Carboxyl-Rich Carbon Dots as Highly Selective and Sensitive Fluorescent Sensor for Detection of Fe3+ in Water and Lactoferrin
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
2.3. Synthesis of COOH-CDs
2.4. Toxicity Experiment
2.5. Fluorescence Selectivity and Sensitive of COOH-CDs towards Fe3+ and Lactoferrin
2.6. Theoretical Calculations
3. Results and Discussion
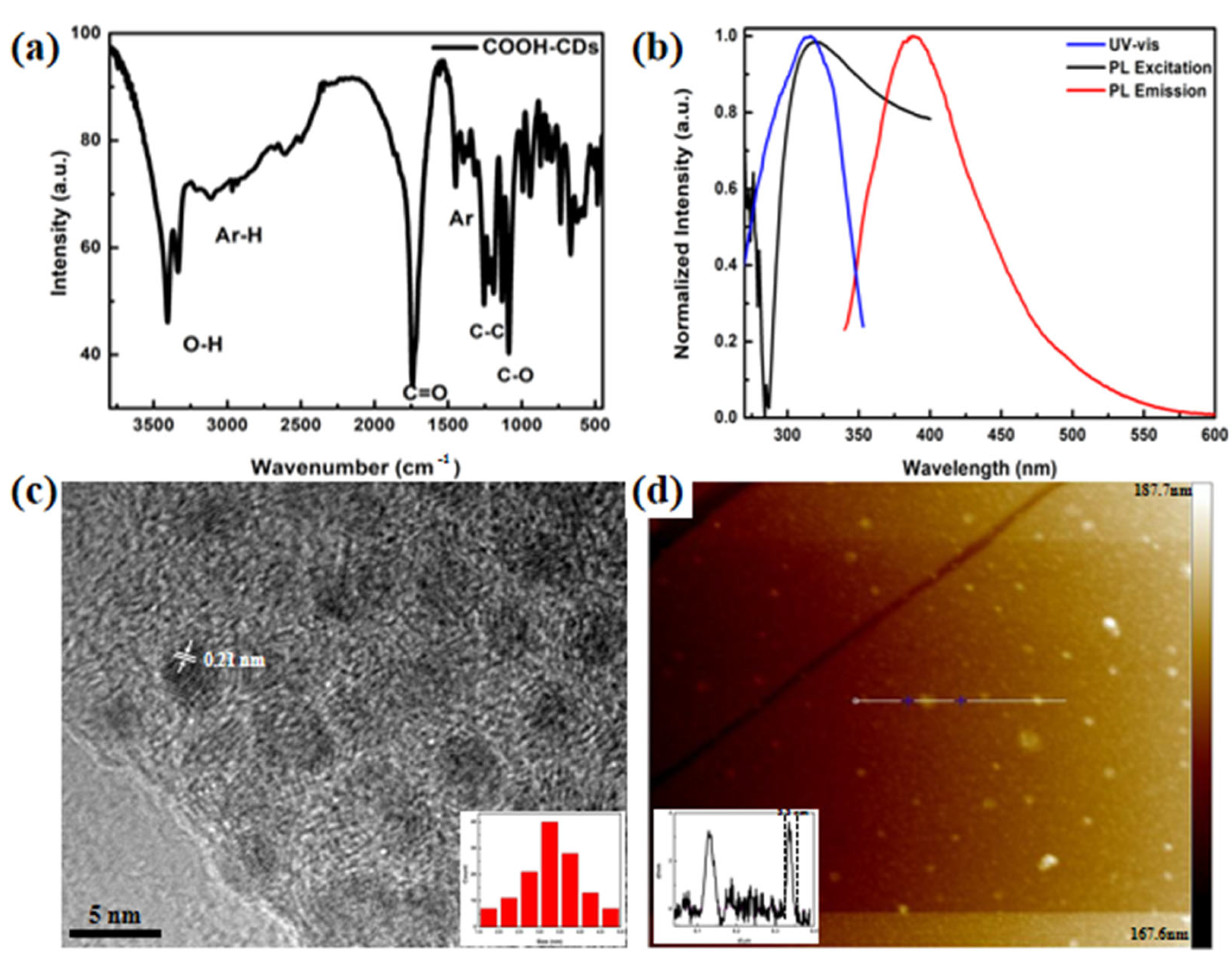
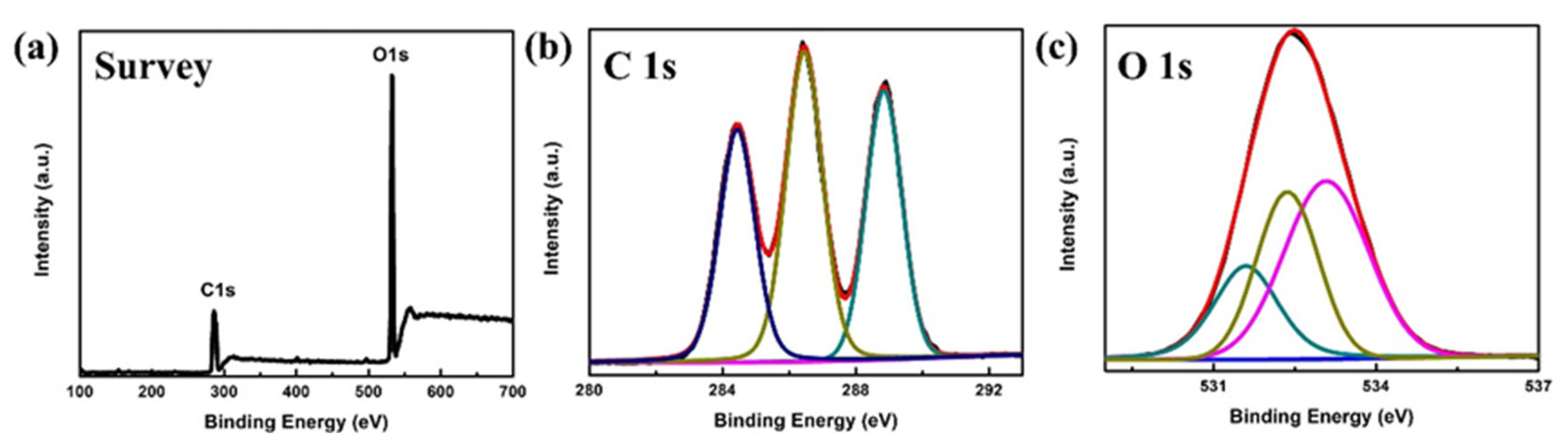
3.1. Characterizations of COOH-CDs
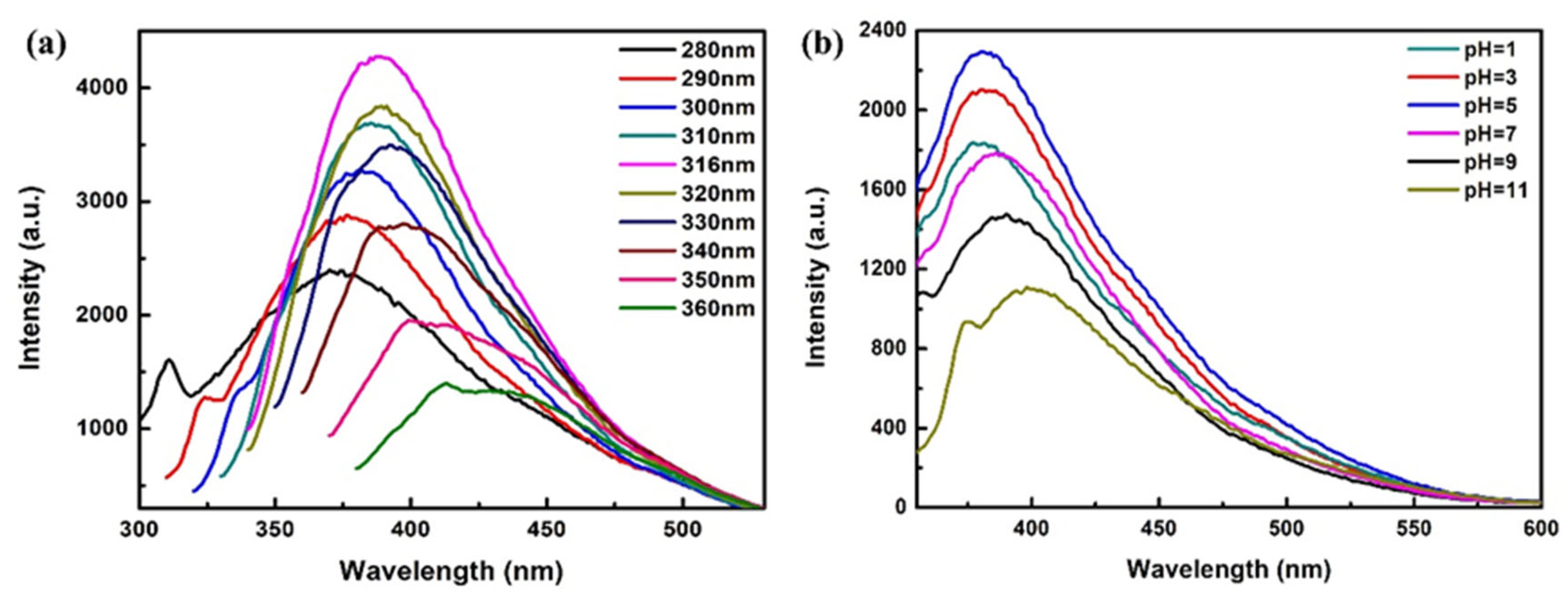
3.2. Effects of Excitation Wavelength and pH on Fluorescent Emission
3.3. Toxicity Study of COOH-CDs
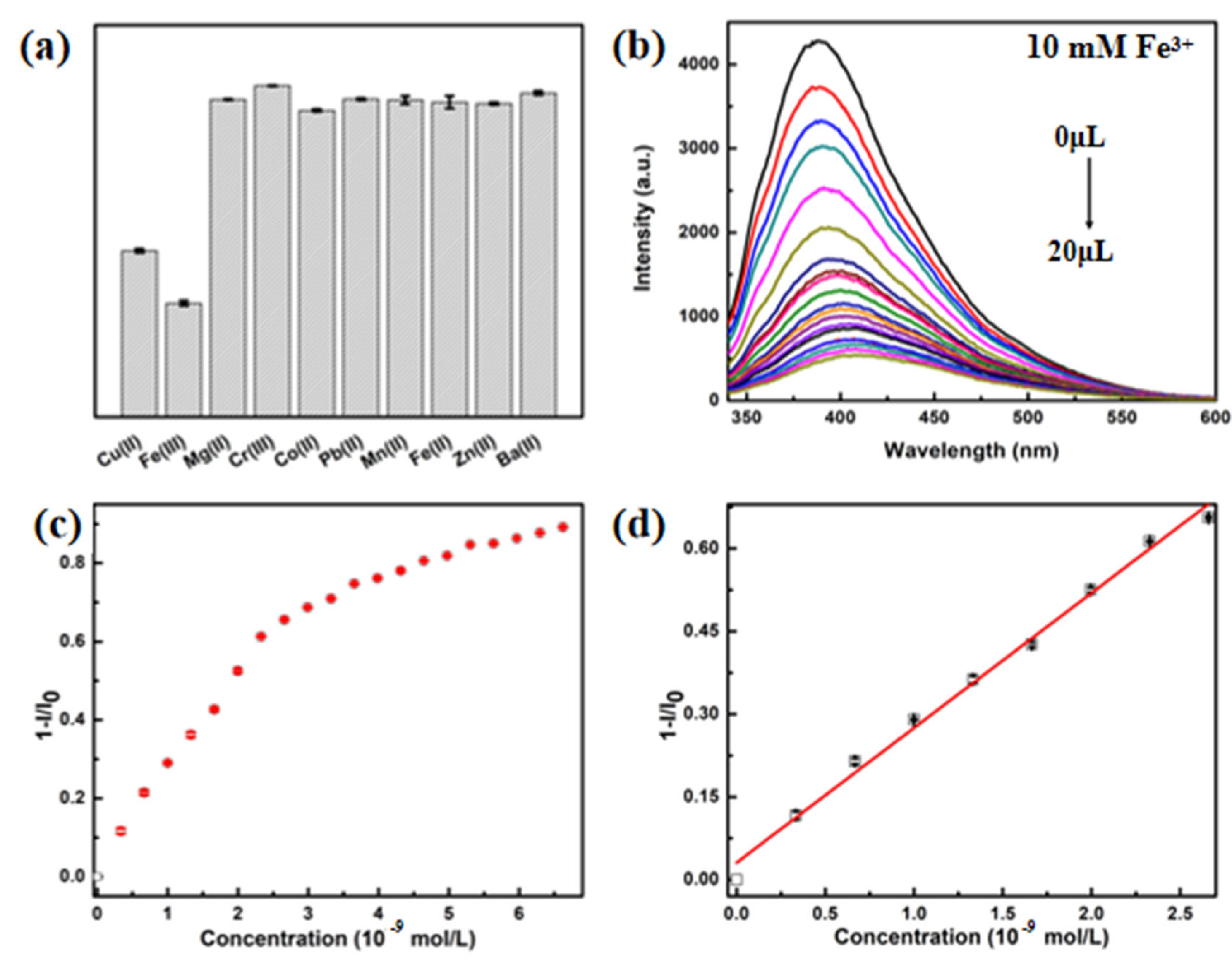
3.4. Fluorescent Selectivity and Sensitivity toward Fe3+
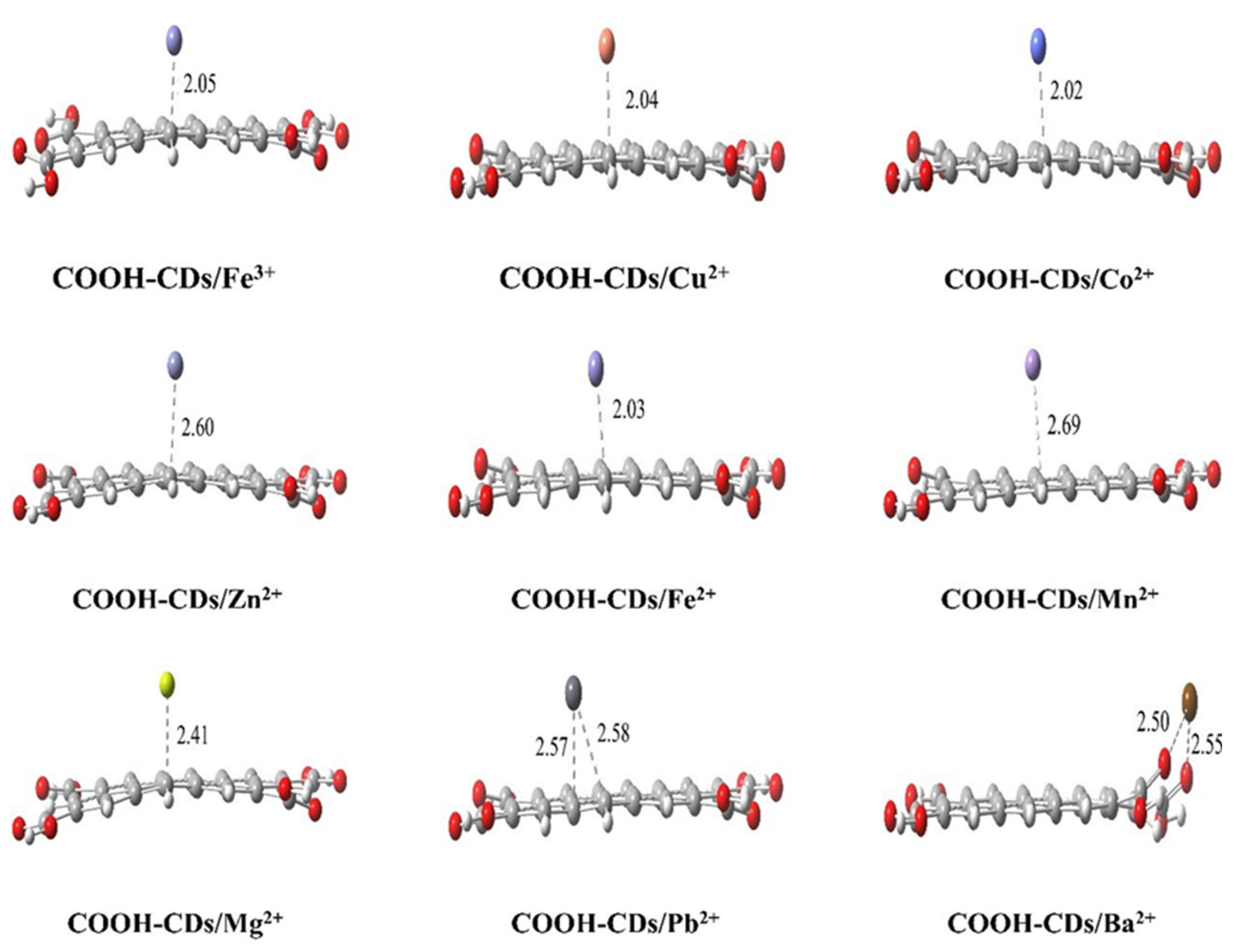
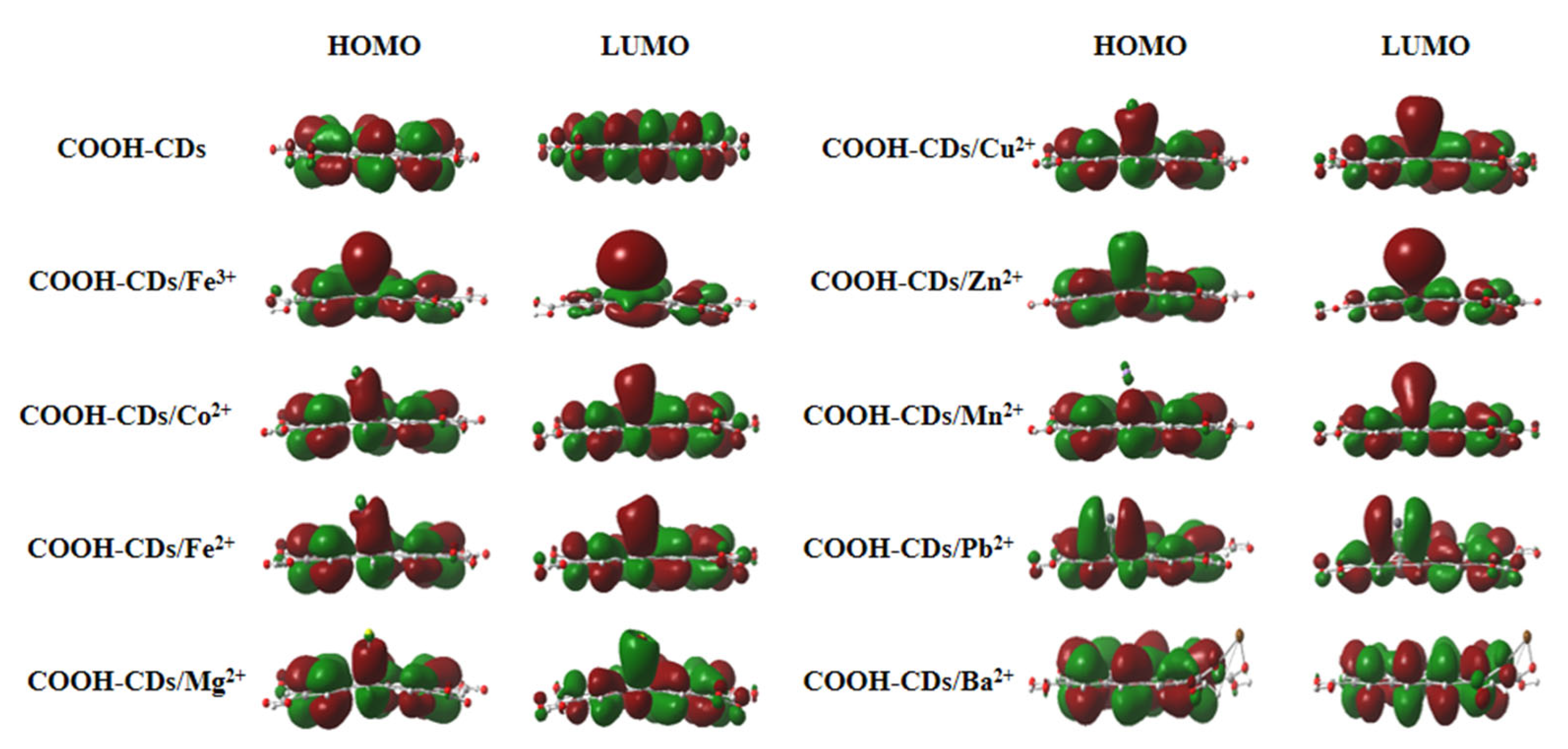
3.5. Theoretical Analysis for the Selectivity of COOH-CDs toward Metal Ions
3.6. Fluorescent Sensing Performance of LF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.J.; Xiao, Q.Q.; Yan, H.; Cao, Y.Q.; Miao, J.J.; Wang, S.M.; Li, X.F.; Li, H.; Cheng, Z.Z. Bovine Lactoferrin Quantification in Dairy Products by a Simple Immunoaffinity Magnetic Purification Method Coupled with High-Performance Liquid Chromatography with Fluorescence Detection. J. Agric. Food. Chem. 2020, 68, 892–898. [Google Scholar] [CrossRef]
- Habing, G.; Harris, K.; Schuenemann, G.M.; Pineiro, J.M.; Lakritz, J.; Clavijo, X.A. Lactoferrin reduces mortality in preweaned calves with diarrhea. J. Dairy Sci. 2017, 100, 3940–3948. [Google Scholar] [CrossRef]
- Chang, R.; Ng, T.B.; Sun, W.Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Ibrahim, S.; Zaim, A.; Ibrahim, W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharm. Ther. 2021, 136, 111228. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin Against SARS-CoV-2: In Vitro and In Silico Evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef] [PubMed]
- Indyk, H.E.; McGrail, I.J.; Watene, G.A.; Filonzi, E.L. Optical biosensor analysis of the heat denaturation of bovine lactoferrin. Food Chem. 2007, 101, 838–844. [Google Scholar] [CrossRef]
