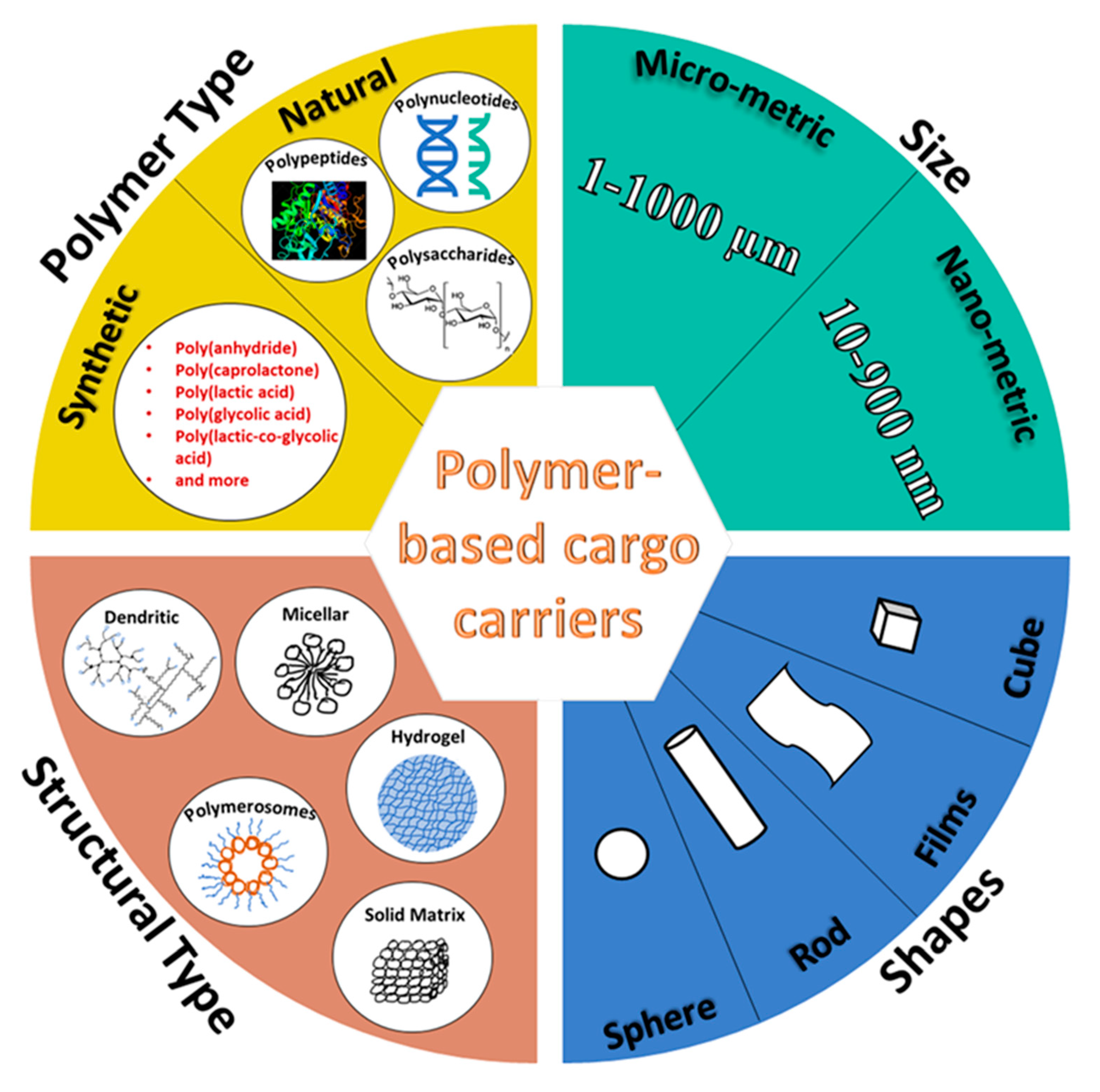
Designing Natural Polymer-Based Capsules and Spheres for Biomedical Applications—A Review
Abstract
:1. Introduction
| Polymer Class | Polymer |
|---|---|
| Polysaccharides | Cellulose |
| Cellulose derivatives | |
| Alginate | |
| Gellan gum | |
| Pectin | |
| Gum Arabica | |
| Gaur gum | |
| Locust bean gum | |
| Starch | |
| Carrageenan | |
| Chitin | |
| Chitosan | |
| Xanthan gum | |
| Shellac | |
| Dextran | |
| Cashew gum | |
| Pullulan | |
| Polypeptides | Gelatin |
| Bovine serum albumin | |
| Human serum albumin | |
| Egg albumin | |
| Casein | |
| Collagen | |
| Keratin | |
| Elastin | |
| Resilin | |
| Soy protein | |
| Gliadin | |
| Hyaluronic acid | Hyaluronic acid |
| Phospholipids | Liposomes |
| Polynucleotides | Ribonucleic acid |
| Deoxyribonucleic acid |
2. Chemical Aspects of Designing Natural Polymer-Based Spherical Capsules and Spheres
2.1. Function-Specific Carrier Design
2.1.1. Structural Configurations and Carrier Materials
Desired Functions
2.1.2. Modes of Encapsulation
2.2. Synthesis Approaches and Mechanisms of Carrier Formation
2.2.1. Solid Templating
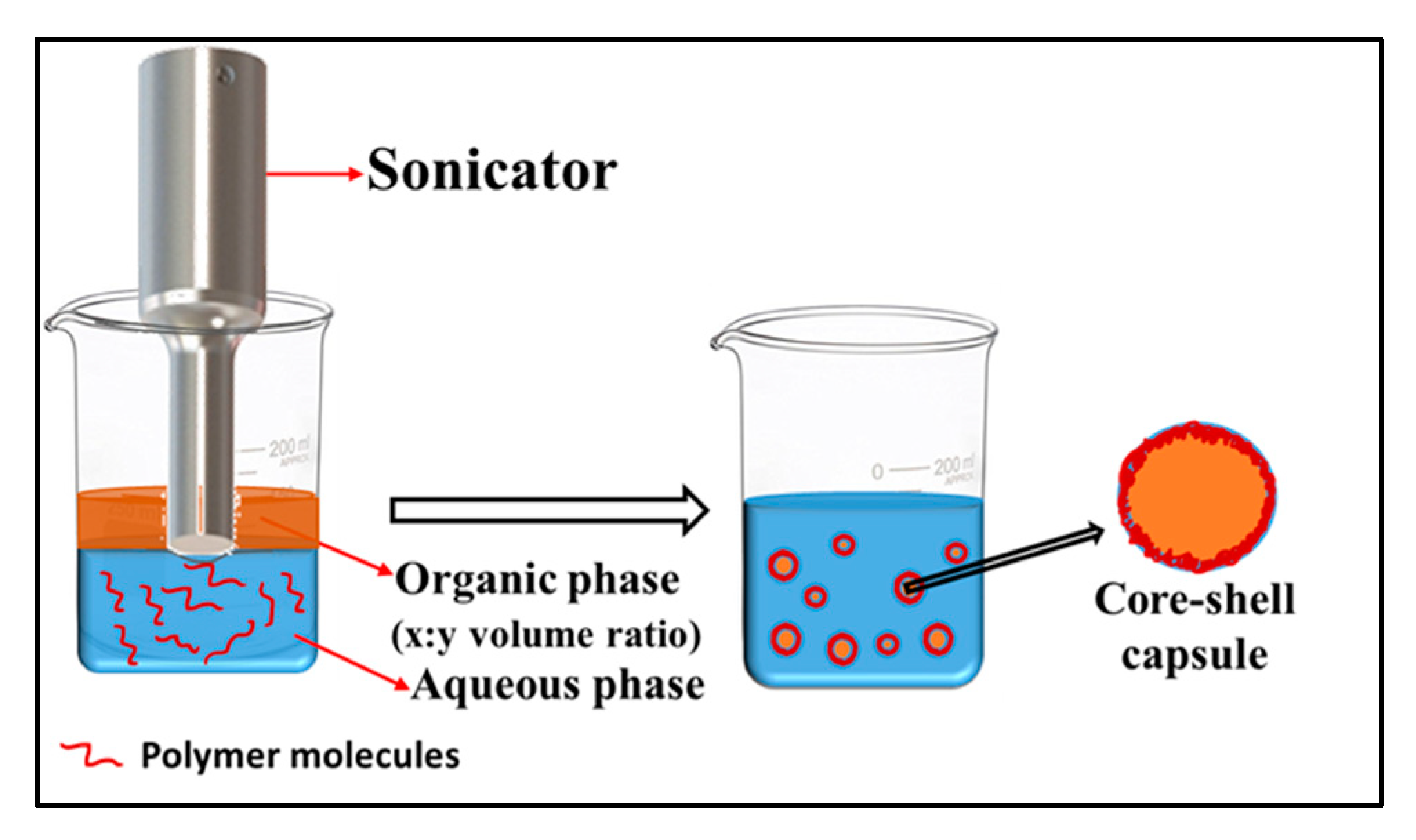
2.2.2. Emulsion Templating
Emulsion–Diffusion–Evaporation
Emulsion-Coacervation
Emulsion-Interfacial Deposition
Emulsion-Spray Drying
2.2.3. Other Techniques
Coextrusion–Coacervation
Microfluidics
| Polymer 1/Polyelectrolyte 1 | Polymer 2/Polyelectrolyte 2 | Solid Template/Core | Template Dissolving Agent | Template/Core Synthesis Method | Shell-Type and Deposition | APC and Location | EE (%) | Capsule Surface Charge (mV) | Template/Core Size and Capsule Size | Core-Polymer and Polymer–Polymer Interactions | Crosslinking between Core and Layers | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BSA polycation (+5.05 mV) | Alginate polyanion (−24.6 mV) | Template: amine modified-SiO2 (+11.8 mV) | NH4F/HF | Stöber process | Multiwalled (seven alternate layers of BSA and Alginate) | Betamethasone disodium phosphate (BSP); shell; post-synthesis introduction | 56% | +5.05 mV | ~128 nm; ~170 to188 nm | Non-covalent (hydrogen bonding, electrostatic, van der Waals, and hydrophobic interaction) | - | [17] |
| BSA | Tannic Acid | Template: CaCO3. Core: BSA | Ethyl-enediaminetetraacetic acid trisodium salt (EDTA) | Co-precipitation | Multiwalled (six bilayers of BSA/Tannic Acid) | Tetramethylrhodamine-isothiocyanate labeled BSA; core; co-precipitated with the solid template during synthesis | - | (−30 ± 1.9) mV | - | Hydrogen bonding | - | [33] |
| Silk fibroin (anionic) | Aminopropyl triethoxysilane (APTES) (cationic) | Template: polystyrene | N,N-dimethyl formamide (DMF) | - | Multiwalled (nine layers of Silk fibroin) | chlorin e6 (Ce6) and doxorubicin (DOX); shell; post-synthesis introduction | DOX = 80% Ce6 = 90% | − | ~150 to 250 nm; ~230 nm | Electrostatic interactions | - | [8] |
| Silk fibroin | - | Solid core: poly(lactic-co-glycolic acid) | - | Single emulsion-solvent evaporation method | Single layer of silk fibroin | Simvastatin; Core; in-synthesis encapsulation | 59.4% to 70.3% | - | ~15.3 μm | Covalent bonding | Chemical crosslinking by Glutaraldehyde | [24] |
| calcium cross-linked k-carrageenan | k-carrageenan and chitosan polyelectrolyte complex | Template: CaCO3. Core: BSA | EDTA | Co-precipitation | Multiwalled | Curcumin; after core synthesis, before layer assembly | 6.25 to 8% | - | - | Electrostatic interactions | - | [100] |
| Gelatin A | (−)-epigallocatechin gallate (EGCG) | Template: MnCO3 | EDTA | - | Multiwalled (four layers) | - | - | −25 mV | ~4.0 μm; ~4–5 μm | Non-covalent (hydrophobic and electrostatic interactions) | - | [36] |
| Chitosan polycation | Alginate Polyanion | Template: E. coli cells (−32.70 ± 3.2 mV) | Lysis buffer (0.1% Triton X-100, 2 mM EDTA in 10 mM Tris-pH8) | Cultured | Multiwalled (four bilayers of chitosan–alginate) | - | - | (−36.08 ± 8.8) mV | - | Electrostatic interactions | - | [56] |
| Thiolated-chitosan polycation | Thiolated-hyaluronic acid polyanion | Template: CaCO3 −15.8 mV | EDTA | Co-precipitation | Multiwalled (four bilayers of chitosan/hyaluronic acid) | BSA and Dextran; Core; Co-precipitated with the solid template during synthesis | 20.2% | −11 to −25 mV | 3.0 µm; 4 to 6 µm | Covalent interactions by disulfide bonding | Enzymatic crosslinking using horseradish peroxidase and tyramine hydrochloride | [55] |
| Chitosan | - | Solid; Ca-alginate | - | Extrusion | A single layer of chitosan | Insulin and probiotic cells; post-synthesis | - | - | -- | - | Electrostatic interactions | [101] |
| Polymer Matrix | Porogen | Preparation Method | Porogen Removal Process | Crosslinkers; Precipitants | APC | Pore Size | Sphere Size | Ref. |
|---|---|---|---|---|---|---|---|---|
| Silk fibroin | Ice crystals ~(−195 °C) | Microinjection into liquid nitrogen and freeze-drying | Sublimation | - | Basic fibroblast growth factor (bFGF) | 1.5–7.0 µm | 95 µm to 260 µm | [39] |
| Ice crystals (−20 °C) | w/o emulsion, rapid cooling, and freeze-drying | Sublimation | - | Strontium | (20 ± 5) to (34.8 ± 6.5) μm | - | [26] | |
| Microinjection into liquid nitrogen and freeze-drying | Sublimation | Ethanol-assisted precipitation | - | 0.3–10.7 μm | 208.4–727.3 μm | [102] | ||
| Chitosan | Ice crystals (−20 °C) | w/o emulsion, low temperature, thermally-induced phase separation, and pH-assisted coacervation | Drying under vacuum | - | - | 20–50 μm | ca. 150 μm | [74] |
| Ice crystals ~(−195 °C) | Microinjection into liquid nitrogen and freeze-drying | Sublimation | Saturated sodium tripolyphosphate (STPP) crosslinker | - | <30 μm | <400 μm | [96] | |
| Chitosan/poly(L-glutamic acid) (PLGA) polyelectrolyte complex | Ice crystals (−20 °C) | w/o emulsion, low temperature, thermally-induced phase separation | Drying | - | - | (47.5 ± 5.4) μm | 250 μm | [75] |
