Effects of Monomer Composition of Urethane Methacrylate Based Resins on the C=C Degree of Conversion, Residual Monomer Content and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
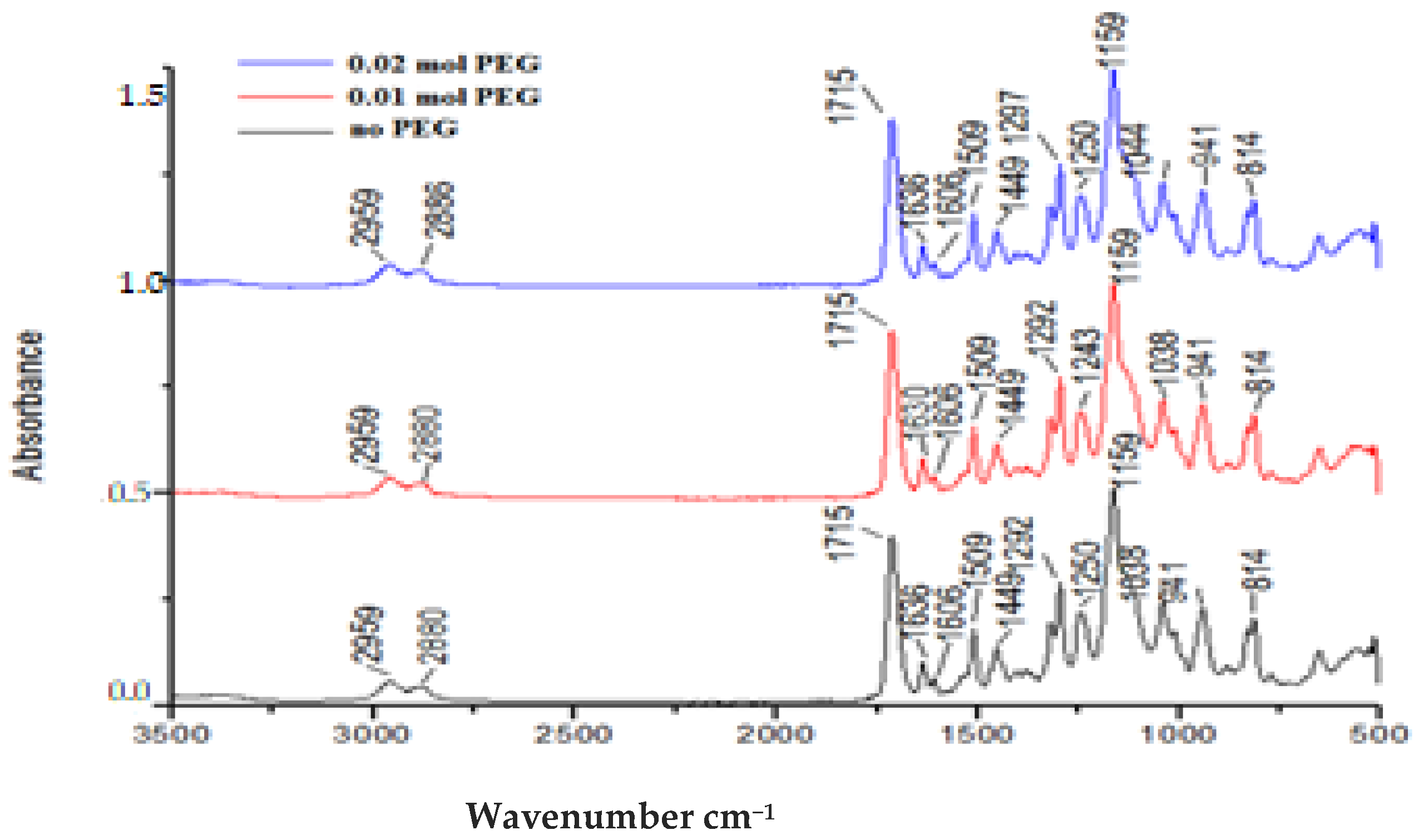
2.2. Syntheses of Dimethacrylates
2.3. Preparation of Experimental Resin Formulations
2.4. In Vitro Analysis Methods