- Ke, X.; Chen, Q.; Pan, X.D.; Zhang, J.S.; Mo, W.M.; Ren, Y.P. Quantification of lactoferrin in breast milk by ultra-high performance liquid chromatography-tandem mass spectrometry with isotopic dilution. RSC Adv. 2016, 6, 12280–12285. [Google Scholar] [CrossRef]
- Kudo, H.; Maejima, K.; Hiruta, Y.; Citterio, D. Microfluidic Paper-Based Analytical Devices for Colorimetric Detection of Lactoferrin. Slas Technol. 2020, 25, 47–57. [Google Scholar] [CrossRef] [PubMed]
- El-Hawiet, A. A Simple, Sensitive, and Label-Free Platform for the Quantification of Lactoferrin in Camel and Goat Milk Based on Thin-Layer Chromatography. Chromatographia 2017, 80, 1797–1804. [Google Scholar] [CrossRef]
- Chen, M.X.; Wen, F.; Zhang, Y.D.; Li, P.; Zheng, N.; Wang, J.Q. Determination of native lactoferrin in milk by HPLC on HiTrap Heparin HP column. Food Anal. Method. 2019, 12, 2518–2526. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.W.M.; El Mkami, H.; Cammack, R.; Evans, R.W. Pulsed ELDOR determination of the intramolecular distance between the metal binding sites in dicupric human serum transferrin and lactoferrin. J. Am. Chem. Soc. 2007, 129, 4868. [Google Scholar] [CrossRef]
- Gibbons, J.A.; Kanwar, J.R.; Kanwar, R.K. Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer 2015, 15, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Pyo, C.W.; Hahm, D.H.; Kim, J.; Choi, S.Y. Iron-saturated lactoferrin stimulates cell cycle progression through PI3K/Akt pathway. Mol. Cells 2009, 28, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hancock, R.E.W. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Sui, B.W.; Zhang, Y.P.; Huang, L.; Chen, Y.X.; Li, D.W.; Li, Y.F.; Yang, B. Fluorescent Nanofibrillar Hydrogels of Carbon Dots and Cellulose Nanocrystals and Their Biocompatibility. ACS Sustain. Chem. Eng. 2020, 8, 18492–18499. [Google Scholar] [CrossRef]