| Collagen/cellulose | Solid polystyrene | w/o emulsion | Washing with acetone | n-butyl al-cohol as precipitant | BSA | ~198.5 nm | 8–12 μm | [62] |
| Alginate | NaCl | w/o emulsion, freeze drying | - | Calcium chloride as crosslinker | - | 200–300 nm | ~158 μm | [73] |
| Soy protein | CaCO3 | Solid templating over porogen by incubation | Dissolution by EDTA | Transglutaminase as crosslinker | - | - | 3–12 μm | [61] |
| Silk sericin and hydroxylapatite | Silk sericin | Nucleation and growth of hydroxyapatite, induced by the sericin template in simulated body fluid | - | - | Doxorubicin | - | 1–3 μm | [103] |
| Polymer Shell | Core & Type | Template & Organic Solvent | Emulsion Type | Method | APC & Location | Interactions | Crosslinkers; Stabilizers; & Surfactants | Surface Charge | Size | Encapsulation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human serum albumin (HSA) | Lauroglycol 90; oily | Lauroglycol 90; Acetone | o/w single emulsion | Diffusion-evaporation | Exemestane and hesperetin; core | Electrostatic interactions | None; 1:1w/w poloxamer/Tween 80 mixture; benzalkonium chloride | 20.7 ± 1.26 mV | 172.4 ± 8.6 nm | 95–98% | [10] |
| Folic acid-functionalized HSA | Oily; dodecane | Dodecane | o/w single emulsion | Ultrasonic emulsification | - | Oxidative crosslinking | - | −20 mV | ~440 nm | - | [22] |
| Wheat germ agglutinin-functionalized HSA | Biocompatible plant oils; oily | Almond oil, rapeseed oil, olive oil, and linseed oil | o/w single emulsion | Ultrasonic emulsification | - | Oxidative crosslinking | - | −12.4 ± 9.4 mV | (662.1 ± 7.6) nm to (862.2 ± 59.5 nm) | - | [104] |
| Fluorescently tagged bovine serum albumin (BSA) shell; Shell filled with PLGA and unsaturated fatty linoleic acid | Lecithin; aqueous | Dichloromethane and ethanol | w/o/w double emulsion | Double emulsion–evaporation | lipophilic paclitaxel in the oily shell and hydrophilic transcription factor p53 in the aqueous core | - | Pluronic F-68 & Lecithin | −36.4 mV | ~180 nm | - | [25] |
| BSA | Soya bean oil; oily | Soya bean oil | o/w single emulsion | Ultrasonic emulsification | Ribonucleic acid (RNA); shell | Oxidative crosslinking | - | −40 meV | (0.5 μm to 2.5 μm) | ~60% | [16] |
| Polyvinyl alcohol (PVA) functionalized-BSA | 0 meV | ||||||||||
| Polyethyleneimine (PEI) functionalized-BSA | +20 meV | ||||||||||
| Silk fibroin | Sodium alginate; solid | Paraffin oil | w/o single emulsion | Emulsion-coacervation | - | Chemical crosslinking using glutaraldehyde | Span 80 | - | Avg. 141.839 μm. | - | [14] |
| Collagen and PLGA layers | Hollow | Dichloromethane | o/w single emulsion | Emulsion–evaporation | MnO2 nanoparticles; shell | Carbodiimide initiated covalent crosslinking | Crosslinking facilitated by N-(3-Dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride (EDC), N-Hydroxysuccinimide (NHS); stabilizer: polyvinyl alcohol (PVA) | - | - | - | [18] |
| Anti-epidermal growth factor receptor (EGFR) modified-BSA | Dodecane; oily | dodecane | o/w single emulsion | Ultrasonic emulsification | Gemcitabine; shell | Oxidative crosslinking | - | - | ~1.1 μm | 30% | [20] |
| Whey protein isolate (WPI) | Sunflower oil; solid | Sunflower oil | o/w single emulsion | Spray- and freeze-drying | Vitamin E; core | - | - | - | ~145.3 µm | 89.3% | [12] |
| Gelatin | Citric acid; solid | Dichloromethane and ethanol | o/w single emulsion | Spray drying | Itraconazole; core | Physical crosslinking | - | - | - | - | [21] |
| Tetramethylrhodamine-isothiocyanate labeled-BSA, tannic acid, and BSA layers | Sunflower oil; oily | Sunflower oil | o/w single emulsion | Emulsion-coacervation | 3,4,9,10-tetra-(hectoxy-carbonyl)-perylene (THCP); core | Hydrogen bonding between the shell layers | - | (−30 ± 1.9) mV | - | - | [33] |
| Chitosan | Soybean oil, oily | Soybean oil; benzyl benzoate | o/w single emulsion | Emulsion-microfluidic | Tea tree oil; core | Covalent interactions by chemical crosslinking | Terephthalaldehyde (TPA) | - | ~106 μm | 19.5–49.3% | [105] |
| Gelatin and gum arabica | Soybean oil; aqueous | Soybean oil | w/o/w double emulsion | Emulsion-complex coacervation | Sucralose; core | Covalent interactions | Lecithin | 81 to 113 μm | 43.04 to 89.44% | [106] | |
| Folic acid-modified hyaluronic acid | Ethyl acetate; oily | Ethyl acetate | o/w single emulsion | Ultrasonication | Curcumin; core | Oxidative crosslinking | - | - | 400 to 600 nm | 91.3 to 93.2% | [107] |
| Soy protein and gum arabica | (80 vol% NEOBEE M5 + 20 vol% limonene); oily | 80 vol% NEOBEE M5 + 20 vol% limo-nene | o/w single emulsion | Complex coacervation | - | Heat-induced gelation crosslinking | - | - | - | - | [108] |
| Pea protein isolate and sugar beat pectin | Hemp seed oil; oily | Hemp seed oil | o/w single emulsion | Complex coacervation, followed by spray-drying | Hempseed oil | pH-induced crosslinking | - | - | (12.80 ± 2.17) to (23.70 ± 1.23) μm | (79.65 ± 5.99) to (94.42 ± 6.63)% | [109] |
3. Natural Polymer-Based Capsule Characterization
3.1. General Characteristics
3.1.1. Size
3.1.2. Stability
3.1.3. Moisture Content
3.1.4. Surface Charge
3.1.5. Encapsulation Efficiency
3.1.6. Drug-Loading Capacity
3.1.7. Cytotoxicity
3.1.8. Blood Compatibility
3.1.9. Flowability
3.1.10. Pore Size and Porosity
3.2. Function-Specific Characteristics
3.2.1. Drug Release and Kinetics
3.2.2. Swelling Ratio
3.2.3. Cell Survival Number
3.2.4. In-Vivo Bioavailability
3.2.5. Dissolution Profile of the Capsules
4. Recent Advances in Protein-Based Spherical Capsules towards Biomedical Applications
4.1. Liquid-Core Protein-Shell Capsules
4.2. Spherical Protein Capsules with a Solid Core
4.3. Porous/Hollow Core Protein Capsules
4.4. Multiwalled Core–Shell Protein Capsules
| Protein | Shell Composition | Core Type | Shell-Bound API | Core API | Biomedical Function | Type of Therapy | Ref. |