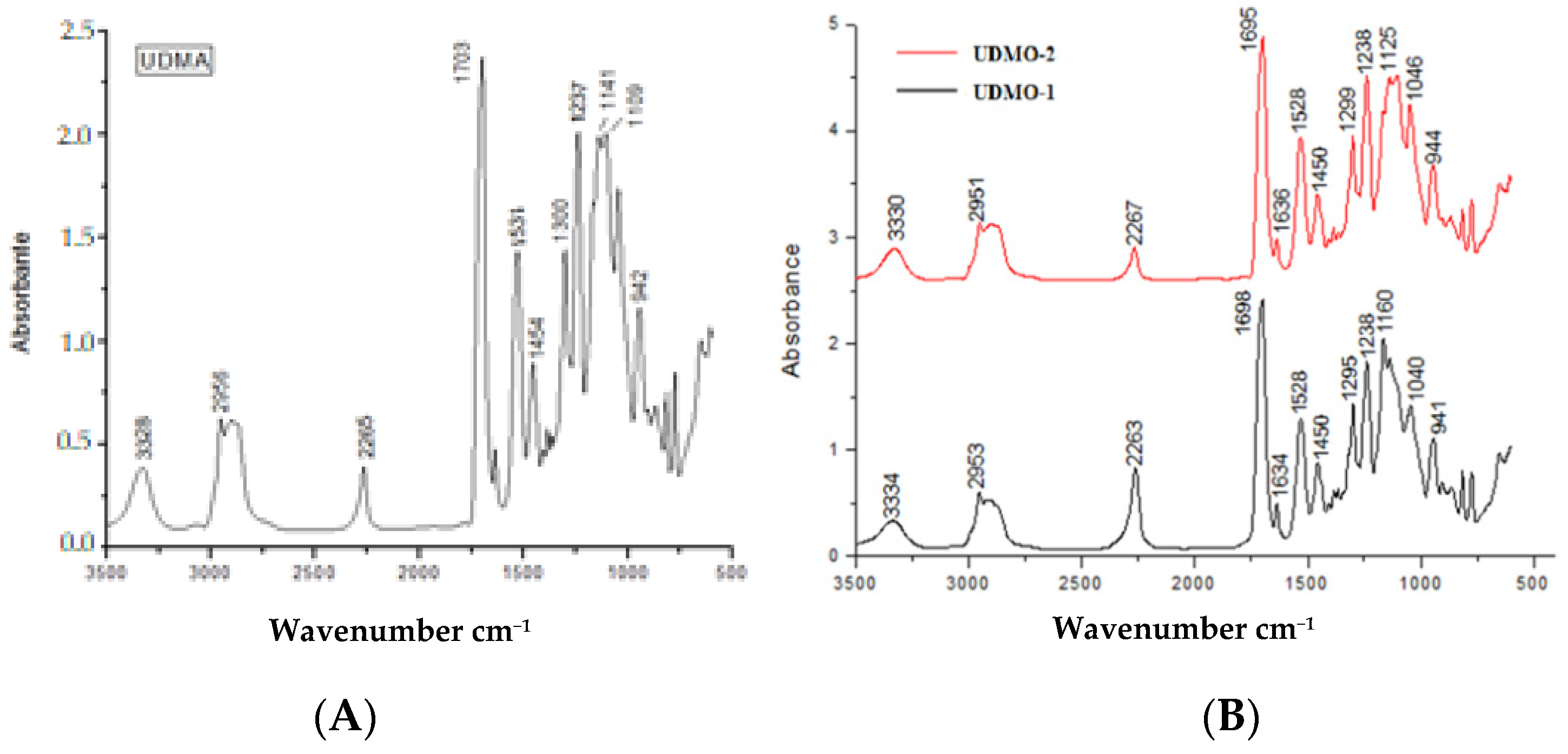
2.4.1. Fourier Transmission Infra-Red Spectroscopy (FTIR)
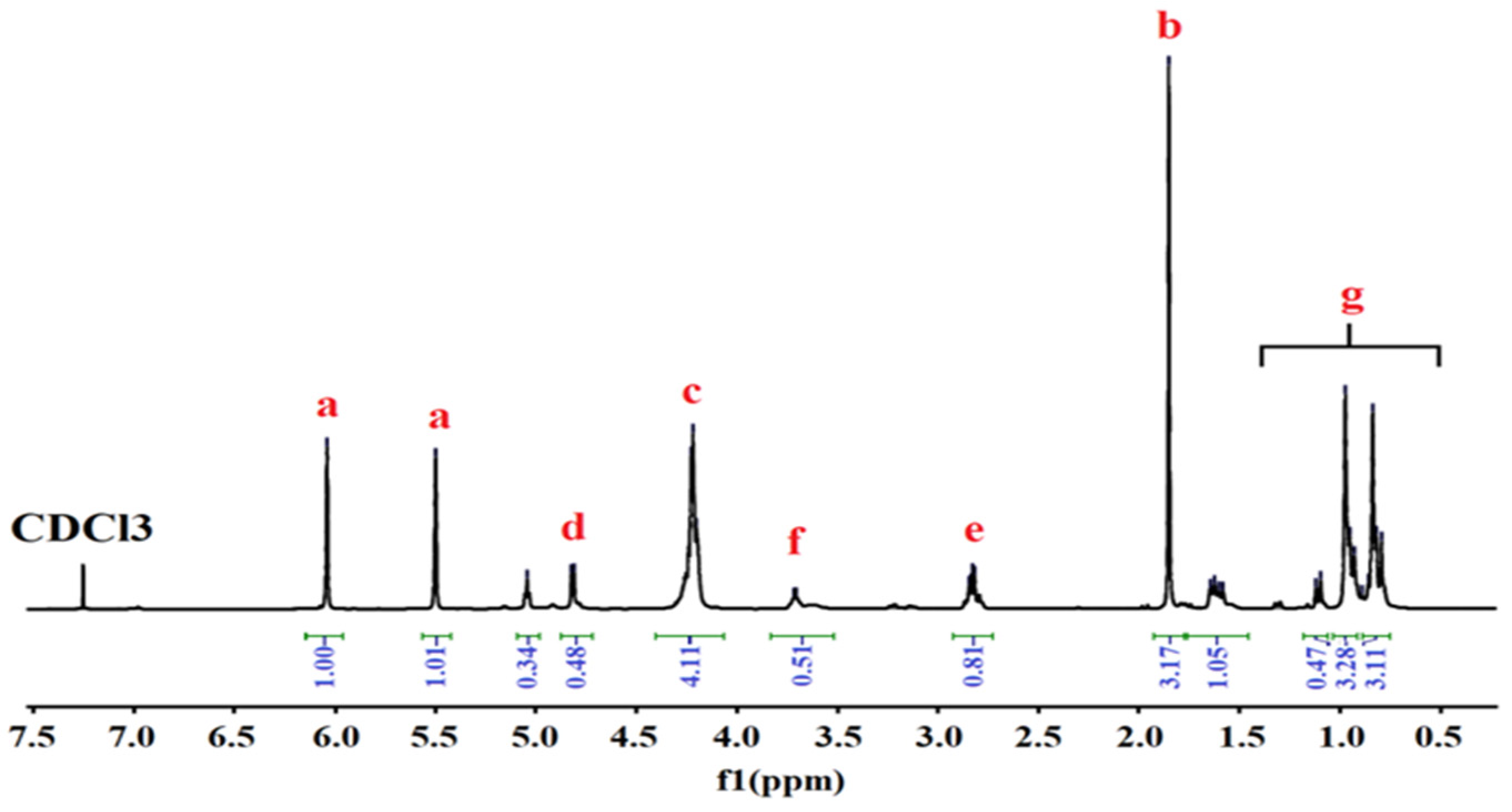
2.4.2. 1H-NMR Analysis
2.4.3. Measurement of Double Bond Conversion
2.4.4. Mechanical Properties
2.4.5. Residual Monomer Determination
3. Results
3.1. Fourier Transmission Infra-Red Spectroscopy (FTIR)
3.2. 1H-NMR Analysis
3.3. Double Bond Conversion
3.4. Mechanical Properties
3.5. Residual Monomer Determination
4. Discussion
5. Conclusions
- (1)
- Isocyanate double bond consumption in the process of urethane methacrylate synthesis can be improved by adding PEG in the optimal molar ratio of isophorone diisocyanate:HEMA:PEG = 2:2:1.