- Yang, Y.S.; Gu, B.L.; Liu, Z.D.; Chen, D.; Zhao, Y.; Guo, Q.L.; Wang, G. Hydrothermal synthesis of N, P co-doped graphene quantum dots for high-performance Fe3+ detection and bioimaging. J. Nanopart. Res. 2021, 23, 40. [Google Scholar] [CrossRef]
- Ge, L.; Yu, H.L.; Ren, H.T.; Shi, B.; Guo, Q.; Gao, W.S.; Li, Z.Q.; Li, J.G. Photoluminescence of carbon dots and their applications in Hela cell imaging and Fe3+ ion detection. J. Mater. Sci. 2017, 52, 9979–9989. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, L.X.; Tang, K.; Guan, Y.T.; Wang, T.; Wang, H.; Yu, W.W. Nitrogen-doped carbon dots used as an “on-off-on” fluorescent sensor for Fe3+ and glutathione detection. Dye. Pigment. 2020, 178, 108358. [Google Scholar] [CrossRef]
- Chu, X.H.; Wu, F.; Sun, B.H.; Zhang, M.; Song, S.J.; Zhang, P.; Wang, Y.L.; Zhang, Q.C.; Zhou, N.L.; Shen, J. Genipin cross-linked carbon dots for antimicrobial, bioimaging and bacterial discrimination. Colloid. Surf. B 2020, 190, 110930. [Google Scholar] [CrossRef]
- Abdel-Kader, N.S.; Abdel-Latif, S.A.; El-Ansary, A.L.; Sayed, A.G. Spectroscopic studies, density functional theory calculations, non-linear optical properties, biological activity of 1-hydroxy-4-((4-(N-(pyrimidin-2-yl)sulfamoyl)phenyl)diazenyl)-2-naphthoic acid and its chelates with Nickel (II), Copper (II), Zinc (II) and Palladium (II) metal ions. J. Mol. Struct. 2021, 1223, 129203. [Google Scholar]
- Rao, J.S.; Sastry, G.N. Structural and Energetic Preferences of pi, sigma, and Bidentate Cation Binding (Li+, Na+, and Mg2+) to Aromatic Amines (Ph-(CH2)(n)-NH2, n=2-5): A Theoretical Study. J. Phys. Chem. A 2009, 113, 5446–5454. [Google Scholar] [CrossRef]
- Yadegari, A.; Khezri, J.; Esfandiari, S.; Mandavi, H.; Karkhane, A.A.; Rahighi, R.; Heidarimoghadam, R.; Tayebi, L.; Hashemi, E.; Farmany, A. Bottom-up synthesis of nitrogen and oxygen co-decorated carbon quantum dots with enhanced DNA plasmid expression. Colloid. Surf. B 2019, 184, 110543. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Madni, A.; Kashif, P.M.; Rehman, M.; Raza, A.; Khan, M.I.; Rahim, M.A.; Jabar, A. Formulation and compatibility assessment of PLGA/lecithin based lipid-polymer hybrid nanoparticles containing doxorubicin. Acta Pol. Pharm. 2017, 74, 1563–1572. [Google Scholar]
