Preparation and Characterization of Intrinsic Low-κ Polyimide Films
Abstract
:1. Introduction
2. Materials and Methods
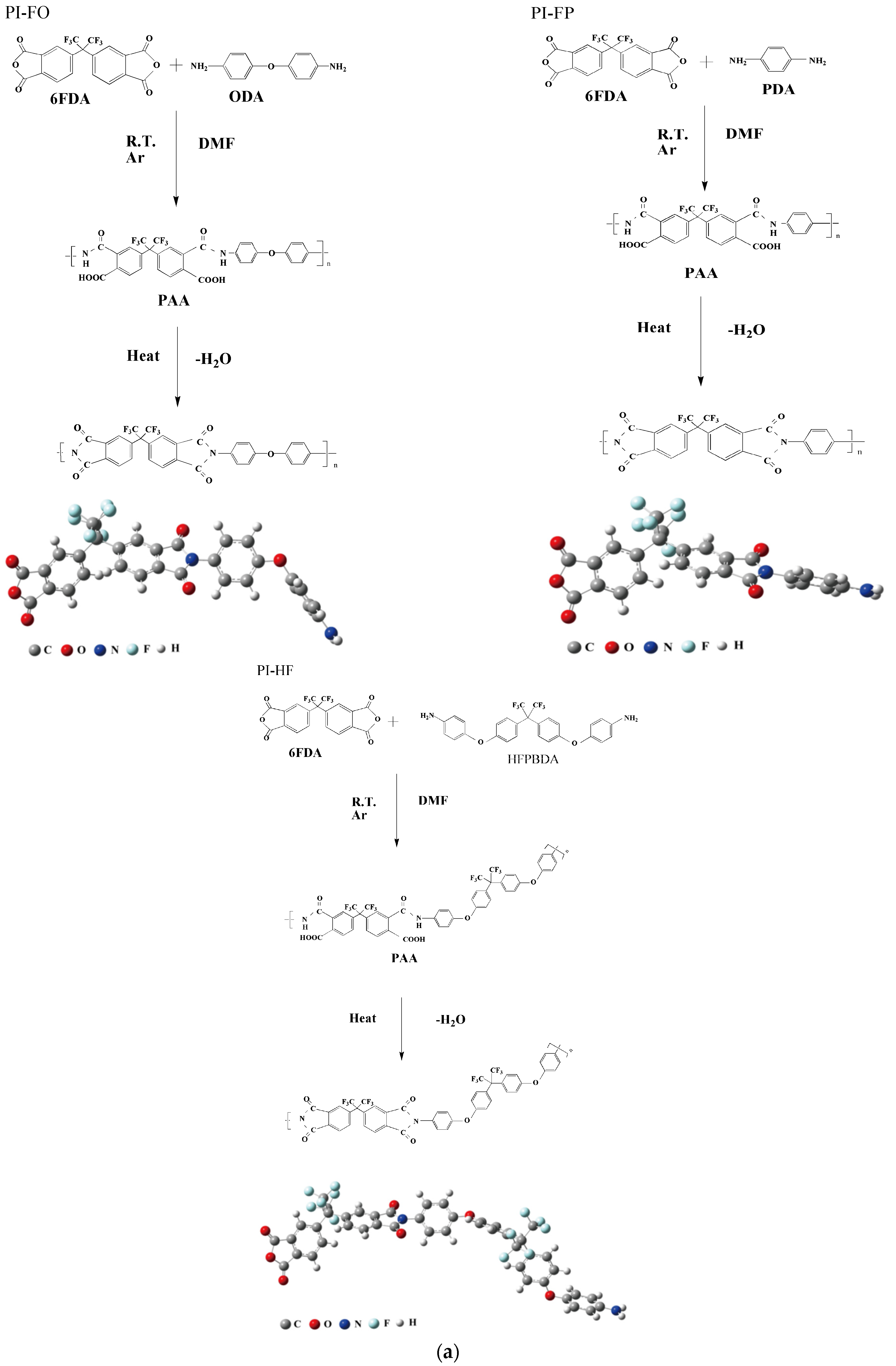
2.1. Materials and PI Film Fabrication
2.2. Characterization
3. Results and Discussion
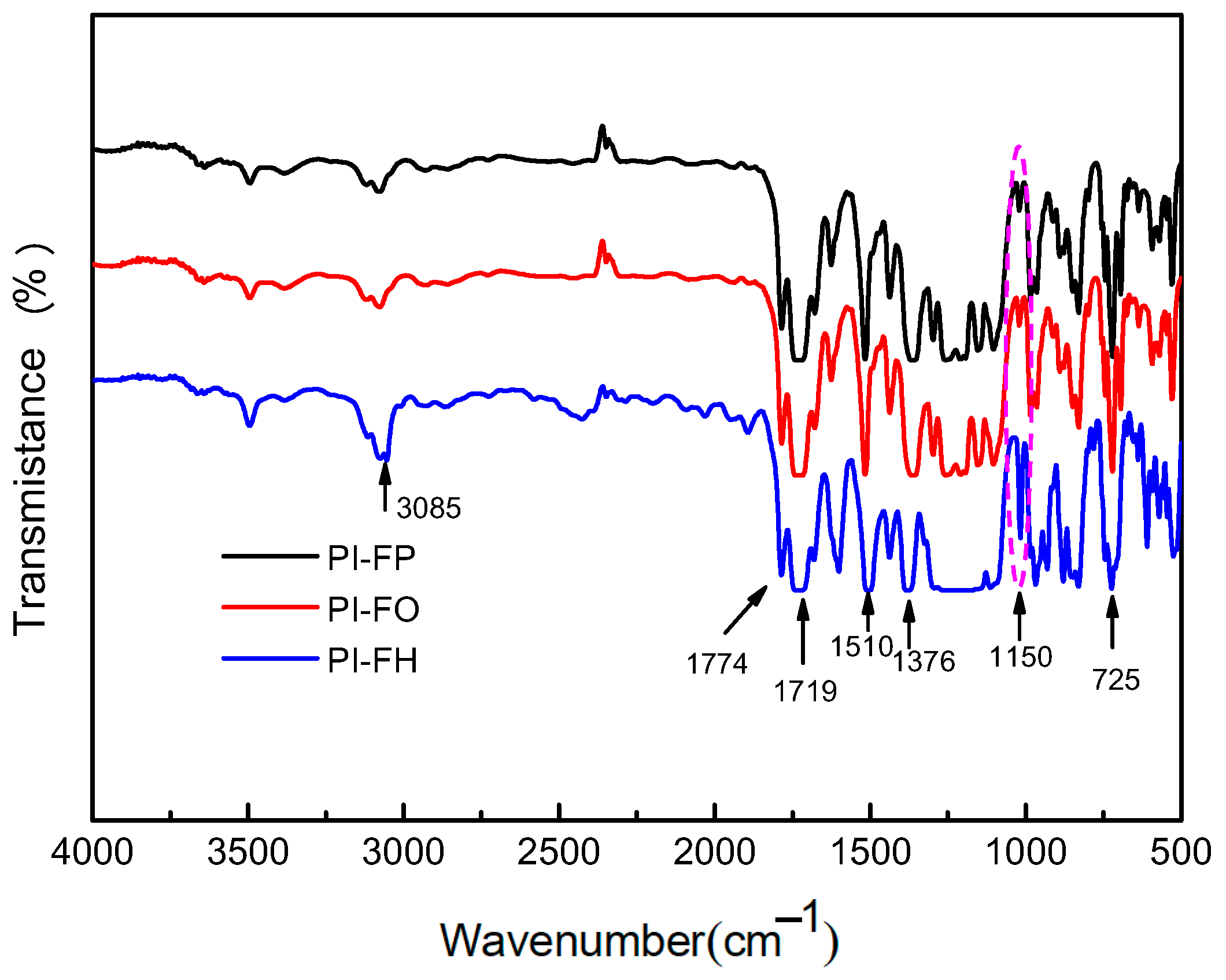
3.1. FT-IR Spectra and EDX
3.2. Positron Annihilation Analysis
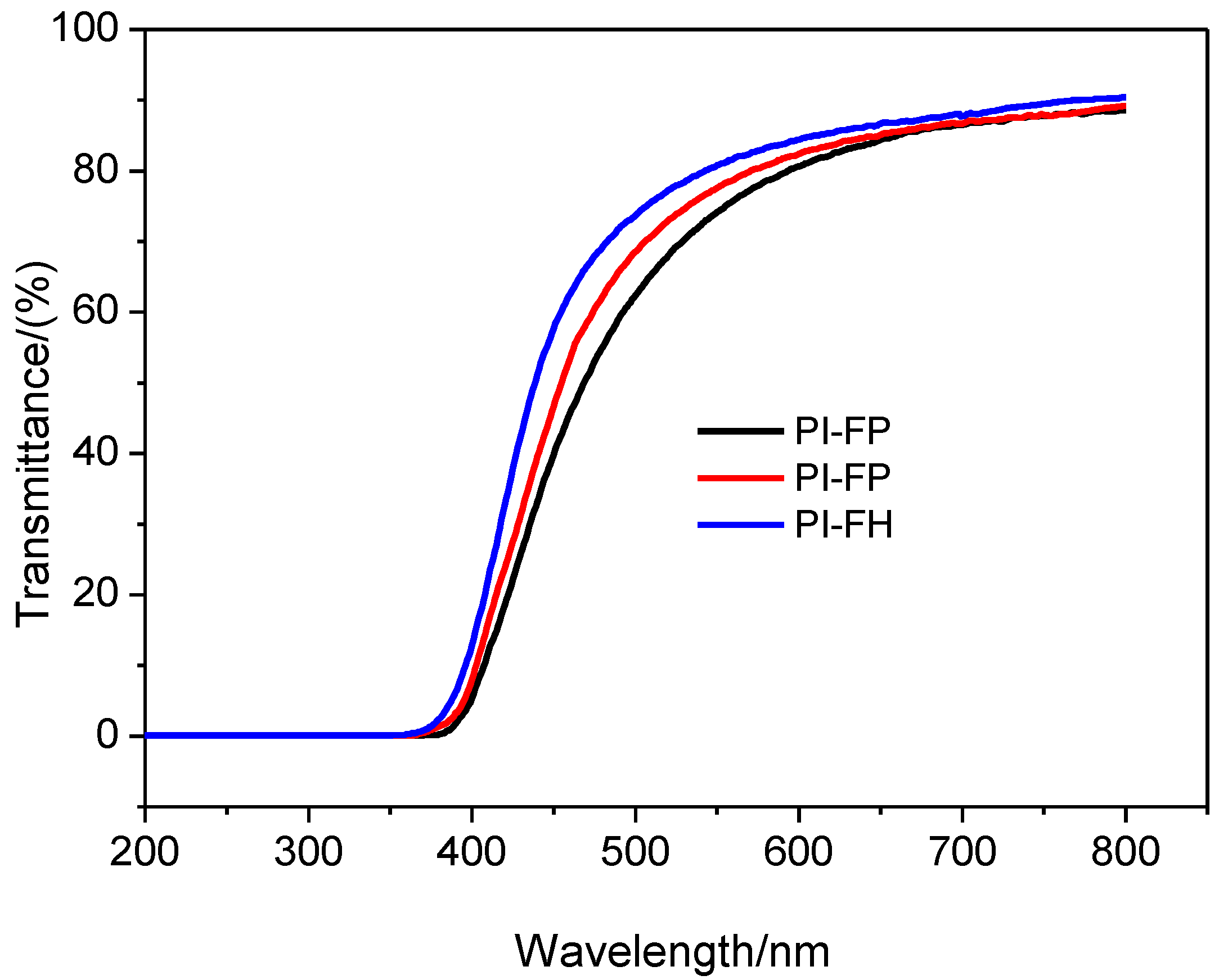
3.3. UV-Visible Transmission Spectra
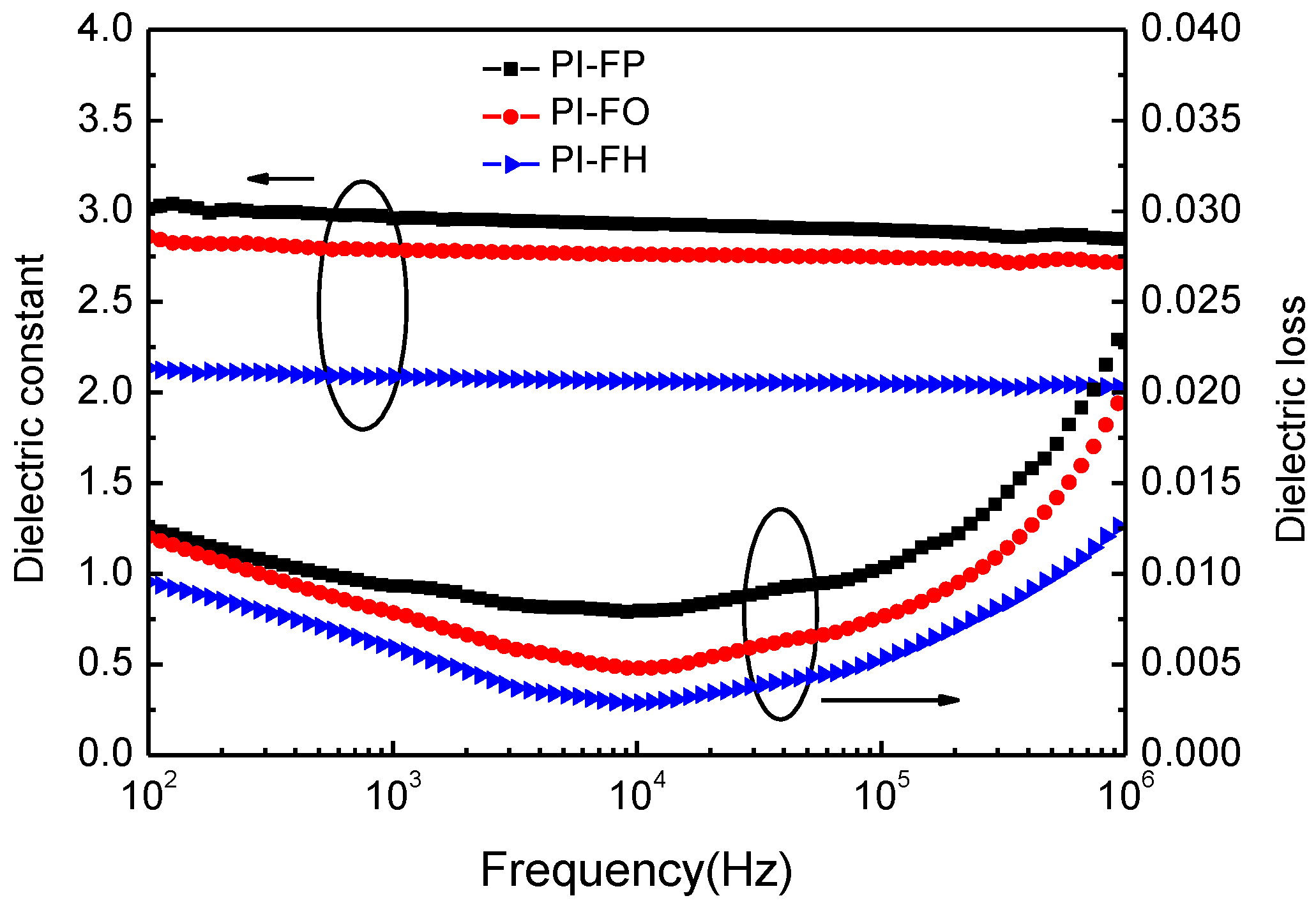
3.4. Dielectric Properties
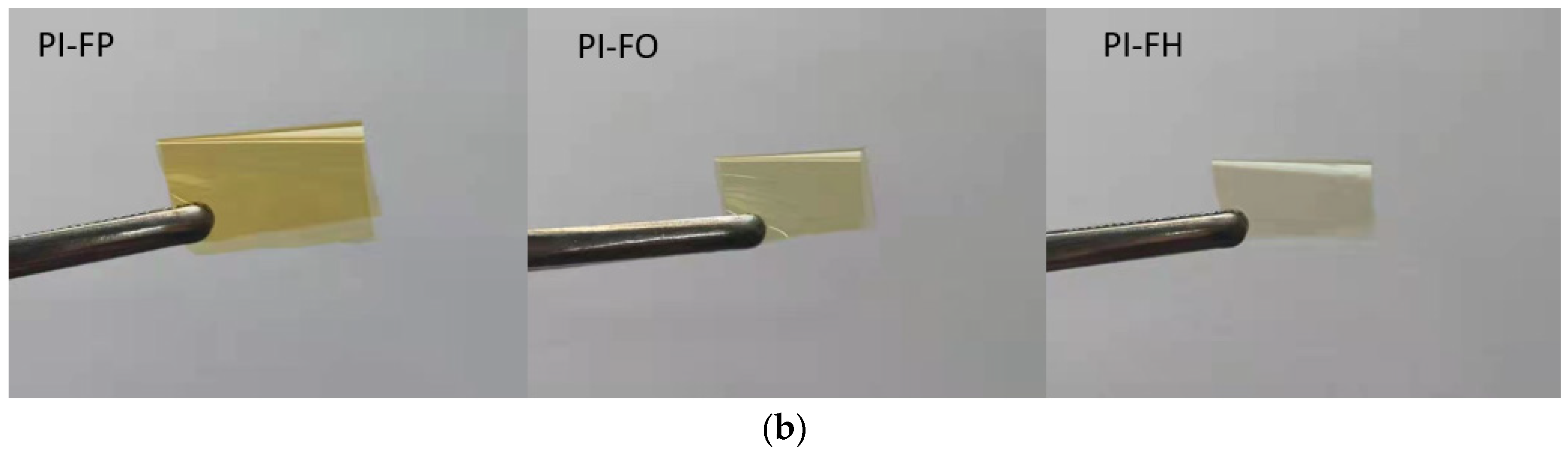
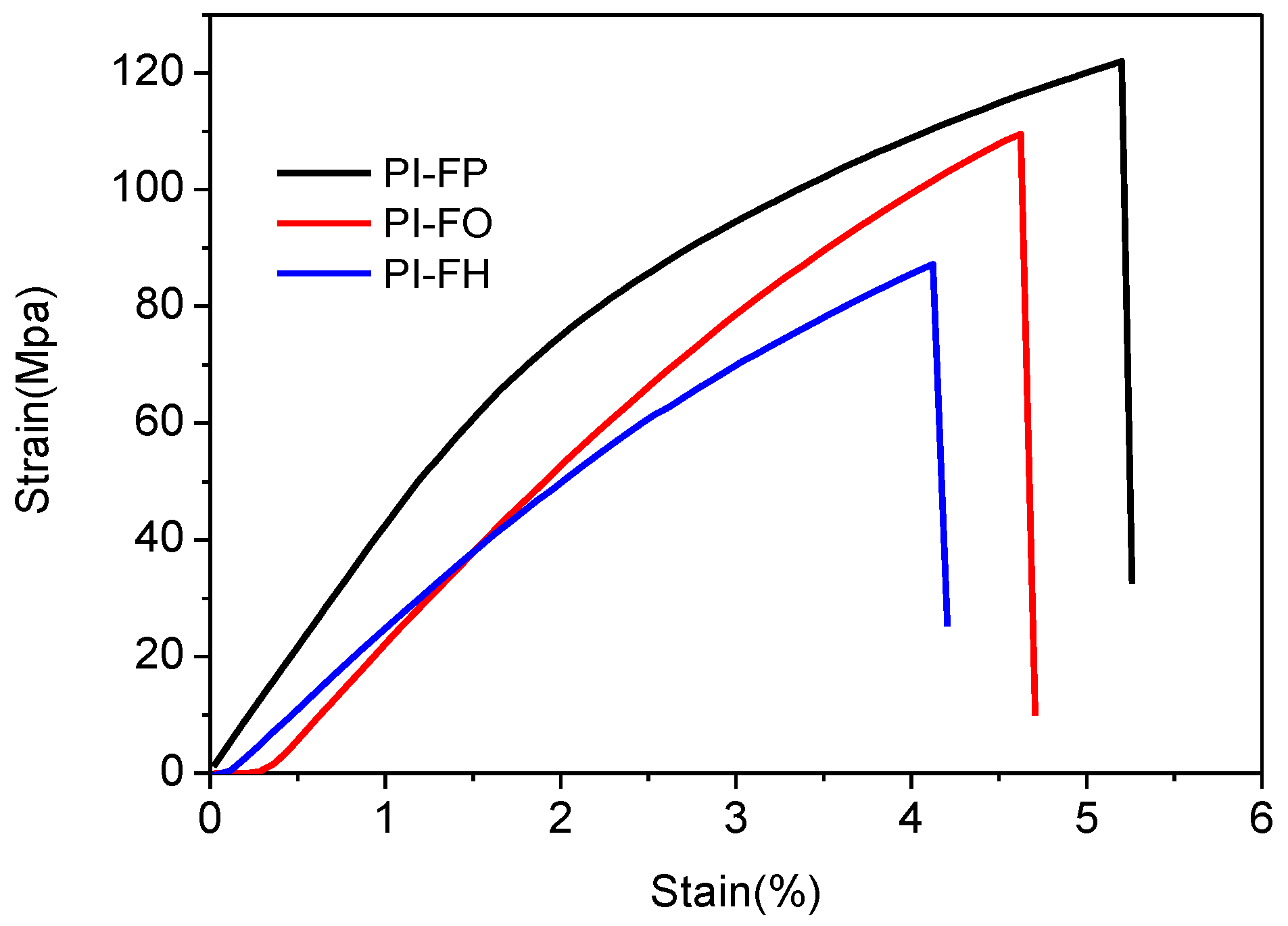
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.J.; Huang, J.M.; Kuo, S.W.; Chang, F.C. Low-dielectric, nanoporous polyimide films prepared from PEO-POSS nanoparticles. Polymer 2005, 46, 10056–10065. [Google Scholar] [CrossRef]
- Volksen, W.; Miller, R.D.; Dubois, G. Low dielectric constant materials. Chem. Rev. 2010, 110, 56–110. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, K.K.; Li, K.; Diao, S.; Tong, J.W.; Fang, Q. Non-porous low-k dielectric films based on a new structural amorphous fluoropolymer. Adv. Mater. 2013, 25, 4875–4878. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.W.; Diao, S.; Jin, K.K.; Yuan, C.; Wang, J.J.; Sun, J.; Fang, Q. Benzocyclobutene-functionalized poly(m-phenylene): A novel polymer with low dielectric constant and high thermostability. Polymer 2014, 55, 3628–3633. [Google Scholar] [CrossRef]