|---|---|---|---|---|---|---|---|
| Albumins | BSA | Liquid, organic (soybean oil) | RNA | - | Controlled release of RNA and its protection from the outer cellular environment | Gene expression and function | [16] |
| PVA and PEI functionalized-BSA protein | Liquid, organic (soybean oil) | RNA | - | Targeted delivery of RNA to the cell nucleus, controlled release, and protection from the outer cellular environment | Gene expression and function | [35] | |
| Anti-EGFR-modified BSA | Liquid, organic (dodecane) | Gemcitabine | - | Sustained-release of Gemcitabine and EGFR blocking | Pancreatic-cancer therapy | [20] | |
| FITC-BSA bound liquid organic shell filled with PLGA-linolic acid | Liquid Aqueous | Paclitaxel | Transcription factor p53 | Sustained-release synergistic apoptotic effect of hydrophilic and hydrophobic drugs on HeLa cells | Cancer theragnostic | [25] | |
| Multiwalled, BSA polycation–alginate polyanion layered alternatively | Hollow | Betamethasone disodium phosphate (BSP) | - | Sustained-release of BSP having metabolic, immunosuppressive, and anti-inflammatory activity | Rheumatoid arthritis, Crohn’s disease, etc. | [17] | |
| Multiwalled, BSA-Tannic acid layered alternatively | Solid, hydrophilic tetramethylrhodamine-isothiocyanate labeled BSA (TRITC-BSA) | - | TRITC-BSA | - | - | [33] | |
| Multiwalled, BSA-Tannic acid layered alternatively | Liquid, organic (sunflower oil) | TRITC-BSA | 3,4,9,10-tetra-(hectoxy-carbonyl)-perylene (THCP) | Co-encapsulation of hydrophobic and hydrophilic drugs for sustained-release and their protection by polyphenol Tannic Acid | All types of therapies | [33] | |
| 3-aminophenylboronic acid functionalized-HSA | Liquid, organic | - | Exemestane and Hesperetin | Cell-specific internalization and Targeted delivery into MCF-7 cell lines and sustained-release | Breast-cancer therapy | [10] | |
| Folic acid-functionalized HSA | Liquid, organic (dodecane) | Folic acid | - | Cell-specific internalization and Targeted delivery into folic-receptor macrophages | Rheumatoid arthritis | [22] | |
| Whey Protein Isolate (WPI) | WPI | Solid Hydrophobic (sunflower oil) | - | vitamin E ((+)-α-tocopherol) | Enhanced bioavailability of water-insoluble vitamin E | Nutritional therapy | [12] |
| Collagen | MnO2 functionalized-collagen-PLGA | Hollow | - | - | Prevention of oxidative stress-induced protein-, lipid- or DNA damage and cell apoptosis | Cancer therapy, cardiovascular and neurological disorders treatment | [18] |
| Silk Fibroin | Silk fibroin protein | Solid Hydrophilic (alginate) | Adriamycin hydrochloride | - | Transcatheter arterial chemoembolizing by the microcapsules and controlled release of adriamycin hydrochloride | Liver cancer therapy | [14] |
| Silk fibroin protein | Solid Hydrophobic (PLGA) | - | Simvastatin | sustained-release of cholesterol-reducing and osteoinductive simvastatin | Bone regeneration | [24] | |
| Multiwalled, silk fibroin-APTES layered alternatively | Hollow | chlorin e6 (Ce6) and doxorubicin (DOX) | - | Sustained-release of anti-tumor drug DOX and photosensitizer Ce6 | Chemophototherapy | [8] | |
| Zein | Citric acid-modified zein | Solid Hydrophilic (alginate) | - | Lactobacillus acidophilus | Protection of probiotic L. acidophilus from the gastric environment | Nutritional therapy | [11] |
| Gelatin | Gelatin | Solid hydrophilic (citric acid) | - | Itraconazole | Enhanced bioavailability of water-insoluble itraconazole | Treatment of mycotic infections | [21] |
| Hyaluronic acid-graft gelatin hydrophobic shell embedding SPIO | Hollow (hydrophilic) | - | - | Chondrocyte cells 3D-culture platforms to form cartilage tissue-mimicking pellets, magnetic field, and magnetic stress-induced gene expression | Tissue repair (correction of articular cartilage damage) | [23] | |
| Multiwalled gelatin–epigallocatechin gallate (EGCG) LbL | Hollow | - | - | EGCG layers introduce antioxidant properties to the microcapsules to prolong the lifetime and enhance the effectiveness of encapsulated APIs | Cancer therapy and more | [36] |
| Protein | Composition | Biomedical Cargo | Biomedical Function | Type of Therapy | Ref. |
|---|---|---|---|---|---|
| Collagen | Collagen microspheres | Recombinant human vascular endothelial growth factor (rhVEGF) | Sustained-release of signal protein rhVEGF | Cardiac muscle repair | [28] |
| Steroidal saponins | Sustained-release of Steroidal saponins | Osteogenesis and bone regeneration | [133] | ||
| Oligodendrocyte progenitor cells (OPC) | Culturing OPC and their delivery to lesion-affected tissue for the repair of the neurite myelin sheath | Tissue regeneration | [13] | ||
| Mesenchymal stem cells, mesenchymal stromal cells, osteoarthritis chondrocytes, and neuroblastoma cells | 3D cell culture platform for stem cell culture, differentiation, and delivery | Stem cell therapy | [134,135] | ||
| Bone marrow mesenchymal stromal cells | Integration into platelet-rich blood clots and implantation at the nonunion lesion site | Bone regeneration for nonunion fractures | [136,137] | ||
| Silk Fibroin | Porous silk fibroin (SF) microspheres | Basic fibroblast growth factor (bFGF) | Sustained-release of bFGF and lowering of biodegradability | Tissue repair | [39] |
| Strontium loaded porous SF microspheres | Strontium and mesenchymal stem cell (MSC) | Sustained-release of osteogenic strontium and the culture of MSC | Bone regeneration | [26] |
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkman, J.; Long, D.M. The use of silicone rubber as a carrier for prolonged drug therapy. J. Surg. Res. 1964, 4, 139–142. [Google Scholar] [CrossRef]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Gregoriadis, G. Drug entrapment in liposomes. FEBS Lett. 1973, 36, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Kramer, P.A. Albumin Microspheres as Vehicles for Achieving Specificity in Drug Delivery. J. Pharm. Sci. 1974, 63, 1646–1647. [Google Scholar] [CrossRef]