- Kime, G.; Zhou, K.G.; Hardman, S.J.O.; Nair, R.R.; Novoselov, K.S.; Andreeva, D.V.; Binks, D.J. pH Dependence of Ultrafast Charge Dynamics in Graphene Oxide Dispersions. J. Phycs. Chem. C 2019, 123, 10677–10681. [Google Scholar] [CrossRef] [Green Version]
- He, S.J.; Turnbull, M.J.; Nie, Y.T.; Sun, X.H.; Ding, Z.F. Band structures of blue luminescent nitrogen-doped graphene quantum dots by synchrotron-based XPS. Surf. Sci. 2018, 676, 51–55. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Silva, R.C.; Valenzuela, M.A.; Agarwal, V. 4-nitrophenol optical sensing with N doped oxidized carbon dots. J. Hazard. Mater. 2020, 386, 121643. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xin, Z.J.; Lei, Y.J.; Yang, Y.; Yan, X.Y.; Lu, Y.B.; Li, C.B.; Wang, H.Y. Thin Copper-Based Film for Efficient Electrochemical Hydrogen Production from Neutral Aqueous Solutions. ACS Sustain. Chem. Eng. 2017, 5, 7496–7501. [Google Scholar] [CrossRef]
- Lee, D.S.; Park, S.J. Water-mediated modulation of TiO2 decorated with graphene for photocatalytic degradation of trichloroethylene. Curr. Appl. Phys. 2015, 15, 144–148. [Google Scholar] [CrossRef]
- Mohai, M.; Laszlo, K.; Bertoti, I. Reduction and covalent modification of graphene-oxide by nitrogen in glow discharge plasma. Surf. Interface Anal. 2018, 50, 1207–1212. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yan, Z.W.; Zhou, X.Y.; Zhang, F.J. Purification of PTCDA by Vacuum Sublimation and Spectral Test and Analysis. Spectrosc. Spect. Anal. 2015, 35, 885–888. [Google Scholar]
- Wang, J.L.; Wang, Y.L.; Zheng, J.X.; Yu, S.P.; Yang, Y.Z.; Liu, X.G. Mechanism, Tuning and Application of Excitation-Dependent Fluorescence Property in Carbon Dots. Prog. Chem. 2018, 30, 1186–1201. [Google Scholar]
