Future-Oriented Experimental Characterization of 3D Printed and Conventional Elastomers Based on Their Swelling Behavior
Abstract
:1. Introduction
2. Swelling Behavior of Additively Manufactured Soft Polymers in Water and in the Fuel Component Toluene
2.1. Elastomers in the AM Scenario
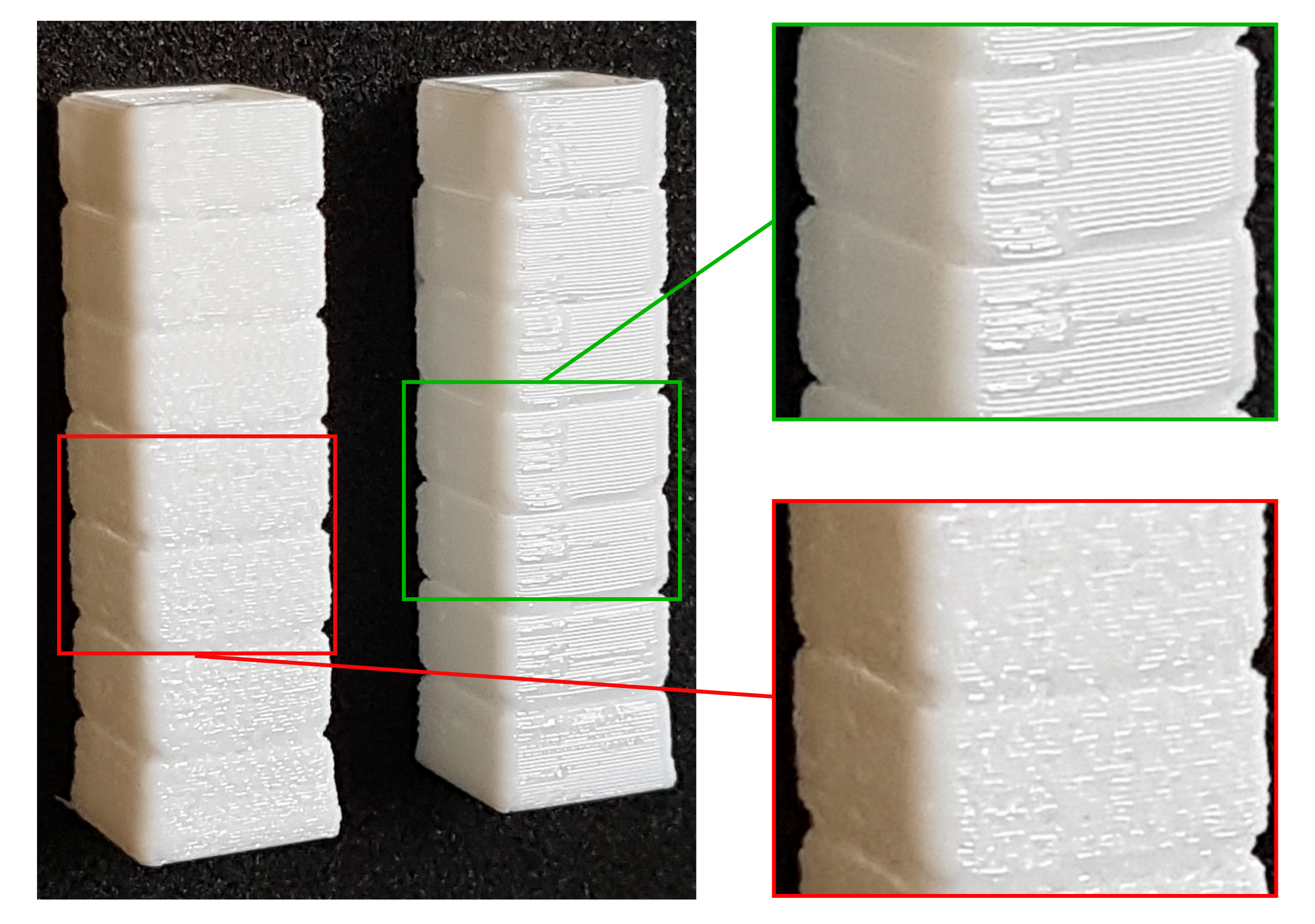
2.2. Overview of the Investigated 3D Printed Elastomers
2.3. Chemical Composition Analysis via IR Spectroscopy
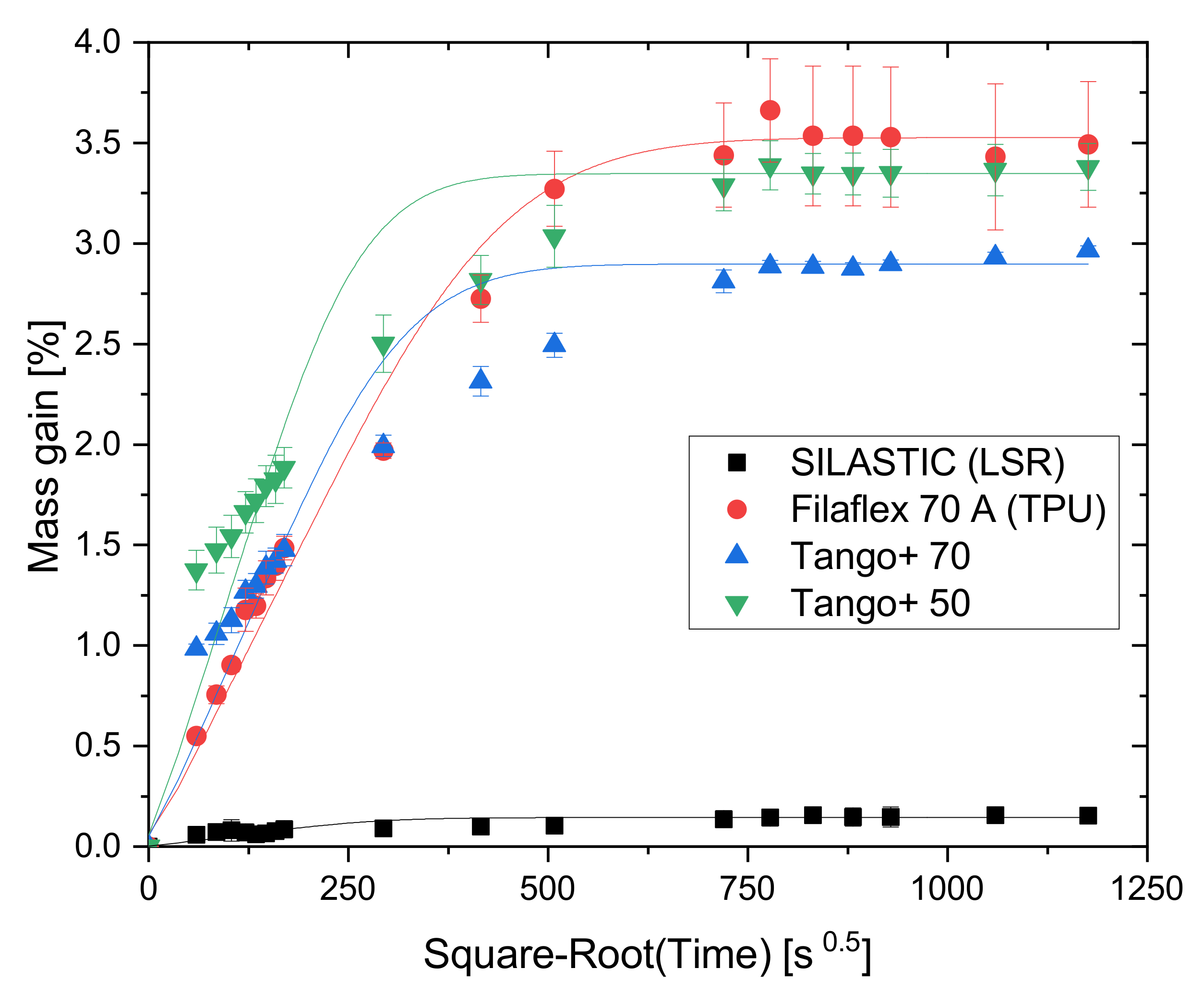
2.4. Absorption Experiments with Toluene and Water
2.4.1. Absorption Test with Toluene as Solvent
2.4.2. Absorption Test with Water
2.5. Mechanical Behavior Analysis via Uniaxial Tensile Tests
2.6. Intermediate Conclusions and Remarks
3. Swelling Behavior of Pristine and Thermo-Oxidatively Aged Elastomers in Synthetic Fuel
3.1. Kerosene and Synthetic Aviation Fuels
3.2. Testing the Interactions of Elastomers with Synthetic Fuels
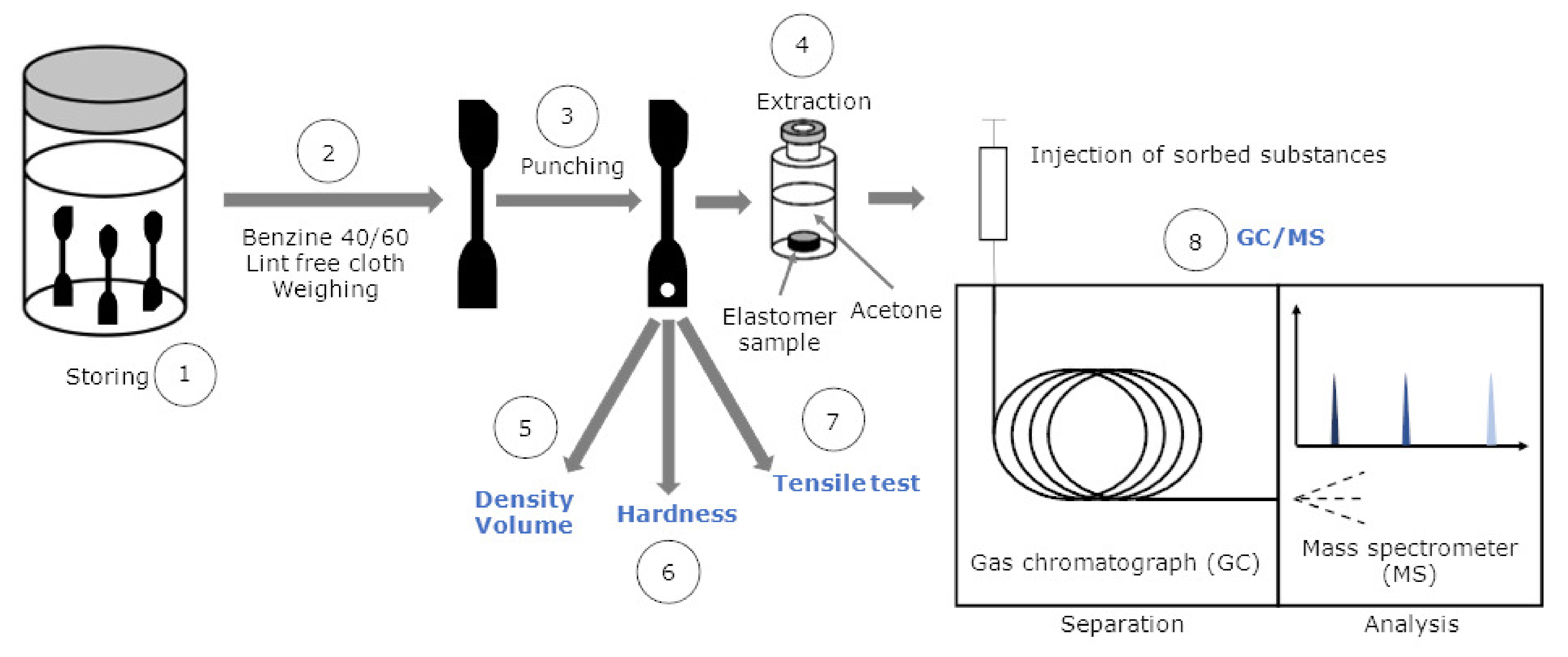
3.3. Experimental Part
3.3.1. Testing Procedure
3.3.2. Gas Chromatography/Mass Spectrometry (GC/MS)
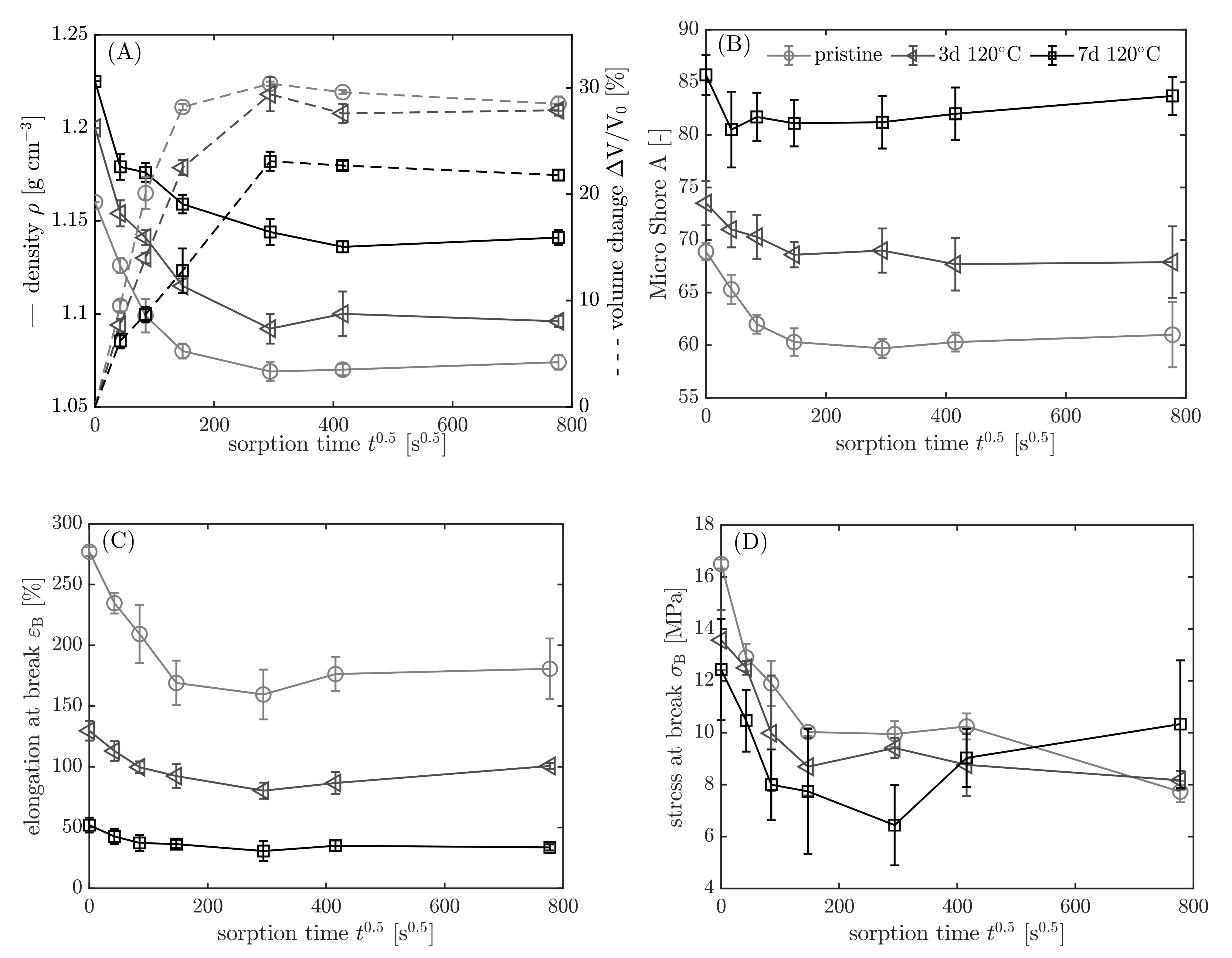
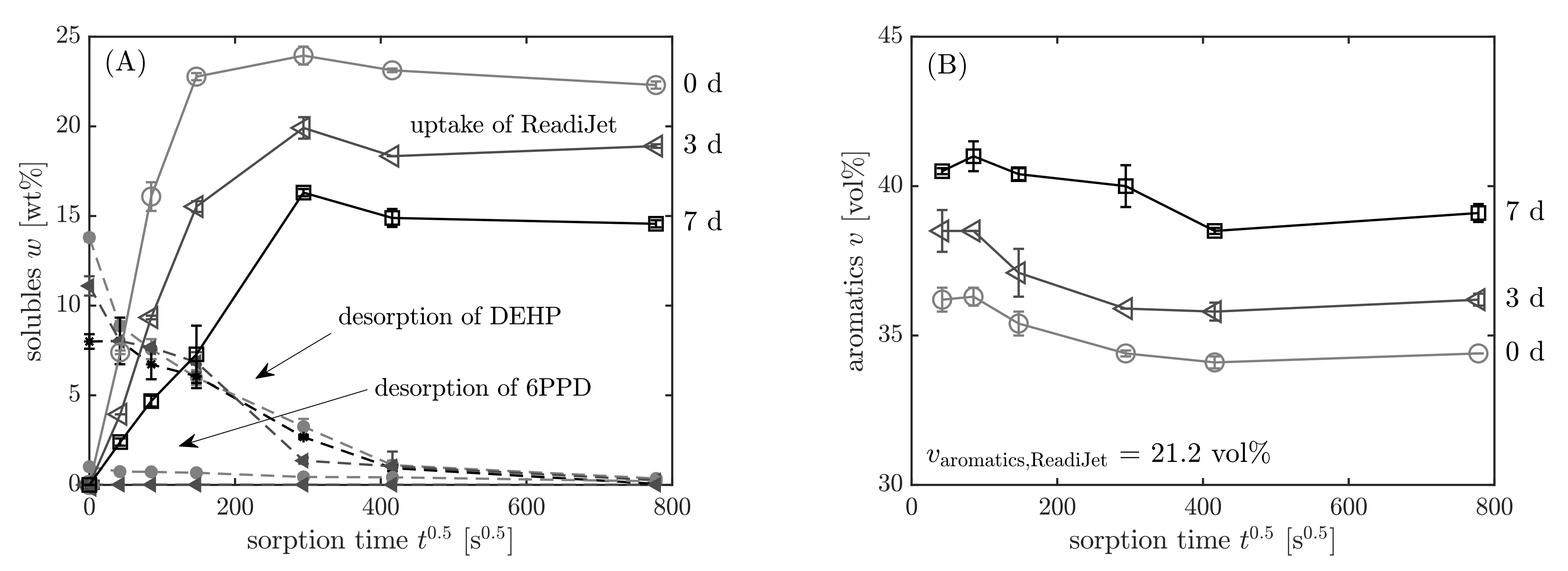
3.3.3. Results
3.4. Intermediate Conclusions and Remarks
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6PPD | Antioxidant N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine |
| AM | Additive Manufacturing |
| ARA | Applied Research Associates |
| ATR | Attenuated total Reflectance |
| CtL | Coal-to-Liquid |
| DEHP | Plasticizer Di(2-ethylhexyl)phthalate |
| FDM | Fused Deposition Modeling |
| FFF | Fused Filament Fabrication |
| FTIR | Fourier Transform Infrared Spectrometer |
| GC/MS | Gas Chromatography coupled with Mass Spectrometry |
| IR | Infrared |
| IATA | International Air Transport Agency |