- (2)
- Monomer conversion following the photopolymerization process affects both the strength and mechanical properties of final dental composites and also results in pendant methacrylate groups and unreacted monomers trapped in the material.
- (3)
- The concentration of leached out monomer depends not only on the concentration of unreacted monomers but also on the morphology of the tested material and the associated differences in its chemical composition: the structural differences of the used base monomer, curing time, and final network characteristics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iftikhar, S.; Jahanzeb, N.; Saleem, M.; ur Rehman, S.; Matinlinna, J.P.; Khan, A.S. The trends of dental biomaterials research and future directions: A mapping review. Saudi Dent. J. 2021, 33, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Kumar, M. Dental restorative composite materials: A review. J. Oral Biosci. 2019, 61, 78–83. [Google Scholar] [CrossRef]
- Kim, K.-H.; Ong, J.L.; Okuno, O. The effect of filler loading andmorphology on the mechanical properties of contemporary composites. J. Prosthet. Dent. 2002, 87, 642–649. [Google Scholar] [CrossRef]
- Pfeifer, C.S.; Shelton, Z.R.; Braga, R.R.; Windmoller, D.; MacHado, J.C.; Stansbury, J.W. Characterization of dimethacrylate polymeric networks: A study of the crosslinked structure formed by monomers used in dental composites. Eur. Polym. J. 2011, 47, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Combe, E.; Burke, F.J.; Bernard, T.; Douglas, W. Dental Biomaterials; Kluwer Academic Publishers: Boston, MA, USA, 1999. [Google Scholar]
- Huang, Q.; Lin, Z.; Liang, X.; Liu, F.; He, J. Preparation and characterization of antibacterial dental resin with UDMQA-12. Adv. Polym. Technol. 2014, 33, 21395. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Vallittu, P.K.; Lassila, L.V. Preparation and Evaluation of Dental Resin with Antibacterial and Radio-Opaque Function. Int. J. Mol. Sci. 2013, 14, 5445–5460. [Google Scholar] [CrossRef]
- Peutzfeldt, A. Resin composites in dentistry: The monomer systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef]
- Ilie, N.; Hilton, T.J.; Heintze, S.D.; Hickel, R.; Watts, D.C.; Silikas, N.; Stansbury, J.W.; Cadenaro, M.; Ferracane, J.L. Academy of Dental Materials guidance—Resin composites: Part I—Mechanical properties. Dent. Mater. 2017, 33, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin Composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, M. Structure–property relationship in new photo-cured dimethacrylate-based dental resins. Dent. Mater. 2012, 28, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Charasseangpaisarn, T.; Wiwatwarrapan, C.; Leklerssiriwong, N. Ultrasonic cleaning reduces the residual monomer in acrylic resins. J. Dent. Sci. 2016, 11, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Leprince, J.G.; Palin, W.M.; Hadis, M.A.; Devaux, J.; Leloup, G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent. Mater. 2013, 29, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, F.J.; Serrano-Belmonte, I.; Pérez Calvo, J.C.; Coronado-Parra, M.T.; Bernabeu-Esclapez, A.; Moraleda, J.M. Effects of two low-shrinkage composites on dental stem cells (viability, cell damaged or apoptosis and mesenchymal markers expression). J. Mater. Sci. Mater. Med. 2013, 24, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Cebe, M.A.; Cebe, F.; Cengiz, M.F.; Cetin, A.R.; Arpag, O.F.; Ozturk, B. Elution of monomer from different bulk fill dental composite resins. Dent. Mater. 2015, 31, e141–e149. [Google Scholar] [CrossRef]