- Zhou, H.; Wei, D.Y.; Fan, Y.; Chen, H.; Yang, Y.S.; Yu, J.J.; Jin, L.G. Dielectric properties of polyimide/SiO2 hollow spheres composite films with ultralow dielectric constant. Mat. Sci. Eng. Chem. 2016, 203, 13–18. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, Y.P.; Guo, W.M.; Sun, X.N.; Feng, Y.; Sun, D.; Liu, Y.Y.; Yan, K.; Wu, Z.H.; Su, B.; et al. Dielectric and mechanical properties of polyimide composite films reinforced with graphene nanoribbon. Surf. Coat. Tech. 2017, 320, 497–502. [Google Scholar] [CrossRef]
- Yang, K.S.; Kang, Y.Y.; Ahn, H.J.; Kim, D.G.; Park, N.K.; Choi, S.Y.Q.; Won, J.C.; Kim, Y.H. Porous boron nitride/polyimide composite films with high thermal diffusivity and low dielectric properties via high internal phase Pickering emulsion method. J. Ind. Eng. Chem. 2020, 82, 173–179. [Google Scholar] [CrossRef]
- Liu, Y.W.; Qian, C.; Qu, L.J.; Wu, Y.; Zhang, Y.; Wu, X.H.; Zou, B.; Chen, W.X.; Chen, Z.Q.; Chi, Z.G.; et al. A bulk dielectric polymer film with intrinsic ultralow dielectric constant and outstanding comprehensive properties. Chem. Mater. 2015, 27, 6543–6549. [Google Scholar] [CrossRef]
- Huang, Y.W.; Wei, X.N.; Liu, L.L.; Yu, H.T.; Yang, J.X. A novel pore-free strategy via interfacial effects in nanocomposites to produce polyethylene with ultra-low dielectric constants. Mater. Lett. 2018, 232, 86–91. [Google Scholar] [CrossRef]
- Cao, K.; Yang, L.; Huang, Y.W.; Chang, G.J.; Yang, J.X. High temperature thermosets derived from benzocyclobutene-containing main-chain oligomeric carbosilanes. Polymer 2014, 55, 5680–5688. [Google Scholar] [CrossRef]