| LAM | Liquid Additive Manufacturing |
| LSR | Liquid Silicone Rubber |
| MJ | Material Jetting |
| NBR | Acrylonitrile-butadiene-rubber |
| TPE | Thermoplastic Elastomer |
| TPU | Thermoplastic Polyurethane |
References
- Lacuve, M.; Colin, X.; Perrin, L.; Flandin, L.; Notingher, P.; Tourcher, C.; Hassine, M.B.; Tanzeghti, H. Investigation and modelling of the water transport properties in unfilled EPDM elastomers. Polym. Degrad. Stab. 2019, 168, 108949. [Google Scholar] [CrossRef] [Green Version]
- Gong, B.; Tu, Y.; Zhou, Y.; Li, R.; Zhang, F.; Xu, Z.; Liang, D. Moisture absorption characteristics of silicone rubber and its effect on dielectric properties. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Chenzhen, China, 20–23 October 2013. [Google Scholar]
- Förster, T.; Blivernitz, A. Migration of mineral oil in elastomers. J. Rubber Res. 2021, 24, 257–269. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Graham, J.L.; Striebich, R.C.; Myers, K.J.; Minus, D.K.; Harrison, W.E. Swelling of nitrile rubber by selected aromatics blended in a synthetic jet fuel. Energy Fuels 2006, 20, 759–765. [Google Scholar] [CrossRef]
- Blivernitz, A.; Förster, T.; Eibl, S. Simultaneous and time resolved investigation of diffusion processes of individual model fuel components in acrylonitrile-butadiene-rubber in the light of swelling phenomena. Polym. Test. 2018, 70, 47–56. [Google Scholar] [CrossRef]
- Bikas, H.; Stavropoulos, P.; Chryssolouris, G. Additive manufacturing methods and modelling approaches: A critical review. Int. J. Adv. Manuf. Technol. 2016, 83, 389–405. [Google Scholar] [CrossRef] [Green Version]