- Gurny, R.; Peppas, N.A.; Harrington, D.D.; Banker, G.S. Development of Biodegradable and Injectable Latices for Controlled Release of Potent Drugs. Drug Dev. Ind. Pharm. 1981, 7, 1–25. [Google Scholar] [CrossRef]
- Zheng, H.; Duan, B.; Xie, Z.; Wang, J.; Yang, M. Inventing a facile method to construct Bombyx mori (B. mori) silk fibroin nanocapsules for drug delivery. RSC Adv. 2020, 10, 28408–28414. [Google Scholar] [CrossRef]
- Cheng, C.; Teasdale, I.; Brüggemann, O. Stimuli-Responsive Capsules Prepared from Regenerated Silk Fibroin Microspheres. Macromol. Biosci. 2014, 14, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.; Hany, M.; Mokhtar, S.; Helmy, M.W.; Elkodairy, K.A.; Elzoghby, A.O. Boronic-targeted albumin-shell oily-core nanocapsules for synergistic aromatase inhibitor/herbal breast cancer therapy. Mater. Sci. Eng. C 2019, 105, 110099. [Google Scholar] [CrossRef]
- Laelorspoen, N.; Wongsasulak, S.; Yoovidhya, T.; Devahastin, S. Microencapsulation of Lactobacillus acidophilus in zein–alginate core–shell microcapsules via electrospraying. J. Funct. Foods 2014, 7, 342–349. [Google Scholar] [CrossRef]
- Parthasarathi, S.; Anandharamakrishnan, C. Enhancement of oral bioavailability of vitamin E by spray-freeze drying of whey protein microcapsules. Food Bioprod. Process. 2016, 100, 469–476. [Google Scholar] [CrossRef]
- Yao, L.; Phan, F.; Li, Y. Collagen microsphere serving as a cell carrier supports oligodendrocyte progenitor cell growth and differentiation for neurite myelination in vitro. Stem Cell Res. Ther. 2013, 4, 109. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wei, R.; Huang, X.; Wang, F.; Chen, Z. Synthesis and assessment of sodium alginate-modified silk fibroin microspheres as potential hepatic arterial embolization agent. Int. J. Biol. Macromol. 2020, 155, 1450–1459. [Google Scholar] [CrossRef]
- Ortiz, M.; Jornada, D.S.; Pohlmann, A.R.; Guterres, S.S. Development of Novel Chitosan Microcapsules for Pulmonary Delivery of Dapsone: Characterization, Aerosol Performance, and In Vivo Toxicity Evaluation. AAPS PharmSciTech 2015, 16, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Shimanovich, U.; Tkacz, I.D.; Eliaz, D.; Cavaco-Paulo, A.; Michaeli, S.; Gedanken, A. Encapsulation of RNA Molecules in BSA Microspheres and Internalization into Trypanosoma Brucei Parasites and Human U2OS Cancer Cells. Adv. Funct. Mater. 2011, 21, 3659–3666. [Google Scholar] [CrossRef]
- Mashoofnia, A.; Mohamadnia, Z.; Kompany-Zareh, M. Application of Multivariate and Spectroscopic Techniques for Investigation of the Interactions between Polyelectrolyte Layers in Layer-by-Layer Assembled pH-Sensitive Nanocapsules. Macromol. Chem. Phys. 2021, 222, 2100107. [Google Scholar] [CrossRef]
- Tapeinos, C.; Larrañaga, A.; Sarasua, J.-R.; Pandit, A. Functionalised collagen spheres reduce H2O2 mediated apoptosis by scavenging overexpressed ROS. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Y.; Nie, W.; Song, L.; Li, J.; Yang, B. Fabrication of uniform “smart” magnetic microcapsules and their controlled release of sodium salicylate. J. Mater. Chem. B 2013, 1, 4331. [Google Scholar] [CrossRef]
- Grinberg, O.; Gedanken, A.; Mukhopadhyay, D.; Patra, C.R. Antibody modified Bovine Serum Albumin microspheres for targeted delivery of anticancer agent Gemcitabine. Polym. Adv. Technol. 2013, 24, 294–299. [Google Scholar] [CrossRef]
- Li, D.X.; Park, Y.-J.; Oh, D.H.; Joe, K.H.; Lee, J.H.; Yeo, W.H.; Yong, C.S.; Choi, H.-G. Development of an itraconazole-loaded gelatin microcapsule with enhanced oral bioavailability: Physicochemical characterization and in-vivo evaluation. J. Pharm. Pharmacol. 2010, 62, 448–455. [Google Scholar] [CrossRef]
- Rollett, A.; Reiter, T.; Nogueira, P.; Cardinale, M.; Loureiro, A.; Gomes, A.; Cavaco-Paulo, A.; Moreira, A.; Carmo, A.M.; Guebitz, G.M. Folic acid-functionalized human serum albumin nanocapsules for targeted drug delivery to chronically activated macrophages. Int. J. Pharm. 2012, 427, 460–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, K.-T.; Liu, T.-Y.; Chiang, M.-Y.; Chen, C.-Y.; Chang, S.-J.; Chen, S.-Y. Cartilage Tissue-Mimetic Pellets with Multifunctional Magnetic Hyaluronic Acid-Graft-Amphiphilic Gelatin Microcapsules for Chondrogenic Stimulation. Polymers 2020, 12, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, F.; Zhang, J.; Wang, J.; Du, B.; Huang, X.; Pang, L.; Zhou, Z. Silk fibroin-coated PLGA dimpled microspheres for retarded release of simvastatin. Colloids Surf. B Biointerfaces 2017, 158, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Wu, J.; Zhang, E.; Zhang, Y.; Qian, K. Biodegradable double nanocapsule as a novel multifunctional carrier for drug delivery and cell imaging. Int. J. Nanomed. 2015, 10, 4149–4157. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Wang, D.; Hu, F.; Li, X.; Zou, X.; Xie, J.; Zhou, Z. Strontium mineralized silk fibroin porous microcarriers with enhanced osteogenesis as injectable bone tissue engineering vehicles. Mater. Sci. Eng. C 2021, 128, 112354. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Cheng, H.W.; Cheung, K.M.C.; Chan, D.; Chan, B.P. Mesenchymal stem cell-collagen microspheres for articular cartilage repair: Cell density and differentiation status. Acta Biomater. 2014, 10, 1919–1929. [Google Scholar] [CrossRef]
- Nagai, N.; Kumasaka, N.; Kawashima, T.; Kaji, H.; Nishizawa, M.; Abe, T. Preparation and characterization of collagen microspheres for sustained release of VEGF. J. Mater. Sci. Mater. Med. 2010, 21, 1891–1898. [Google Scholar] [CrossRef]
- Zhi, Z.L.; Song, L.; Pickup, J.J. Nanolayer encapsulation of insulin-chitosan complexes improves efficiency of oral insulin delivery. Int. J. Nanomed. 2014, 9, 2127–2136. [Google Scholar] [CrossRef] [Green Version]