- Ehtesabi, H.; Hallaji, Z.; Nobar, S.N.; Bagheri, Z. Carbon dots with pH-responsive fluorescence: A review on synthesis and cell biological applications. Microchimi. Acta 2020, 187, 150. [Google Scholar] [CrossRef]
- Hu, Y.P.; Gao, Z.J. Highly Photoluminescent Carbon Dots Derived from Discarded Chewing Gum: Toward Multiple Sensing of pH, Ferric Ion, and Adenosine Triphosphate. Chem. Sel. 2019, 4, 12807–12814. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Liu, J.H.; Hou, P.; Zhou, J.; Huang, C.Z. Preparation of nitrogen-doped carbon dots with high quantum yield from Bombyx mori silk for Fe(III) ions detection. RSC Adv. 2017, 7, 50584–50590. [Google Scholar] [CrossRef] [Green Version]
- Baig, M.M.F.; Chen, Y.C. Bright carbon dots as fluorescence sensing agents for bacteria and curcumin. J. Colloid Interface Sci. 2017, 501, 341–349. [Google Scholar] [CrossRef]
- Liang, Y. A silicon-cored tetraphenyl benzene derivative with aggregation-induced emission enhancement as a fluorescent probe for nitroaromatic compounds detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 216, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, S.; Roy, S.; Chanda, D.K.; Mondal, D.; Das, S.; Das, S. Flexible and reusable carbon dot decorated natural microcline membrane: A futuristic probe for multiple heavy metal induced carcinogen detection. Microchimi. Acta 2021, 188, 134. [Google Scholar] [CrossRef] [PubMed]
- An, Q.X.; Lin, Q.L.; Huang, X.H.; Zhou, R.J.; Guo, X.; Xu, W.Z.; Wang, S.Y.; Xu, D.; Chang, H.T. Electrochemical synthesis of carbon dots with a Stokes shift of 309 nm for sensing of Fe3+ and ascorbic acid. Dye. Pigment. 2021, 185, 108878. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, J.J.; Wang, Y.P.; Li, H.J.; Yin, J.H.; Li, M.Y.; Wang, L.; Sun, H.J.; Chen, L.X. Large-scale direct pyrolysis synthesis of excitation-independent carbon dots and analysis of ferric (III) ion sensing mechanism. Appl. Surf. Sci. 2021, 538, 148151. [Google Scholar] [CrossRef]