- Ford, S.; Despeisse, M. Additive manufacturing and sustainability: An exploratory study of the advantages and challenges. J. Clean. Prod. 2016, 137, 1573–1587. [Google Scholar] [CrossRef]
- Janusziewicz, R.; Tumbleston, J.R.; Quintanilla, A.L.; Mecham, S.J.; DeSimone, J.M. Layerless fabrication with continuous liquid interface production. Proc. Natl. Acad. Sci. USA 2016, 113, 11703–11708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Muelhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukić, M.; Clarke, J.; Tuck, C.; Whittow, W.; Wells, G. Printability of elastomer latex for additive manufacturing or 3D printing. J. Appl. Polym. Sci. 2016, 133, 42931. [Google Scholar] [CrossRef] [Green Version]
- Hummel, D.O.; Scholl, F.K. Atlas der Polymer- und Kunststoffanalyse, 2nd ed.; Hanser: München, Germany, 1988. [Google Scholar]
- Salih, S.I.; Oleiwi, J.K.; Ali, H.M. Study the Mechanical Properties of Polymeric Blends (SR/PMMA) Using for Maxillofacial Prosthesis Application. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012086. [Google Scholar] [CrossRef]
- Wang, L.; Ju, Y.; Xie, H.; Ma, G.; Mao, L.; He, K. The mechanical and photoelastic properties of 3D printable stress-visualized materials. Sci. Rep. 2017, 7, 10918. [Google Scholar] [CrossRef] [PubMed]
- DIN Deutsches Institut für Normung e.V. DIN 53504:2017-03 Testing of Rubber—Determination of Tensile Strength at Break, Tensile Stress at Yield, Elongation at Break and Stress Values in a Tensile Test; Beuth Verlag GmbH: Berlin, Germany, 2017. [Google Scholar]
- DIN Deutsches Institut für Normung, e.V. DIN ISO 1817:2016-11 Rubber, Vulcanized or Thermoplastic—Determination of the Effect of Liquids (ISO 1817:2015); Beuth Verlag GmbH: Berlin, Germany, 2016. [Google Scholar]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Ardebili, H.; Zhang, J.; Pecht, M.G. (Eds.) Characterization of encapsulant properties. In Encapsulation Technologies for Electronic Applications, 2nd ed.; William Andrew: Norwich, NY, USA, 2018; pp. 221–258. [Google Scholar]