- Zhong, M.; Wu, X.M.; Shu, C.; Wang, Y.L.; Huang, X.Y.; Huang, W. Organosoluble polyimides with low dielectric constant prepared from an asymmetric diamine containing bulky m-trifluoromethyl phenyl group. React. Funct. Polym. 2021, 169, 05065. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, M.Y.; Han, E.L.; Niu, H.Q.; Wu, D.Z. Structure-property relationship of low dielectric constant polyimide fibers containing fluorine groups. Polymer. 2020, 206, 122884. [Google Scholar] [CrossRef]
- Popovici, D.; Hulubei, C.; Cozan, V.; Lisa, G.; Bruma, M. Polyimides containing cycloaliphatic segments for low dielectric material. High Perform. Polym. 2012, 24, 194–199. [Google Scholar] [CrossRef]
- Ma, S.D.; Wang, Y.; Liu, C.; Xu, Q.; Min, Z.H. Preparation and characterization of nanoporous polyimide membrane by the template method as low-k dielectric material. Polym. Adv. Technol. 2016, 27, 414–418. [Google Scholar] [CrossRef]
- Wang, Q.H.; Wang, C.; Wang, T. Controllable low dielectric porous polyimide films templated by silica microspheres: Microstructure, formation mechanism and properties. J. Colloid Interface Sci. 2013, 389, 99–105. [Google Scholar] [CrossRef]
- Devaraju, S.; Vengatesan, M.R.; Selvi, M.; Kumar, A.A. Synthesis and characterization of bisphenol—A ether diamine-based polyimide POSS nanocomposites for low k dielectric and flame-retardant. Appl. High Perform. Polym. 2012, 24, 85–96. [Google Scholar] [CrossRef]