- Nanbedeh, S.; Faghihi, K. Synthesis and Characterization of New Mesoporous Polyurethane-Nitrogen Doped Carbon Dot Nanocomposites: Ultrafast, Highly Selective and Sensitive Turn-off Fluorescent Sensors for Fe3+ Ions. J. Fluoresc. 2021, 31, 517–539. [Google Scholar] [CrossRef] [PubMed]
- Song, S.M.; Hu, J.H.; Li, M.L.; Gong, X.J.; Dong, C.; Shuang, S.M. Fe3+ and intracellular pH determination based on orange fluorescence carbon dots co-doped with boron, nitrogen and sulfur. Mat. Sci. Eng. C-Mater. 2021, 118, 111478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Li, C.H.; Zhao, S.L.; Pang, H.Y.; Han, Y.; Luo, X.L.; Tang, W.Z.; Li, Z.H. S doped silicon quantum dots with high quantum yield as a fluorescent sensor for determination of Fe3+ in water. Opt. Mater. 2020, 110, 110461. [Google Scholar] [CrossRef]
- Guo, X.P.; Pan, Q.W.; Song, X.Q.; Guo, Q.Y.; Zhou, S.X.; Qiu, J.R.; Dong, G.P. Embedding carbon dots in Eu3+-doped metal-organic framework for label-free ratiometric fluorescence detection of Fe3+ ions. J. Am. Ceram. Soc. 2021, 104, 886–895. [Google Scholar] [CrossRef]
- Zhang, L.X.; Zhang, Z.S.; Gao, Z.W.; Xie, Y.; Shu, S.; Ke, Y.E.; Wang, Y.; Deng, B.; Yu, R.J.; Geng, H.L. Facile synthesis of N,B-co-doped carbon dots with the gram-scale yield for detection of iron (III) and E. coli. Nanotechnology 2020, 31, 10. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.H.; Lang, K.; Ouyang, H.; Zhang, W.Z.; Bai, L.M.; Chen, S.J.; Zhang, Z.F.; Gao, Y.Y.; Mu, Z.H.; Zhao, X.D. Facile synthesis of N, P-doped carbon dots from maize starch via a solvothermal approach for the highly sensitive detection of Fe3+. RSC Adv. 2020, 10, 33483–33489. [Google Scholar] [CrossRef]
- Han, S.Y.; Ni, J.X.; Han, Y.Q.; Ge, M.; Zhang, C.L.; Jiang, G.Q.; Peng, Z.B.; Cao, J.; Li, S.J. Biomass-Based Polymer Nanoparticles With Aggregation-Induced Fluorescence Emission for Cell Imaging and Detection of Fe3+ Ions. Front. Chem. 2020, 8, 563. [Google Scholar] [CrossRef]