- Herzberger, J.; Sirrine, J.M.; Williams, C.B.; Long, T.E. Polymer design for 3D printing elastomers: Recent advances in structure, properties, and printing. Prog. Polym. Sci. 2019, 97, 101144. [Google Scholar] [CrossRef]
- Wang, W.; Tao, L. Bio-jet fuel conversion technologies. Renew. Sustain. Energy Rev. 2016, 53, 801–822. [Google Scholar] [CrossRef] [Green Version]
- Riedel, M.B.U. Alternative fuels in aviation. CEAS Aeronaut. J. 2015, 6, 83–93. [Google Scholar]
- Muzzell, P.; Stavinoha, L.; Chapin, R. Synthetic Fischer-Tropsch (FT). In JP-8/JP-5 Aviation turbine Fuel Elastomer Compatibility; Final Report; Defense Technical Information Center: Fort Belvoir, VA, USA, 2005. [Google Scholar]
- Gormley, R.J.; Link, D.D.; Baltrus, J.P.; Zandhuis, P.H. Interactions of Jet Fuels with Nitrile O-Rings: Petroleum-Derived versus Synthetic Fuels. Energy Fuels 2009, 23, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Ynag, R.; Iervolino, R.; Barbera, S. Changes of Chemical Structure and Mechanical Property Levels during Thermo-oxidative Aging of NBR. Rubber Chem. Technol. 2013, 86, 591–603. [Google Scholar] [CrossRef]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Ghani, A. Comprehensive investigation on hydrogen and fuel cell technology in the aviation and aerospace sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Barzkar, A.; Ghassemi, M. Electric Power Systems in More and All Electric Aircraft: A Review. IEEE Access 2020, 8, 169314–169332. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. A comparative life cycle assessment of alternative aviation fuels. Int. J. Sustain. Aviat. 2016, 2, 181–202. [Google Scholar] [CrossRef]