- Bruschi, M.L. (Ed.) Main mechanisms to control the drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: San Diego, CA, USA, 2015; pp. 37–62. [Google Scholar]
- Liu, T.; Dan, N.; Dan, W. The effect of crosslinking agent on sustained release of bFGF–collagen microspheres. RSC Adv. 2015, 5, 34511–34516. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Lomova, M.V.; Brichkina, A.I.; Kiryukhin, M.V.; Vasina, E.N.; Pavlov, A.M.; Gorin, D.A.; Sukhorukov, G.B.; Antipina, M.N. Multilayer Capsules of Bovine Serum Albumin and Tannic Acid for Controlled Release by Enzymatic Degradation. ACS Appl. Mater. Interfaces 2015, 7, 11732–11740. [Google Scholar] [CrossRef]
- Toublan, F.J.-J.; Boppart, S.; Suslick, K.S. Tumor Targeting by Surface-Modified Protein Microspheres. J. Am. Chem. Soc. 2006, 128, 3472–3473. [Google Scholar] [CrossRef]
- Shimanovich, U.; Eliaz, D.; Zigdon, S.; Volkov, V.; Aizer, A.; Cavaco-Paulo, A.; Michaeli, S.; Shav-Tal, Y.; Gedanken, A. Proteinaceous microspheres for targeted RNA delivery prepared by an ultrasonic emulsification method. J. Mater. Chem. B 2013, 1, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shutava, T.G.; Balkundi, S.S.; Lvov, Y.M. (−)-Epigallocatechin gallate/gelatin layer-by-layer assembled films and microcapsules. J. Colloid Interface Sci. 2009, 330, 276–283. [Google Scholar] [CrossRef]
- Van den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef] [PubMed]
- Sapkal, S.B.; Adhao, V.S.; Thenge, R.R.; Darakhe, R.A.; Shinde, S.A.; Shrikhande, V.N. Formulation and Characterization of Solid Dispersions of Etoricoxib Using Natural Polymers. Turk. J. Pharm. Sci. 2020, 17, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wang, L.; Niu, L.; Lin, J.; Huang, Q.; Jiang, X.; Li, M. Porous Silk Fibroin Microspheres Sustainably Releasing Bioactive Basic Fibroblast Growth Factor. Materials 2018, 11, 1280. [Google Scholar] [CrossRef] [Green Version]
- Gentilini, R.; Munarin, F.; Bloise, N.; Secchi, E.; Visai, L.; Tanzi, M.C.; Petrini, P. Polysaccharide-based hydrogels with tunable composition as 3D cell culture systems. Int. J. Artif. Organs 2018, 41, 213–222. [Google Scholar] [CrossRef]
- De Oliveira, A.C.; Sabino, R.M.; Souza, P.R.; Muniz, E.C.; Popat, K.C.; Kipper, M.J.; Zola, R.S.; Martins, A.F. Chitosan/gellan gum ratio content into blends modulates the scaffolding capacity of hydrogels on bone mesenchymal stem cells. Mater. Sci. Eng. C 2020, 106, 110258. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Yu, H.; Feng, G.; Zhuang, L.; Xi, W.; Ma, M.; Chen, J.; Gu, N.; Zhang, Y. High-Performance Poly(lactic-co-glycolic acid)-Magnetic Microspheres Prepared by Rotating Membrane Emulsification for Transcatheter Arterial Embolization and Magnetic Ablation in VX 2 Liver Tumors. ACS Appl. Mater. Interfaces 2017, 9, 43478–43489. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, G.-H.; Ro, J.; Kuh, H.-J.; Kwak, B.-K.; Lee, J. Recoverability of freeze-dried doxorubicin-releasing chitosan embolic microspheres. J. Biomater. Sci. Polym. Ed. 2013, 24, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Katsumori, T.; Miura, H.; Arima, H.; Hino, A.; Tsuji, Y.; Masuda, Y.; Nishimura, T. Tris-acryl gelatin microspheres versus gelatin sponge particles in uterine artery embolization for leiomyoma. Acta Radiol. 2017, 58, 834–841. [Google Scholar] [CrossRef]
- Sommer, C.M.; Do, T.D.; Schlett, C.L.; Flechsig, P.; Gockner, T.L.; Kuthning, A.; Vollherbst, D.F.; Pereira, P.L.; Kauczor, H.U.; Macher-Göppinger, S. In vivo characterization of a new type of biodegradable starch microsphere for transarterial embolization. J. Biomater. Appl. 2018, 32, 932–944. [Google Scholar] [CrossRef]
- Choi, H.; Choi, B.; Yu, B.; Li, W.; Matsumoto, M.M.; Harris, K.R.; Lewandowski, R.J.; Larson, A.C.; Mouli, S.K.; Kim, D.-H. On-demand degradable embolic microspheres for immediate restoration of blood flow during image-guided embolization procedures. Biomaterials 2021, 265, 120408. [Google Scholar] [CrossRef]
- Guo, L.; Qin, S. Studies on preparations and properties of drug-eluting embolization microspheres made from oxidated alginate and carboxymethyl chitosan. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 844–849. [Google Scholar] [CrossRef]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Piñón-Segundo, E.; Llera-Rojas, V.G.; Leyva-Gómez, G.; Urbán-Morlán, Z.; Mendoza-Muñoz, N.; Quintanar-Guerrero, D. The emulsification-diffusion method to obtain polymeric nanoparticles. In Nanoscale Fabrication, Optimization, Scale-Up and Biological Aspects of Pharmaceutical Nanotechnology; Elsevier: Kidlington, UK, 2018; pp. 51–83. [Google Scholar]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Lensen, D.; Vriezema, D.M.; van Hest, J.C.M. Polymeric Microcapsules for Synthetic Applications. Macromol. Biosci. 2008, 8, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Front. 2021, 2, 1–17. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Baggett, J.; Godin, B.; Liu, X.; Kharlampieva, E. Hydrogen-Bonded Multilayers of Silk Fibroin: From Coatings to Cell-Mimicking Shaped Microcontainers. ACS Macro Lett. 2012, 1, 384–387. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, H.; Wang, J.; Fang, Q.; Peng, Z. Enzymatically Disulfide-Crosslinked Chitosan/Hyaluronic Acid Layer-by-Layer Self-Assembled Microcapsules for Redox-Responsive Controlled Release of Protein. ACS Appl. Mater. Interfaces 2018, 10, 33493–33506. [Google Scholar] [CrossRef] [PubMed]
- Yitayew, M.Y.; Tabrizian, M. Hollow Microcapsules Through Layer-by-Layer Self-Assembly of Chitosan/Alginate on E. coli. MRS Adv. 2020, 5, 2401–2407. [Google Scholar] [CrossRef]