- Othman, M.B.H.; Ramli, M.R.; Tyng, L.Y.; Ahmad, Z.; Akil, H.M. Dielectric constant and refractive index of poly (siloxane-imide) block copolymer. Mater. Des. 2011, 32, 3173–3182. [Google Scholar] [CrossRef]
- Chen, Z.G.; Liu, S.M.; Yan, S.J.; Shu, X.; Yuan, Y.C.; Huang, H.H.; Zhao, J.Q. Overall improvement in dielectric and mechanical properties of porous graphene fluoroxide/polyimide nanocomposite films via bubble-stretching approach. Mater. Des. 2017, 117, 150–156. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, S.J.; Li, Y.; Li, F.; Yang, F.C.; Gong, C.L.; Zhao, J.J. Synthesis and characterization of soluble polyimides based on a new fluorinated diamine: 4-Phenyl-2,6-bis[3-(4′-amino-2′-trifluoromethyl-phenoxy)phenyl] pyridine. J. Fluor. Chem. 2010, 131, 724–730. [Google Scholar] [CrossRef]
- Wu, T.T.; Dong, J.; Gan, F.; Fang, Y.; Zhao, X.; Zhang, Q. Low dielectric constant and moisture-resistant polyimide aerogels containing trifluoromethyl pendent groups. Appl. Surf. Sci. 2018, 440, 595–605. [Google Scholar] [CrossRef]
- Wang, Z.H.; Fang, G.Q.; He, J.J.; Yang, H.X.; Yang, S.Y. Semi-aromatic thermosetting polyimide resins containing alicyclic units for achieving low melt viscosity and low dielectric constant. React. Funct. Polym. 2020, 146, 104411. [Google Scholar] [CrossRef]
- Maier, G. Low dielectric constant polymers for microelectronics. Prog. Polym. Sci. 2001, 26, 3–65. [Google Scholar] [CrossRef]