- Scheuermann, S.S.; Forster, S.; Eibl, S. In-Depth Interpretation of Mid-Infrared Spectra of Various Synthetic Fuels for the Chemometric Prediction of Aviation Fuel Blend Properties. Energy Fuels 2017, 31, 2934–2943. [Google Scholar] [CrossRef]
- Zschocke, A.; Scheuermann, S.; Ortner, J. High Biofuel Blends in Aviation (HBBA); Interim Report, ENER C; Deutsche Lufthansa AG: Köln, Germany, 2012. [Google Scholar]
- Pechstein, J.; Zschocke, A. Blending of Synthetic Kerosene and Conventional Kerosene. In Biokerosene; Springer: Berlin/Heidelberg, Germany, 2018; pp. 665–686. [Google Scholar]
- ASTM. ASTM D1655-20: Standard Specification for Aviation Turbine Fuels; Book of Standards Volume: 05.01; ASTM: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Ministry of Defence. Defence Standard 91-091 Turbine Fuel, Aviation Kerosine Type, Jet A-1 NATO Code: F-35 Joint Service Designation: AVTUR; Ministry of Defence: Glasgow, UK, 2019. [Google Scholar]
- ASTM. ASTM D7566-16b: Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons; Book of Standards Volume: 05.04; ASTM: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Wilson, G.R.; Edwards, T.; Corporan, E.; Freerks, R.L. Certification of Alternative Aviation Fuels and Blend Components. Energy Fuels 2013, 27, 962–966. [Google Scholar] [CrossRef]
- Bisignani, G. Annual Report. International Air Transport Association. 2011. Available online: https://www.iata.org/contentassets/c81222d96c9a4e0bb4ff6ced0126f0bb/annual-report-2011.pdf (accessed on 8 November 2021).