- Szafraniec-Szczęsny, J.; Janik-Hazuka, M.; Odrobińska, J.; Zapotoczny, S. Polymer Capsules with Hydrophobic Liquid Cores as Functional Nanocarriers. Polymers 2020, 12, 1999. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajpai, J.; Tiwari, A.; Bajpai, A.K. Designing casein-coated iron oxide nanostructures (CCIONPs) as superparamagnetic core–shell carriers for magnetic drug targeting. Prog. Biomater. 2015, 4, 39–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, P.-H.; Neoh, K.-G.; Kang, E.-T.; Wang, W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials 2008, 29, 1412–1421. [Google Scholar] [CrossRef]
- Deng, C.; Dong, W.-F.; Adalsteinsson, T.; Ferri, J.K.; Sukhorukov, G.B.; Möhwald, H. Solvent-filled matrix polyelectrolyte capsules: Preparation, structure and dynamics. Soft Matter 2007, 3, 1293. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lan, T.; Wang, X.; Zhang, Y.; Jiang, L.; Sui, X. Preparation and characterization of soy protein microspheres using amorphous calcium carbonate cores. Food Hydrocoll. 2020, 107, 105953. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, J.; Zhang, L. Drugs adsorption and release behavior of collagen/bacterial cellulose porous microspheres. Int. J. Biol. Macromol. 2019, 140, 196–205. [Google Scholar] [CrossRef]
- Fan, J.-B.; Huang, C.; Jiang, L.; Wang, S. Nanoporous microspheres: From controllable synthesis to healthcare applications. J. Mater. Chem. B 2013, 1, 2222. [Google Scholar] [CrossRef]
- Yuan, W.; Cai, Y.; Chen, Y.; Hong, X.; Liu, Z. Porous microsphere and its applications. Int. J. Nanomed. 2013, 8, 1111–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Han, B.; Wang, Z.; Gao, C.; Peng, C.; Shen, J. Hollow chitosan-alginate multilayer microcapsules as drug delivery vehicle: Doxorubicin loading and in vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.D. Layer-by-layer hyaluronic acid/chitosan polyelectrolyte coated mesoporous silica nanoparticles as pH-responsive nanocontainers for optical bleaching of cellulose fabrics. Carbohydr. Polym. 2016, 146, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Sukhishvili, S.A.; Granick, S. Layered, Erasable Polymer Multilayers Formed by Hydrogen-Bonded Sequential Self-Assembly. Macromolecules 2002, 35, 301–310. [Google Scholar] [CrossRef]
- Manna, U.; Bharani, S.; Patil, S. Layer-by-Layer Self-Assembly of Modified Hyaluronic Acid/Chitosan Based on Hydrogen Bonding. Biomacromolecules 2009, 10, 2632–2639. [Google Scholar] [CrossRef] [PubMed]
- Liu, P. Stabilization of layer-by-layer engineered multilayered hollow microspheres. Adv. Colloid Interface Sci. 2014, 207, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Mishra, B. Multilayered membrane-controlled microcapsules for controlled delivery of isoniazid. Daru 2011, 19, 41–46. [Google Scholar]
- Pal, K.; Paulson, A.T.; Rousseau, D. Biopolymers in Controlled-Release Delivery Systems. In Handbook of Biopolymers and Biodegradable Plastics; Elsevier: London, UK, 2013; pp. 329–363. [Google Scholar]
- Mu, B.; Lu, C.; Liu, P. Disintegration-controllable stimuli-responsive polyelectrolyte multilayer microcapsules via covalent layer-by-layer assembly. Colloids Surf. B Biointerfaces 2011, 82, 385–390. [Google Scholar] [CrossRef]
- Wang, C.; Luo, W.; Li, P.; Li, S.; Yang, Z.; Hu, Z.; Liu, Y.; Ao, N. Preparation and evaluation of chitosan/alginate porous microspheres/Bletilla striata polysaccharide composite hemostatic sponges. Carbohydr. Polym. 2017, 174, 432–442. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, L.; Jung Poudel, A.; Li, J.; Zhou, P.; Gauthier, M.; Liu, H.; Wu, Z.; Yang, G. Porous chitosan microspheres as microcarriers for 3D cell culture. Carbohydr. Polym. 2018, 202, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Zhang, Y.; Yan, S.; Liu, Z.; He, S.; Cui, L.; Yin, J. Poly(l-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014, 10, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Moinard-Chécot, D.; Chevalier, Y.; Briançon, S.; Beney, L.; Fessi, H. Mechanism of nanocapsules formation by the emulsion–diffusion process. J. Colloid Interface Sci. 2008, 317, 458–468. [Google Scholar] [CrossRef]
- Trojanowska, A.; Nogalska, A.; Valls, R.G.; Giamberini, M.; Tylkowski, B. Technological solutions for encapsulation. Phys. Sci. Rev. 2017, 2, 171–202. [Google Scholar] [CrossRef]
- Grinstaff, M.W.; Suslick, K.S. Air-filled proteinaceous microbubbles: Synthesis of an echo-contrast agent. Proc. Natl. Acad. Sci. USA 1991, 88, 7708–7710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suslick, K.S.; Grinstaff, M.W. Protein microencapsulation of nonaqueous liquids. J. Am. Chem. Soc. 1990, 112, 7807–7809. [Google Scholar] [CrossRef]
- Panigrahi, R.; Srivastava, S.K. Ultrasound assisted synthesis of a polyaniline hollow microsphere/Ag core/shell structure for sensing and catalytic applications. RSC Adv. 2013, 3, 7808. [Google Scholar] [CrossRef]
- Rajabinejad, H.; Patrucco, A.; Caringella, R.; Montarsolo, A.; Zoccola, M.; Pozzo, P.D. Preparation of keratin-based microcapsules for encapsulation of hydrophilic molecules. Ultrason. Sonochem. 2018, 40, 527–532. [Google Scholar] [CrossRef]
- Wong, M.; Suslick, K.S. Sonochemically Produced Hemoglobin Microbubbles. MRS Proc. 1994, 372, 89. [Google Scholar] [CrossRef]
- Sharma, K.; Sadhanala, H.K.; Mastai, Y.; Porat, Z.; Gedanken, A. Sonochemically Prepared BSA Microspheres as Adsorbents for the Removal of Organic Pollutants from Water. Langmuir 2021, 37, 9927–9938. [Google Scholar] [CrossRef]
- Mutalikdesai, A.; Nassir, M.; Saady, A.; Hassner, A.; Gedanken, A. Sonochemically modified ovalbumin enhances enantioenrichment of some amino acids. Ultrason. Sonochem. 2019, 58, 104603. [Google Scholar] [CrossRef]
- Sharma, K.; Saady, A.; Jacob, A.; Porat, Z.; Gedanken, A. Entrapment and release kinetics study of dyes from BSA microspheres forming a matrix and a reservoir system. J. Mater. Chem. B 2020, 8, 10154–10161. [Google Scholar] [CrossRef]
