg (KPa) | Level 1 ≥ 80 (11) Level 2 ≥ 120 (16) Level 3 ≥ 160 (21) | ASTM F1862 | Type I NA Type II NA Type IIR ≥ 120 (16) | ISO 22609 |
| Flammability | Level 1–3 to be Class 1 | 16 CFR 1610 | NA | NA |
| Microbial cleanliness (cfu/g) | NA | NA | Type I ≤ 30 Type II ≤ 30 Type IIR ≤ 30 | ISO 11737-1 |
| Biocompatibility | NA | FDA recommends following ISO 10993 | Pass | ISO 10993-1 ISO 10993-5 ISO 10993-10 |
| Viral filtration efficiency, 3 µm (%) | No standard | Adapted ASTM F2101 using PhiX174 virus at 28.3 L/min; 1700–2000 plaque forming units per test | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhang, M.; Wu, Y.; Tang, P.; Sun, G.; Wang, L.; Mandal, S.; Wang, L.; Lang, J.; Passalacqua, A.; et al. What We Are Learning from COVID-19 for Respiratory Protection: Contemporary and Emerging Issues. Polymers 2021, 13, 4165. https://doi.org/10.3390/polym13234165
Li R, Zhang M, Wu Y, Tang P, Sun G, Wang L, Mandal S, Wang L, Lang J, Passalacqua A, et al. What We Are Learning from COVID-19 for Respiratory Protection: Contemporary and Emerging Issues. Polymers. 2021; 13(23):4165. https://doi.org/10.3390/polym13234165
Chicago/Turabian StyleLi, Rui, Mengying Zhang, Yulin Wu, Peixin Tang, Gang Sun, Liwen Wang, Sumit Mandal, Lizhi Wang, James Lang, Alberto Passalacqua, and et al. 2021. "What We Are Learning from COVID-19 for Respiratory Protection: Contemporary and Emerging Issues" Polymers 13, no. 23: 4165. https://doi.org/10.3390/polym13234165
APA StyleLi, R., Zhang, M., Wu, Y., Tang, P., Sun, G., Wang, L., Mandal, S., Wang, L., Lang, J., Passalacqua, A., Subramaniam, S., & Song, G. (2021). What We Are Learning from COVID-19 for Respiratory Protection: Contemporary and Emerging Issues. Polymers, 13(23), 4165. https://doi.org/10.3390/polym13234165