- Die Ziele von Aireg Bis 2025. Available online: https://aireg.de/themen/aktuelle-projekte/die-ziele-von-aireg-bis-2025/ (accessed on 8 November 2021).
- Buckley, G.S.; Roland, C.M. Influence of liquid media on lifetime predictions of nitrile rubber. J. Appl. Polym. Sci. 2014, 131, 40296. [Google Scholar] [CrossRef] [Green Version]
- Musil, B.; Demmel, B.; Lion, A.; Johlitz, M. A contribution to the chemomechanics of elastomers surrounded by liquid media: Continuum mechanical approach for parameter identification using the example of sorption experiments. J. Rubber Res. 2021, 24, 271–279. [Google Scholar] [CrossRef]
- Corporan, E.; Edwards, T.; Shafer, L.; Dewitt, M.J.; Klingshirn, C.; Zabarnick, S.; West, Z.; Striebich, R.; Graham, J.; Klein, J. Chemical, Thermal Stability, Seal Swell, and Emissions Studies of Alternative Jet Fuels. Energy Fuels 2011, 25, 955–966. [Google Scholar] [CrossRef]
- Kömmling, A.; Jaunich, M.; Pourmand, P.; Wolff, D.; Hedenqvist, M. Analysis of O-Ring Seal Failure under Static Conditions and Determination of End-of-Lifetime Criterion. Polymers 2019, 11, 1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wilson, C.W.; Blakey, S.; Dolmansley, T. Elastomer Compatibility Test of Alternative Fuels Using Stress-Relaxation Test and FTIR Spectroscopy. In Proceedings of the ASME 2011 Turbo Expo: Turbine Technical Conference and Exposition, GT2011, Vancouver, BC, Canada, 6–10 June 2011; pp. 1–11. [Google Scholar]
- ASTM. ASTM D1319-20a: Standard Test Method for Hydrocarbon Types in Liquid Petroleum Products by Fluorescent Indicator Adsorption; Book of Standards Volume: 05.01; ASTM: West Conshohocken, PA, USA, 2020. [Google Scholar]
- DIN Deutsches Institut für Normung, e.V. Rubber, Vulcanized or Thermoplastic—Determination of Indentation Hardness—Part 1: Durometer Method (Shore Hardness) (ISO 7619-1:2010); Beuth Verlag GmbH: Berlin, Germany, 2012. [Google Scholar]