- Saady, A.; Varon, E.; Jacob, A.; Shav-Tal, Y.; Fischer, B. Applying styryl quinolinium fluorescent probes for imaging of ribosomal RNA in living cells. Dyes Pigment. 2020, 174. [Google Scholar] [CrossRef]
- Gedanken, A. Preparation and Properties of Proteinaceous Microspheres Made Sonochemically. Chem. Eur. J. 2008, 14, 3840–3853. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Ferreira, H.; Azoia, N.G.; Shimanovich, U.; Freddi, G.; Gedanken, A.; Cavaco-Paulo, A. Insights on the mechanism of formation of protein microspheres in a biphasic system. Mol. Pharm. 2012, 9, 3079–3088. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.; Ferreira, H.; Cavaco-Paulo, A. Sonoproduction of Liposomes and Protein Particles as Templates for Delivery Purposes. Biomacromolecules 2011, 12, 3353–3368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suslick, K.S.; Grinstaff, M.W.; Kolbeck, K.J.; Wong, M. Characterization of sonochemically prepared proteinaceous microspheres. Ultrason. Sonochem. 1994, 1, S65–S68. [Google Scholar] [CrossRef]
- Abismaïl, B.; Canselier, J.; Wilhelm, A.; Delmas, H.; Gourdon, C. Emulsification by ultrasound: Drop size distribution and stability. Ultrason. Sonochem. 1999, 6, 75–83. [Google Scholar] [CrossRef]
- Shimanovich, U.; Bernardes, G.J.L.; Knowles, T.P.J.; Cavaco-Paulo, A. Protein micro- and nano-capsules for biomedical applications. Chem. Soc. Rev. 2014, 43, 1361–1371. [Google Scholar] [CrossRef] [Green Version]
- Atkin, R.; Davies, P.; Hardy, J.; Vincent, B. Preparation of Aqueous Core/Polymer Shell Microcapsules by Internal Phase Separation. Macromolecules 2004, 37, 7979–7985. [Google Scholar] [CrossRef] [Green Version]
- Dolçà, C.; Ferrándiz, M.; Capablanca, L.; Franco, E.; Mira, E.; López, F.; García, D. Microencapsulation of Rosemary Essential Oil by Co-Extrusion/Gelling Using Alginate as a Wall Material. J. Encapsulation Adsorpt. Sci. 2015, 5, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.-B.; Peng, C.-H.; Huang, F.; Kuang, J.; Yu, S.-L.; Dong, Y.-D.; Han, B.-S. Preparation and characterization of chitosan porous microcarriers for hepatocyte culture. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 509–515. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Mendes, A.C.; Baran, E.T.; Lisboa, P.; Reis, R.L.; Azevedo, H.S. Microfluidic Fabrication of Self-Assembled Peptide-Polysaccharide Microcapsules as 3D Environments for Cell Culture. Biomacromolecules 2012, 13, 4039–4048. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, W.J.; Zieringer, M.; Wagner, O.; Wilking, J.N.; Abbaspourrad, A.; Haag, R.; Weitz, D.A. Microfluidic synthesis of monodisperse porous microspheres with size-tunable pores. Soft Matter 2012, 8, 10636. [Google Scholar] [CrossRef]
- Paşcaləu, V.; Soritau, O.; Popa, F.; Pavel, C.; Coman, V.; Perhaita, I.; Borodi, G.; Dirzu, N.; Tabaran, F.; Popa, C. Curcumin delivered through bovine serum albumin/polysaccharides multilayered microcapsules. J. Biomater. Appl. 2016, 30, 857–872. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Liu, W.; Tromp, R.H. Microencapsulation of probiotics in multi-polysaccharide microcapsules by electro-hydrodynamic atomization and incorporation into ice-cream formulation. Food Struct. 2020, 25, 100147. [Google Scholar] [CrossRef]
- Qu, J.; Wang, L.; Hu, Y.; Wang, L.; You, R.; Li, M. Preparation of Silk Fibroin Microspheres and Its Cytocompatibility. J. Biomater. Nanobiotechnol. 2013, 4, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Shuai, Y.; Yang, S.; Li, C.; Zhu, L.; Mao, C.; Yang, M. In situ protein-templated porous protein–hydroxylapatite nanocomposite microspheres for pH-dependent sustained anticancer drug release. J. Mater. Chem. B 2017, 5, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Skoll, K.; Ritschka, M.; Fuchs, S.; Wirth, M.; Gabor, F. Characterization of sonochemically prepared human serum albumin nanocapsules using different plant oils as core component for targeted drug delivery. Ultrason. Sonochem. 2021, 76, 105617. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.-T.; Ju, X.-J.; Zhang, L.; Huang, X.-B.; Faraj, Y.; Liu, Z.; Wang, W.; Xie, R.; Deng, Y.; Chu, L.-Y. Chitosan microcapsule membranes with nanoscale thickness for controlled release of drugs. J. Membr. Sci. 2019, 590, 117275. [Google Scholar] [CrossRef]
- Rocha-Selmi, G.A.; Theodoro, A.C.; Thomazini, M.; Bolini, H.M.A.; Favaro-Trindade, C.S. Double emulsion stage prior to complex coacervation process for microencapsulation of sweetener sucralose. J. Food Eng. 2013, 119, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Zhong, S.; He, S.; Gao, Y.; Cui, X. Constructing of pH and reduction dual-responsive folic acid-modified hyaluronic acid-based microcapsules for dual-targeted drug delivery via sonochemical method. Colloid Interface Sci. Commun. 2021, 44, 100503. [Google Scholar] [CrossRef]
- Li, X.; van der Gucht, J.; Erni, P.; de Vries, R. Core–Shell Microcapsules from Unpurified Legume Flours. ACS Appl. Mater. Interfaces 2021, 13, 37598–37608. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Ohm, J.-B.; Chen, B.; Rao, J. Microencapsulation of hemp seed oil by pea protein isolate−sugar beet pectin complex coacervation: Influence of coacervation pH and wall/core ratio. Food Hydrocoll. 2021, 113, 106423. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothamasu, P.; Kanumur, H.; Ravur, N.; Maddu, C.; Parasuramrajam, R.; Thangavel, S. Nanocapsules: The weapons for novel drug delivery systems. BioImpacts 2012, 2, 71–81. [Google Scholar] [CrossRef]
- Palomo, M.E.; Ballesteros, M.P.; Frutos, P. Solvent and plasticizer influences on ethylcellulose-microcapsules. J. Microencapsul. 1996, 13, 307–318. [Google Scholar] [CrossRef]