- Jin, Y.X.; Tang, J.; Hu, J.; Xan, X.; Shang, Y.Z.; Liu, H.L. One-step fabrication of ultralow dielectric polyimide films consisting of size-controlled mesoporous nanoparticles. Colloids Surf. A 2011, 392, 178–186. [Google Scholar] [CrossRef]
- Yang, C.P.; Chiang, H.C.; Chen, K.H. Organosoluble and light-colored fluorinated polyimides based on 9,9-Bis[4-(4-amino-2-trifluoromethylphenoxy)phenyl]fluorene and aromatic dianhydrides. Colloid Polym. Sci. 2004, 28, 1347–1358. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Brusa, R.S.; Spagolla, M.; Karwasz, G.P.; Zecca, A. Porosity in low dielectric constant SiOCH films depth profiled by positron annihilation spectroscopy. J. Appl. Phys. 2004, 95, 2348. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, R.; Wu, Z.; Zhang, H.; Zhou, Z.; Li, L.; Dong, Y.; Luo, M.; Ye, B.; Zhang, H. Effect of free volume on cryogenic mechanical properties of epoxy resin reinforced by hyperbranched polymers. Mater. Des. 2021, 202, 109565. [Google Scholar] [CrossRef]
- Zhou, B.; Li, C.Y.; Qi, N.; Jiang, M.; Wang, B.; Chen, Z.Q. Pore structure of mesoporous silica (KIT-6) synthesized at different temperatures using positron as a nondestructive probe. Appl. Surf. Sci. 2018, 450, 31–37. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sellaiyan, S.; Kakizaki, T.; Uedono, A.; Taniguchi, Y.; Hayashi, K. Effect of freevolume holes on dynamic mechanical properties of epoxy resins for carbon-fifiberreinforced polymers. Macromolecules 2017, 50, 3933–3942. [Google Scholar] [CrossRef]