- Fonseca, A.S.Q.S.; Moreira, A.D.L.; de Albuquerque, P.P.A.C.; de Menezes, L.R.; Pfeifer, C.S.; Schneider, L.F.J. Effect of monomer type on the C=C degree of conversion, water sorption and solubility, and color stability of model dental composites. Dent. Mater. 2017, 33, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Vallittu, P.K.; Ruyter, I.E.; Buykuilmaz, S. Effect of polymerization temperature and time on the residual monomer content of denture base polymers. Eur. J. Oral Sci. 1998, 106, 588–593. [Google Scholar] [CrossRef]
- Manojlovic, D.; Radisic, M.; Vasiljevic, T.; Zivkovic, S.; Lausevic, M.; Miletic, V. Monomer elution from nanohybrid and ormocer-based composites cured with different light sources. Dent. Mater. 2011, 27, 371–378. [Google Scholar] [CrossRef]
- Douglas, P.; Albadarin, A.B.; Sajjia, M.; Mangwandi, C.; Kuhs, M.; Collins, M.N.; Walker, G.M. Effect of poly ethylene glycol on the mechanical and thermal properties of bioactive poly(å-caprolactone) melt extrudates for pharmaceutical applications. Int. J. Pharm. 2016, 500, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Herrera-González, A.M.; Caldera-Villalobos, M.; Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; González-López, J.A. Analysis of Double Bond Conversion of Photopolymerizable Monomers by FTIR-ATR Spectroscopy. J. Chem. Educ. 2019, 96, 1786–1789. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 4049:2000 Denstistry—Polymer-Based Filling, Restorative and Luting Materials; ISO: Genève, Switzerland, 2000. [Google Scholar]
- Liang, X.; Huang, Q.; Liu, F.; He, J.; Lin, Z. Synthesis of novel antibacterial monomers (UDMQA) and their potential application in dental resin. J. Appl. Polym. Sci. 2013, 129, 3373–3381. [Google Scholar] [CrossRef]
- Andreani, L.; Silva, L.L.; Witt, M.A.; Meier, M.M.; Joussef, A.C.; Soldi, V. Development of dental resinous systems composed of bisphenol a ethoxylated dimethacrylate and three novel methacrylate monomers: Synthesis and characterization. J. Appl. Polym. Sci. 2013, 128, 725–734. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Dickens, H.S. Determination of double bond conversion in a dental resin by near infrared spectroscopy. Dent. Mater. 2007, 17, 71–79. [Google Scholar] [CrossRef]
- Liu, D.; Liu, F.; He, J.; Lassila, L.V.; Vallittu, P.K. Synthesis of a novel tertiary amine containing urethane dimethacrylate monomer (UDMTA) and its application in dental resin. J. Mater. Sci. Mater. Med. 2013, 24, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Buruiana, T.; Buruiana, E.C.; Melinte, V.; Colceriu, A.; Moldovan, M. Urethane dimethacrylate oligomers for dental composite matrix: Synthesis and properties. Polym. Eng. Sci. 2009, 49, 1127–1135. [Google Scholar] [CrossRef]
- Willems, G.; Lambrechts, P.; Braem, M.; Celis, J.P.; Vanherle, G. A classification of dental composites according to their morphological and mechanical characteristics. Dent. Mater. 1992, 8, 310–319. [Google Scholar] [CrossRef]
- Alshali, R.Z.; Salim, N.A.; Sung, R.; Satterthwaite, J.D.; Silikas, N. Qualitative and quantitative characterization of monomers of uncured bulk-fill and conventional resin-composites using liquid chromatography/mass spectrometry. Dent. Mater. 2015, 31, 711–720. [Google Scholar] [CrossRef]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the Degree of Conversion, Residual Monomers and Mechanical Properties of Some Light-Cured Dental Resin Composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floyd, C.J.E.; Dickens, S.H. Network structure of Bis-GMA- and UDMA- based resin systems. Dent. Mater. 2006, 22, 1143–1149. [Google Scholar] [CrossRef]