- DeWitt, M.J.; Corporan, E.; Graham, J.; Minus, D. Effects of aromatic type and concentration in Fischer-Tropsch fuel on emissions production and material compatibility. Energy Fuels 2008, 22, 2411–2418. [Google Scholar] [CrossRef]
- Blivernitz, A. Untersuchung der Verträglichkeit von Elastomeren mit Synthetischen Flugturbinenkraftstoffen Anhand Ablaufender Diffusionsprozesse. Ph.D. Thesis, Universität der Bundeswehr München, Neubiberg, Germany, 2019. [Google Scholar]
- Loos, K.; Blivernitz, A.; Musil, B.; Rehbein, T.; Johlitz, M.; Lion, A. Challenges in Material Modelling and Testing of Elastomers. In Proceedings of the International Rubber Conference, London, UK, 3–5 September 2019. [Google Scholar]
- Zhao, J.; Yang, R.; Iervolino, R.; Barbera, S. Investigation of crosslinking in the thermooxidative aging of nitrile-butadiene rubber. J. Appl. Polym. Sci. 2015, 132, 41319. [Google Scholar] [CrossRef]
- Lachat, V.; Varshney, V.; Dhinojwala, A.; Yeganeh, M.S. Molecular Origin of Solvent Resistance of Polyacrylonitrile. Macromolecules 2009, 42, 7103–7107. [Google Scholar] [CrossRef] [Green Version]






| Property | SILASTIC | Filaflex 70 A | Tango+ 70 | Tango+ 50 |
|---|---|---|---|---|
| Hardness [Shore A] | 50 | 70 | 70 | 50 |
| Tensile Strength [MPa] | 9.5 | 32 | 3.5–5.0 | 1.9–3.0 |
| Elongation at Break [%] | 480 | 900 | 65–80 | 95–110 |
| Printing Technology | LAM | FFF | MJ | MJ |
| Condition | Property | SILASTIC | Filaflex 70 A | Tango+ 70 | Tango+ 50 |
|---|---|---|---|---|---|
| Before water uptake | Stress [MPa] | 11.69 ± 1.46 | 15.82 ± 1.62 | 3.73 ± 0.09 | 1.94 ± 0.02 |