- Eldrup, M.; Lightbody, D.; Sherwood, J.N. The temperature dependence of positron lifetimes in solid pivalic acid. Chem. Phys. 1981, 63, 51–58. [Google Scholar] [CrossRef]
- Li, T.Y.; Liu, J.J.; Zhao, S.S.; Chen, Z.Q.; Huang, H.H.; Guo, R.L.; Chen, Y.M. Microporous polyimides containing bulky tetra-o-isopropyl and naphthalene groups for gas separation membranes. J. Membr. Sci. 2019, 585, 282–288. [Google Scholar] [CrossRef]
- Ji, M.C.; Zhou, M.T.; Li, Y.J.; Lu, G.L.; Huang, X.Y. Construction of semi-fluorinated polyimides with perfluorocyclobutyl aryl ether-based side chains. Polym. Chem. 2018, 9, 920–930. [Google Scholar] [CrossRef]
- Japip, S.; Lee, G.R.; Chung, T.S. The role of fluorinated aryl ether moiety in polyimide-co-etherimide on gas transport properties. Ind. Eng. Chem. Res. 2020, 59, 5315–5323. [Google Scholar] [CrossRef]
- Khalil, M.; Saeed, S.; Ahmad, Z. Properties of binary polyimide blends containing hexafluoroisopropylidene group. J. Macromol. Sci. A 2006, 44, 55–63. [Google Scholar] [CrossRef]
- Lee, C.K.; Shul, Y.G.; Han, H. Dielectric properties of oxydianiline-based polyimide thin films according to the water uptake. J. Polym. Sci. Pol. Phys. 2002, 40, 2190–2198. [Google Scholar] [CrossRef]
- Liu, X.D.; Hou, Z.L.; Zhang, B.X.; Zhan, K.T.; He, P.; Zhang, K.L.; Song, W.L. A general model of dielectric constant for porous materials. Appl. Phys. Lett. 2016, 108, 102902. [Google Scholar] [CrossRef]