- Kerby, R.E.; Knobloch, L.A.; Schricker, S.; Gregg, B. Synthesis and evaluation of modified urethane dimethacrylate resins with reduced water sorption and solubility. Dent. Mater. 2009, 25, 302–313. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 20795-1:2013 Specification Dentistry—Denture Base Polymers; International Organization for Standardization: Genève, Switzerland, 2013. [Google Scholar]
- Łagocka, R.; Jakubowska, K.; Chlubek, D.; Buczkowska-Radlińska, J. Elution study of unreacted TEGDMA from bulk-fill composite (SDR™ Dentsply) using HPLC. Adv. Med. Sci. 2015, 60, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Denis, A.B.; Diagone, C.A.; Plepis, A.M.; Viana, R.B. The effect of the polymerization initiator and light source on the elution of residual Bis-GMA and TEGDMA monomers: A study using liquid chromatography with UV detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Lempel, E.; Czibulya, Z.; Kovács, B.; Szalma, J.; Tóth, Á.; Kunsági-Máté, S.; Varga, Z.; Böddi, K. Degree of Conversion and BisGMA, TEGDMA, UDMA Elution from Flowable Bulk Fill Composites. Int. J. Mol. Sci. 2016, 17, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Resin Formulation | Monomer Composition (%) | Photoinitiator System (%) | Light Curing Time (s) | |||||
|---|---|---|---|---|---|---|---|---|
| UDMA | UDMO1 | UDMO2 | Bis-GMA | TEGDMA | DMAEM | CQ | ||
| L.1.1. | 20 | - | - | 45 | 33.5 | 1 | 0.5 | 20 |
| L.1.2. | 20 | - | - | 45 | 33.5 | 1 | 0.5 | 40 |
| L.2.1. | - | 20 | - | 45 | 33.5 | 1 | 0.5 | 20 |
| L.2.2. | - | 20 | - | 45 | 33.5 | 1 | 0.5 | 40 |
| L.3.1. | - | - | 20 | 45 | 33.5 | 1 | 0.5 | 20 |
| L.3.2. | - | - | 20 | 45 | 33.5 | 1 | 0.5 | 40 |
| Resin Formulation | DC (%) ± SD |
|---|---|
| L.1.1. | 56.704 ± 0.845 a |
| L.1.2. | 63.199 ± 1.006 b |
| L.2.1. | 44.585 ± 1.055 c |
| L.2.2. | 56.182 ± 1.014 a |
| L.3.1. | 56.157 ± 0.729 a |
| L.3.2. | 67.418 ± 0.553 d |
| p | 1.11793 × 10−11 |
| Resin Formulation | Bis-GMA (%) | TEGDMA (%) | UDMA (%) |
|---|---|---|---|
| L.1.1. | 0.586 | 0.246 | 0.332 |
| L.1.2. | 0.504 | 0.204 | 0.288 |
| L.2.1. | 0.524 | 0.216 | 0.307 |
| L.2.2. | 0.462 | 0.226 | 0.265 |
| L.3.1. | 0.536 | 0.276 | 0.284 |
| L.3.2. | 0.589 | 0.275 | 0.341 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarosi, C.; Moldovan, M.; Soanca, A.; Roman, A.; Gherman, T.; Trifoi, A.; Chisnoiu, A.M.; Cuc, S.; Filip, M.; Gheorghe, G.F.; et al. Effects of Monomer Composition of Urethane Methacrylate Based Resins on the C=C Degree of Conversion, Residual Monomer Content and Mechanical Properties. Polymers 2021, 13, 4415. https://doi.org/10.3390/polym13244415
Sarosi C, Moldovan M, Soanca A, Roman A, Gherman T, Trifoi A, Chisnoiu AM, Cuc S, Filip M, Gheorghe GF, et al. Effects of Monomer Composition of Urethane Methacrylate Based Resins on the C=C Degree of Conversion, Residual Monomer Content and Mechanical Properties. Polymers. 2021; 13(24):4415. https://doi.org/10.3390/polym13244415
Chicago/Turabian StyleSarosi, Codruta, Marioara Moldovan, Andrada Soanca, Alexandra Roman, Timea Gherman, Ancuta Trifoi, Andrea Maria Chisnoiu, Stanca Cuc, Miuta Filip, Georgiana Florentina Gheorghe, and et al. 2021. "Effects of Monomer Composition of Urethane Methacrylate Based Resins on the C=C Degree of Conversion, Residual Monomer Content and Mechanical Properties" Polymers 13, no. 24: 4415. https://doi.org/10.3390/polym13244415
APA StyleSarosi, C., Moldovan, M., Soanca, A., Roman, A., Gherman, T., Trifoi, A., Chisnoiu, A. M., Cuc, S., Filip, M., Gheorghe, G. F., & Chisnoiu, R. M. (2021). Effects of Monomer Composition of Urethane Methacrylate Based Resins on the C=C Degree of Conversion, Residual Monomer Content and Mechanical Properties. Polymers, 13(24), 4415. https://doi.org/10.3390/polym13244415