- Murugan, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off ‘ sensor probe for selective detection of Cu2+. Appl. Surf. Sci. 2019, 476, 468–480. [Google Scholar] [CrossRef]
- Baker, H.M.; Baker, E.N. A structural perspective on lactoferrin function. Biochem. Cell Biol. 2012, 90, 320–328. [Google Scholar] [CrossRef]
- Tsakali, E.; Petrotos, K.; Chatzilazarou, A.; Stamatopoulos, K.; D’Alessandro, A.G.; Goulas, P.; Massouras, T.; Van Impe, J.F.M. Short communication: Determination of lactoferrin in Feta cheese whey with reversed-phase high-performance liquid chromatography. J. Dairy Sci. 2014, 97, 4832–4837. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.Q.; Kong, D.Z.; Xing, C.R.; Zhang, X.; Kuang, H.; Xu, C.L. Sandwich immunoassay for lactoferrin detection in milk powder. Anal. Methods 2014, 6, 4742–4745. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.M.; Xu, E.M.; Chen, L.; Feng, H.; Chen, L.; Deng, L.G.; Guo, D.L. Determination of Lactoferrin in Camel Milk by Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry Using an Isotope-Labeled Winged Peptide as Internal Standard. Molecules 2019, 24, 4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sensor System | Analyte | Limit of Detection (LoD) | Refs. |
|---|---|---|---|
| Carbon dot decorated natural microcline membrane | Fe3+ | 61.6 μM | [39] |
| Carbon dots | Fe3+ | 0.16 μM | [40] |
| Carbon dots | Fe3+ | 0.703 μM | [41] |
| Polyurethane–nitrogen-doped carbon dot nanocomposites | Fe3+ | 10.10 μM | [42] |
| B, N, S-doped carbon dots | Fe3+ | 87 nM | [43] |
| S-doped silicon quantum dots | Fe3+ | 0.21 μM | [44] |
| Eu3+-doped metal–organic framework | Fe3+ | 0.897 μM | [45] |
| N, B-co-doped carbon dots | Fe3+ | 0.74 μM | [46] |
| N, P-doped carbon dots | Fe3+ | 0.1 μM | [47] |
| Biomass-based polymer nanoparticles | Fe3+ | 0.17 μM | [48] |
| COOH-CDs/Mn+ Complexes | BEs (kcal/mol) | Egs (eV) | Charge Distributions at Mn+ Surface (Δ a) (a.u.) |
|---|---|---|---|
| COOH-CDs/Fe3+ | −593.82 | 1.87 | 0.87 (−2.13) |
| COOH-CDs/Cu2+ | −291.52 | 2.11 | 0.78 (−1.22) |