- Dunne, M.; Corrigan, O.I.; Ramtoola, Z. Influence of particle size and dissolution conditions on the degradation properties of polylactide-co-glycolide particles. Biomaterials 2000, 21, 1659–1668. [Google Scholar] [CrossRef]
- Visscher, G.E.; Pearson, J.E.; Fong, J.W.; Argentieri, G.J.; Robison, R.L.; Maulding, H.V. Effect of particle size on thein vitro andin vivo degradation rates of poly(DL-lactide-co-glycolide) microcapsules. J. Biomed. Mater. Res. 1988, 22, 733–746. [Google Scholar] [CrossRef]
- Saravanan, M.; Rao, K.P. Pectin–gelatin and alginate–gelatin complex coacervation for controlled drug delivery: Influence of anionic polysaccharides and drugs being encapsulated on physicochemical properties of microcapsules. Carbohydr. Polym. 2010, 80, 808–816. [Google Scholar] [CrossRef]
- Jégat, C.; Taverdet, J.L. Stirring speed influence study on the microencapsulation process and on the drug release from microcapsules. Polym. Bull. 2000, 44, 345–351. [Google Scholar] [CrossRef]
- Kristmundsdóttir, T.; Ingvarsdóttir, K. Influence of emulsifying agents on the properties of cellulose acetate butyrate and ethylcellulose microcapsules. J. Microencapsul. 1994, 11, 633–639. [Google Scholar] [CrossRef]
- Valot, P.; Baba, M.; Nedelec, J.-M.; Sintes-Zydowicz, N. Effects of process parameters on the properties of biocompatible Ibuprofen-loaded microcapsules. Int. J. Pharm. 2009, 369, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, Y.; Meloan, C.E. Determination of Moisture. In Food Analysis; Springer: Boston, MA, USA, 1994; pp. 575–601. [Google Scholar]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbán, P.; Liptrott, N.J.; Bremer, S. Overview of the blood compatibility of nanomedicines: A trend analysis of in vitro and in vivo studies. WIREs Nanomed. Nanobiotechol. 2019, 11, e1546. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Biomaterials. In Nanotechnology Applications for Tissue Engineering; William Andrew: Oxford, UK, 2015; pp. 21–44. [Google Scholar]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Faisant, N.; Siepmann, J.; Richard, J.; Benoit, J.P. Mathematical modeling of drug release from bioerodible microparticles: Effect of gamma-irradiation. Eur. J. Pharm. Biopharm. 2003, 56, 271–279. [Google Scholar] [CrossRef]
- Lee, P.I. Modeling of drug release from matrix systems involving moving boundaries: Approximate analytical solutions. Int. J. Pharm. 2011, 418, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.L.; Peppas, N.A.; Boey, F.Y.C.; Venkatraman, S.S. Modeling of drug release from bulk-degrading polymers. Int. J. Pharm. 2011, 418, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Keraliya, R.A.; Patel, C.; Patel, P.; Keraliya, V.; Soni, T.G.; Patel, R.C.; Patel, M.M. Osmotic Drug Delivery System as a Part of Modified Release Dosage Form. ISRN Pharm. 2012, 2012, 528079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arifin, D.Y.; Lee, L.Y.; Wang, C.H. Mathematical modeling and simulation of drug release from microspheres: Implications to drug delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 1274–1325. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, H.; Baumans, V.; Brandt, C.J.W.M.; Boere, H.A.G.; Hesp, A.P.M.; van Lith, H.A.; Schurink, M.; Beynen, A.C. Blood sampling from the retro-orbital plexus, the saphenous vein and the tail vein in rats: Comparative effects on selected behavioural and blood variables. Lab. Anim. 2001, 35, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Iyer, C.; Kailasapathy, K.; Peiris, P. Evaluation of survival and release of encapsulated bacteria in ex vivo porcine gastrointestinal contents using a green fluorescent protein gene-labelled E. coli. LWT Food Sci. Technol. 2004, 37, 639–642. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J. Preparation and characterization of collagen microspheres for sustained release of steroidal saponins. Mater. Res. 2014, 17, 1644–1650. [Google Scholar] [CrossRef] [Green Version]
- Yeung, P.; Sin, H.S.; Chan, S.; Chan, G.C.F.; Chan, B.P. Microencapsulation of Neuroblastoma Cells and Mesenchymal Stromal Cells in Collagen Microspheres: A 3D Model for Cancer Cell Niche Study. PLoS ONE 2015, 10, e0144139. [Google Scholar] [CrossRef] [Green Version]
- Yeung, P.; Cheng, K.H.; Yan, C.H.; Chan, B.P. Collagen microsphere based 3D culture system for human osteoarthritis chondrocytes (hOACs). Sci. Rep. 2019, 9, 12453. [Google Scholar] [CrossRef]
- Wittig, O.; Romano, E.; González, C.; Diaz-Solano, D.; Marquez, M.E.; Tovar, P.; Aoun, R.; Cardier, J.E. A method of treatment for nonunion after fractures using mesenchymal stromal cells loaded on collagen microspheres and incorporated into platelet-rich plasma clots. Int. Orthop. 2016, 40, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Wittig, O.; Diaz-Solano, D.; Cardier, J. Viability and functionality of mesenchymal stromal cells loaded on collagen microspheres and incorporated into plasma clots for orthopaedic application: Effect of storage conditions. Injury 2018, 49, 1052–1057. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, K.; Porat, Z.; Gedanken, A. Designing Natural Polymer-Based Capsules and Spheres for Biomedical Applications—A Review. Polymers 2021, 13, 4307. https://doi.org/10.3390/polym13244307
Sharma K, Porat Z, Gedanken A. Designing Natural Polymer-Based Capsules and Spheres for Biomedical Applications—A Review. Polymers. 2021; 13(24):4307. https://doi.org/10.3390/polym13244307
Chicago/Turabian StyleSharma, Kusha, Ze’ev Porat, and Aharon Gedanken. 2021. "Designing Natural Polymer-Based Capsules and Spheres for Biomedical Applications—A Review" Polymers 13, no. 24: 4307. https://doi.org/10.3390/polym13244307
APA StyleSharma, K., Porat, Z., & Gedanken, A. (2021). Designing Natural Polymer-Based Capsules and Spheres for Biomedical Applications—A Review. Polymers, 13(24), 4307. https://doi.org/10.3390/polym13244307