- Vangchangyia, S.; Swatsitang, E.; Thongbai, P.; Pinitsoontorn, S.; Yamwong, T.; Maensiri, S.; Amornkitbamrung, V. Very low loss tangent and high dielectric permittivity in pure-CaCu3Ti4O12 ceramics prepared by a modifified sol-gel process. J. Am. Ceram. Soc. 2012, 95, 1497–1500. [Google Scholar] [CrossRef]
- Fu, J.; Hou, Y.D.; Zheng, M.P.; Wei, Q.Y.; Zhu, M.K.; Yan, H. Improving dielectric properties of PVDF composites by employing surface modified strong polarized BaTiO3 particles derived by molten salt method. ACS Appl. Mater. Interfaces 2015, 44, 24480–24491. [Google Scholar] [CrossRef]
- Neyertz, S.; Brown, D.; Douanne, A.; Bas, C.; Albérola, N.D. The molecular structure and dynamics of short oligomers of PMDA-ODA and BCDA-ODA polyimides in the absence and presence of water. J. Phys. Chem. B 2002, 106, 4617–4631. [Google Scholar] [CrossRef]


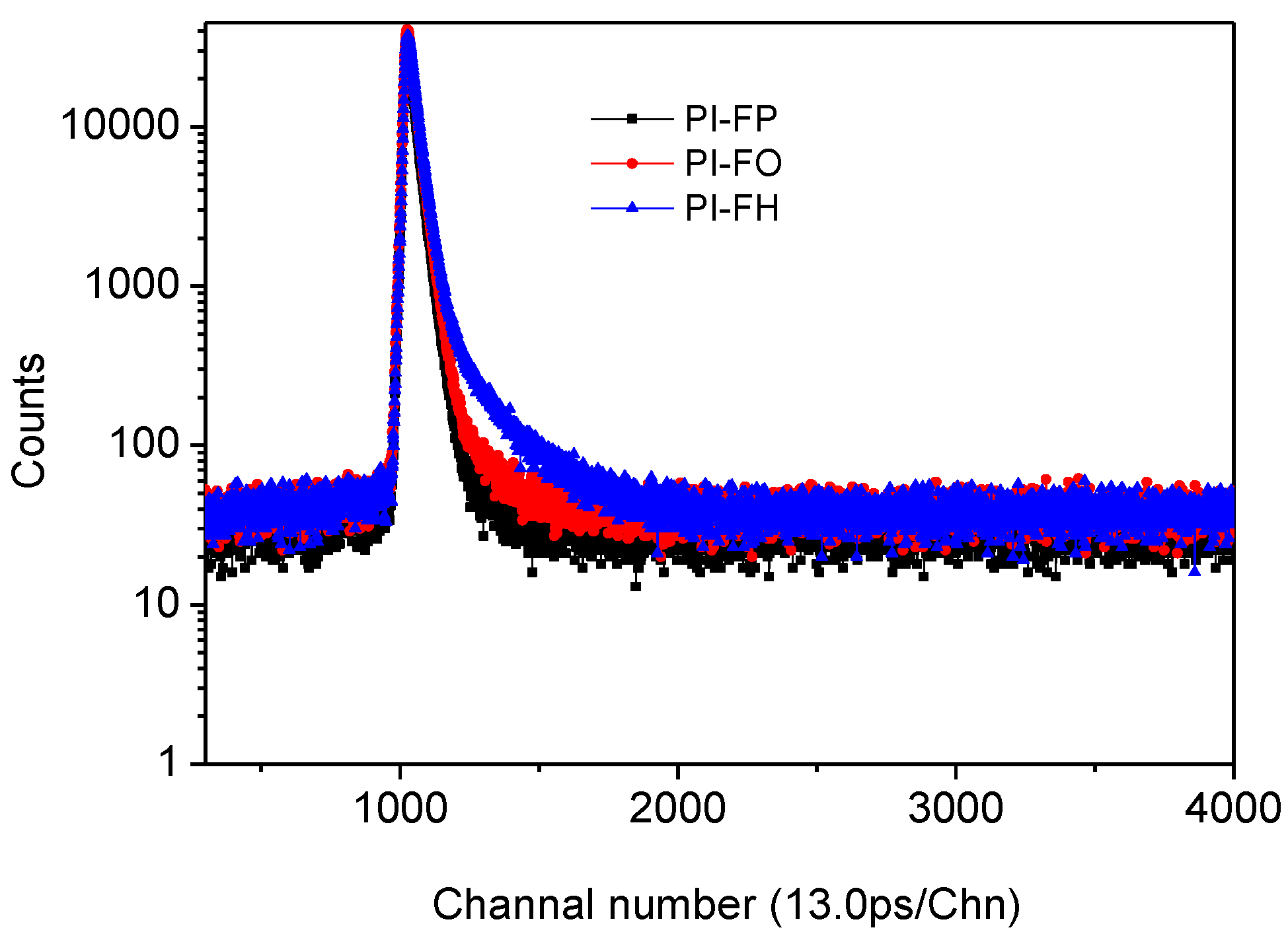


| PI Films | τ1/ps | τ2/ps | τ3/ps | I1/% | I2/% | I3/% |
|---|---|---|---|---|---|---|
| PI-FP | 210.5 ± 4.6 | 429.6 ± 3.7 | 2031.4 ± 30.3 | 36.4 ± 2.3 | 58.6 ± 2.1 | 5.0 ± 0.6 |
| PI-FO | 220.6 ± 5.4 | 424.6 ± 3.5 | 2059.3 ± 28.2 | 32.7 ± 1.4 | 61.0 ± 1.3 | 5.9 ± 0.8 |
| PI-FH | 225.4 ± 9.0 | 444.9 ± 9.2 | 2246.2 ± 29.9 | 30.6 ± 3.6 | 59.7 ± 3.5 | 9.7 ± 1.4 |
| PI Films | R/Å | Vf/Å3 | fr/% |
|---|---|---|---|
| PI-FP | 2.88 ± 0.02 | 99.76 ± 2.83 | 0.90 ± 0.02 |
| PI-FO | 2.90 ± 0.03 | 102.39 ± 2.68 | 1.09 ± 0.02 |
| PI-FH | 3.06 ± 0.02 | 120.48 ± 2.96 | 2.10 ± 0.04 |
| Films | Highest Transmittance/% | Eg/eV |