| COOH-CDs/Co2+ | −229.54 | 2.15 | 1.34 (−0.63) |
| COOH-CDs/Zn2+ | −211.19 | 2.14 | 1.70 (−0.30) |
| COOH-CDs/Fe2+ | −198.64 | 2.33 | 1.47 (−0.53) |
| COOH-CDs/Mn2+ | −169.55 | 2.52 | 1.68 (−0.32) |
| COOH-CDs/Mg2+ | −146.23 | 2.40 | 1.77 (−0.23) |
| COOH-CDs/Pb2+ | −122.68 | 2.45 | 1.28 (−0.72) |
| COOH-CDs/Ba2+ | −114.39 | 2.68 | 1.47 (−0.53) |
| COOH-CDs/Cr3+ | -- b | -- b | -- b |
| Detect Method | Analyte | Limit of Detection (LoD) | Refs. |
|---|---|---|---|
| Thin-Layer Chromatography | LF | 3.5 µg/mL | [10] |
| Colorimetric | LF | 110 µg/mL | [9] |
| HPLC-UV | LF | 35.4 µg/mL | [51] |
| HPLC | LF | 0.57 mg/L | [11] |
| Sandwich Immunoassay | LF | 3.23 ng/mL | [52] |
| Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry | LF | 3.8 mg/kg | [53] |
| Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry | LF | 1 mg/100 g | [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, Y.; Wang, T.; Liang, Y.; Zhao, X.; Tang, K.; Guan, Y.; Wang, H. Carboxyl-Rich Carbon Dots as Highly Selective and Sensitive Fluorescent Sensor for Detection of Fe3+ in Water and Lactoferrin. Polymers 2021, 13, 4317. https://doi.org/10.3390/polym13244317
Wang X, Zhao Y, Wang T, Liang Y, Zhao X, Tang K, Guan Y, Wang H. Carboxyl-Rich Carbon Dots as Highly Selective and Sensitive Fluorescent Sensor for Detection of Fe3+ in Water and Lactoferrin. Polymers. 2021; 13(24):4317. https://doi.org/10.3390/polym13244317
Chicago/Turabian StyleWang, Xinxin, Yanan Zhao, Ting Wang, Yan Liang, Xiangzhong Zhao, Ke Tang, Yutong Guan, and Hua Wang. 2021. "Carboxyl-Rich Carbon Dots as Highly Selective and Sensitive Fluorescent Sensor for Detection of Fe3+ in Water and Lactoferrin" Polymers 13, no. 24: 4317. https://doi.org/10.3390/polym13244317
APA StyleWang, X., Zhao, Y., Wang, T., Liang, Y., Zhao, X., Tang, K., Guan, Y., & Wang, H. (2021). Carboxyl-Rich Carbon Dots as Highly Selective and Sensitive Fluorescent Sensor for Detection of Fe3+ in Water and Lactoferrin. Polymers, 13(24), 4317. https://doi.org/10.3390/polym13244317