| Strain [%] | 674.47 ± 59.10 | 575.02 ± 16.57 | 78.23 ± 0.50 | 105.93 ± 0.71 | |
| After water uptake | Stress [MPa] | 10.28 ± 1.23 | 14.57 ± 1.41 | 1.84 ± 0.02 | 1.35 ± 0.04 |
| Strain [%] | 636.61 ± 51.35 | 630.99 ± 26.93 | 69.05 ± 0.28 | 97.27 ± 3.70 |
| Component | Content/phr |
|---|---|
| Perbunan 1846 | 100 |
| Di(2-ethylhexyl) phthalate (DEHP) | 20 |
| N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6-PPD) | 2 |
| Carbon black (type: N550) | 60 |
| Zinc oxide | 5 |
| Stearic acid | 1 |
| Sulfur | 2 |
| N-Cyclohexyl-2-benzothiazole sulfenamide (CBS) | 1.5 |
| TMTM-80 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loos, K.; Bruère, V.M.; Demmel, B.; Ilmberger, Y.; Lion, A.; Johlitz, M. Future-Oriented Experimental Characterization of 3D Printed and Conventional Elastomers Based on Their Swelling Behavior. Polymers 2021, 13, 4402. https://doi.org/10.3390/polym13244402
Loos K, Bruère VM, Demmel B, Ilmberger Y, Lion A, Johlitz M. Future-Oriented Experimental Characterization of 3D Printed and Conventional Elastomers Based on Their Swelling Behavior. Polymers. 2021; 13(24):4402. https://doi.org/10.3390/polym13244402
Chicago/Turabian StyleLoos, Klara, Vivianne Marie Bruère, Benedikt Demmel, Yvonne Ilmberger, Alexander Lion, and Michael Johlitz. 2021. "Future-Oriented Experimental Characterization of 3D Printed and Conventional Elastomers Based on Their Swelling Behavior" Polymers 13, no. 24: 4402. https://doi.org/10.3390/polym13244402
APA StyleLoos, K., Bruère, V. M., Demmel, B., Ilmberger, Y., Lion, A., & Johlitz, M. (2021). Future-Oriented Experimental Characterization of 3D Printed and Conventional Elastomers Based on Their Swelling Behavior. Polymers, 13(24), 4402. https://doi.org/10.3390/polym13244402