|---|---|---|
| PI-FP | 88.5 ± 2.0 | 2.56 |
| PI-FO | 88.8 ± 2.3 | 2.58 |
| PIF-H | 90.2 ± 2.1 | 2.69 |
| Films | k/10 kHz | D/10 kHz (10−2) | Electrical Breakdown (kV/mm) |
|---|---|---|---|
| PI-FP | 2.92 ± 0.08 | 0.83 ± 0.03 | 65.4 ± 2.8 |
| PI-FO | 2.76 ± 0.07 | 0.52 ± 0.03 | 61.3 ± 2.5 |
| PI-FH | 2.05 ± 0.07 | 0.34 ± 0.03 | 51.6 ± 2.1 |
| Films | Tensile Strength/MPa | Tensile Modulus/GPa | Elongation at Break (%) |
|---|---|---|---|
| PI-FP | 122.5 ± 3.7 | 2.86 ± 0.08 | 5.1 ± 1.4 |
| PI-FO | 109.6 ± 3.4 | 2.42 ± 0.07 | 4.6 ± 1.2 |
| PI-FH | 88.4 ± 3.1 | 2.11 ± 0.05 | 4.1 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, T.; Dai, H.; Wang, M.; Xue, R.; Chen, J.; Liu, D. Preparation and Characterization of Intrinsic Low-κ Polyimide Films. Polymers 2021, 13, 4174. https://doi.org/10.3390/polym13234174
Sun Y, Li T, Dai H, Wang M, Xue R, Chen J, Liu D. Preparation and Characterization of Intrinsic Low-κ Polyimide Films. Polymers. 2021; 13(23):4174. https://doi.org/10.3390/polym13234174
Chicago/Turabian StyleSun, Yu, Tao Li, Haiyang Dai, Manman Wang, Renzhong Xue, Jing Chen, and Dewei Liu. 2021. "Preparation and Characterization of Intrinsic Low-κ Polyimide Films" Polymers 13, no. 23: 4174. https://doi.org/10.3390/polym13234174
APA StyleSun, Y., Li, T., Dai, H., Wang, M., Xue, R., Chen, J., & Liu, D. (2021). Preparation and Characterization of Intrinsic Low-κ Polyimide Films. Polymers, 13(23), 4174. https://doi.org/10.3390/polym13234174





